Imaging of Ulcerative Colitis: The Role of Diffusion-Weighted Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Overview of the DWI Method and Its Advancements
Advantages of DWI in IBD
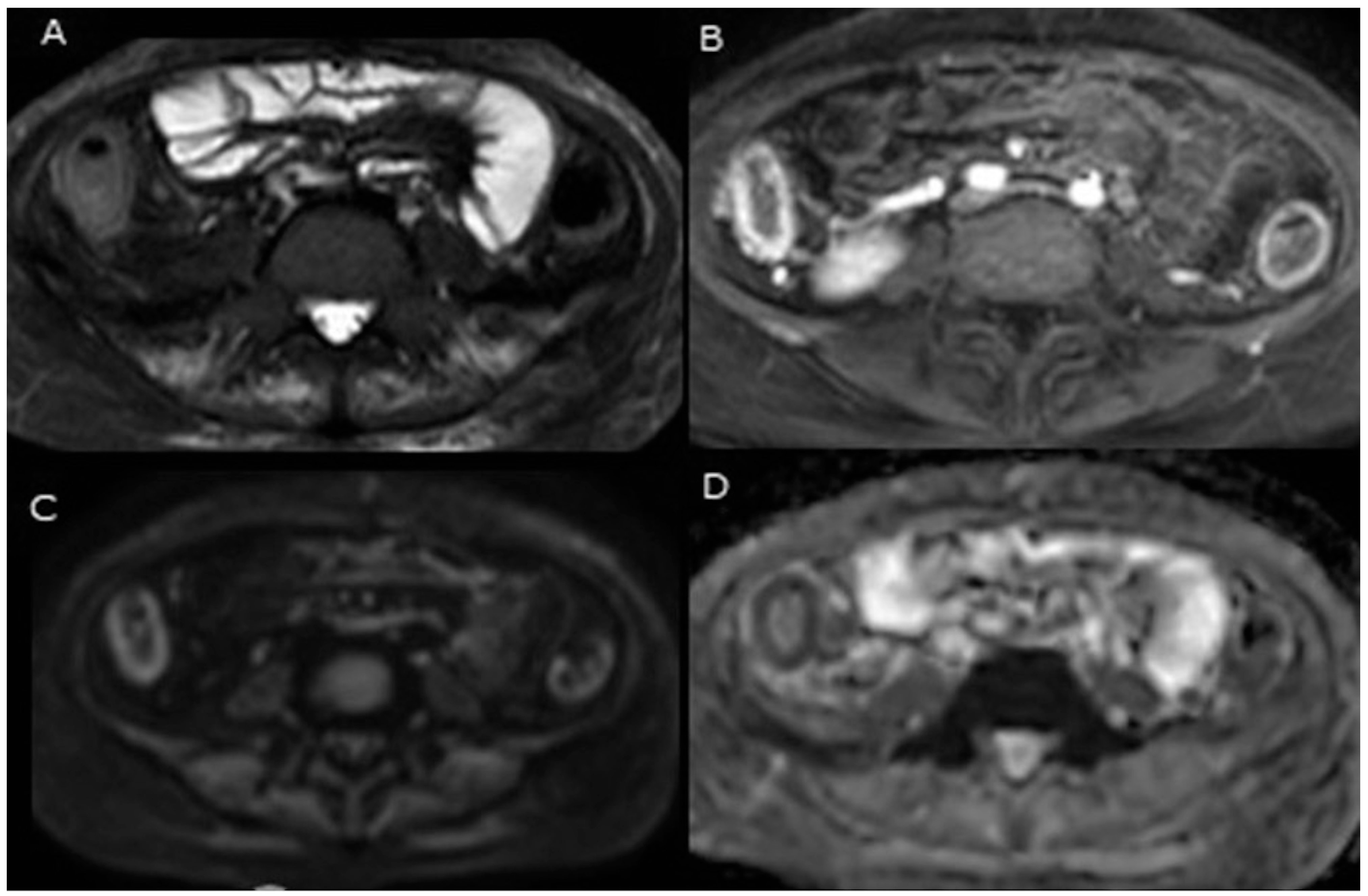
- Bowel preparation is not required for the detection of colonic inflammation using DWI [24]. While most magnetic resonance colonography (MRC) protocols typically involve colonic distension using water or contrast enemas, DWI-MRC stands out as an exception, as it does not require colonic distension [34].
- There is no need for intravenous contrast material. The main advantage of DWI in comparison to conventional MRI is its ability to detect bowel wall inflammation without the need for intravenous contrast material [24].
- Unlike endoscopy, which can be uncomfortable and invasive, DWI uses MRI to assess disease activity without exposure to ionising radiation [35].
- DWI offers a distinctive advantage in its capacity to evaluate tissue cellularity and microstructural integrity. Through the analysis of water molecule diffusion, MRI can provide valuable information regarding the cellular environment within tumours. This enables differentiation among various tissue types and facilitates the early detection of therapeutic response-related changes. The functional imaging capability of DWI complements conventional anatomical imaging, thereby enabling a more comprehensive assessment of different diseases, including IBD.
3. The Role of MRI in UC Detection and Management
| Imaging Modality | Pros | Cons |
|---|---|---|
| MRI | Safe and non-invasive No exposure to harmful ionizing radiation Ability to image the bowel repeatedly over time Exceptional soft tissue contrast enables superior evaluation of disease activity and the identification of penetrating disease complications Able to identify both luminal and extraluminal abnormalities [56] | Long image acquisition, which often needs sedation for neurologically impaired and young patients Need bowel preparation Use contrast agent (if requested) Patients with metallic foreign bodies or MRI-incompatible devices cannot perform MRI [56] |
| US | Safe Non-invasive Lacks radiation exposure Portable [56] | Operator-dependent Assessment of deep bowel loops is hindered by acoustic absorbance in tissues, limiting the effectiveness of ultrasound [57] The sensitivity remains uncertain or inadequately established in pregnancy [56] |
| CT | Short image acquisition The capacity to image paediatric patients without requiring sedation CTE exhibits high sensitivity and specificity in detecting active inflammation within the small intestine, accompanied by improved interobserver agreement and more consistent image quality, including enhanced spatial and contrast resolution, when compared to MRE [56] | The potential exposure of patients to ionizing radiation |
| DWI | Short scan time Safe and non-invasive sequence Quantitative and qualitative analysis No need for bowel preparation or contrast agents [56] | Sensitive to motion artifact T2 shine artifact Poor anatomical visualization |
4. Assessing Disease Activity in UC Using DWI
5. Monitoring UC Using DWI
| Reference | Subject (Bowel Segments) Type of Study | Standard Ref. b-Values s/mm2 | Bowel Preparation | Findings |
|---|---|---|---|---|
| Laurent et al. [59] | 29 ulcerative colitis (UC) patients Prospective, observational study | Colonoscopy 0 and 600 | No | Nancy score < 7 indicated a sensitivity, specificity and accuracy (75%, 67% and 73%, respectively, in the diagnosis of mucosal healing (area under the receiver operating characteristic curve. AUROC]): 0.72; 95% confidence interval [CI], 0.56–0.88; p = 0.0063). In patients who achieved MH, both the Mayo endoscopic subscore and Nancy score showed significant reductions. Specifically, the Mayo endoscopic subscore decreased from 2.4 at baseline to 0.6 at re-evaluation (p = 0.02) and the Nancy score decreased from 18.2 at baseline to 3 at re-evaluation (p = 0.006). Conversely, in active patients at reassessment, the Nancy score did not exhibit significant changes. The association between the total Nancy score and the Mayo Endoscopic Subscore was not good at the first assessment [r = 0.10; p = 0.61] but was good at the second assessment [r = 0.62; p < 0.005]. For the total Nancy score, the intra-class correlation coefficient for inter–intra-observer agreements at baseline and reassessment were 0.89 [0.76–0.95]; 0.79 [0.60–0.90] and 0.99 [0.97–0.99]; 0.99 [0.98–0.99], respectively. The study concluded that the Nancy score is a highly responsive, reliable tool for assessing treatment response in UC patients. |
| Oussalah et al. [24] | 28 UC patients (105 segments) Prospective study | Colonoscopy 0 and 600 | No | The presence of hyperintensity on diffusion-weighted imaging (DWI) demonstrates a significant predictive value for endoscopic inflammation, as indicated by an odds ratio (OR) of 13.26 (95% CI: 3.6–48.93) and an area under the curve (AUC) of 0.854 (p = 0.0001). A total segmental magnetic resonance (MR) score of greater than 1 meant a sensitivity, specificity and AUROC of 89.47%, 86.67% and 0.920, respectively. The proposed total MR score correlated with the Walmsley index (r = 0.678, p < 0.0001) and the total modified Baron score (r = 0.813, p = 0.0001) in those patients. The presence of a DWI hyperintensity (DWI-HI) showed a sensitivity of 90.79% and a specificity of 80% for detecting endoscopic inflammation, with an AUROC of 0.854 (p = 0.0001) In detecting endoscopic inflammation, the DWI hyperintensity had the same accuracy as gadolinium-based contrast agent enhancement. The study concluded that the MR-DWI colonography rectal preparation or without oral could represent a non-invasive tool in evaluating colonic inflammation in UC. |
| Jesuratnam-Nielsen et al. [65]. | 25 UC patients (No available) Prospective study | MRE 0, 100, 200, 500, 700, 800 and 1000 | No | The findings of the study reported that the DWI’s sensitivity, specificity and accuracy ranged from 0 to 52%, 83 to 94% and 76 to 92%, respectively. DWI-MRI in the colon exhibited significant false-positive findings attributed to the T2 shine-through phenomenon. |
| Yu et al. [61] | 20 UC patients (100 segments) Prospective observational study | Colonoscopy 0, 400, 600, 800, and 1000 | No | DWI hyperintensity at a b-value of 800 s/mm2 reliably indicated the presence of endoscopic colonic inflammation, with a sensitivity of 93.0%, specificity of 79.3% and statistically significant AUC of 0.867 (p < 0.0001). The segmental MR score (MR-score-S) exhibited a significant positive correlation with the segmental modified Baron score (Baron-S) (r = 0.761, p < 0.0001). Similarly, the total magnetic resonance score (MR-score-T) demonstrated a strong positive correlation with the total modified Baron score (Baron-T) (r = 0.875, p < 0.0001). An MR-score-S greater than 1 was found to be indicative of endoscopic colonic inflammation, with a sensitivity and specificity of 85.9% and 82.8%, respectively, and an AUC of 0.929 (p < 0.0001). DWI hyperintensity at a b-value of 800 s/mm2 demonstrated significantly higher diagnostic accuracy compared to b-values of 400, 600 and 1000 s/mm2. The study determined that an ADC value of 2.18 × 10⁻³ mm2/s effectively identifies endoscopic inflammation, demonstrating sensitivity and specificity of 89.7% and 80.3%, respectively. For DWI hyperintensity, the inter-observer agreements across different b values were consistent, with kappa values ranging from 0.719 to 0.825. The ADC measurements obtained by the two radiologists were compared, revealing a Pearson’s correlation coefficient of 0.886 (p < 0.001), which indicates a high level of inter-observer agreement. They concluded that the integration of DWI with conventional MRI, without the need for bowel preparation, offers a quantitative approach to distinguishing actively inflamed intestinal segments from normal mucosa, enabling the detection of UC. |
| Podgórska et al. [25] | 20 UC patients Prospective study | Endoscopic and histopathological scoring 0, 10, 30, 50, 75, 100, 150, 200, 500 and 900 | Yes | The analysis did not reveal any statistically significant correlation between the Mayo endoscopic subscore and the measured parameters of D*, D or f as active and non-active diseases were compared endoscopically. Statistical analyses found that there were statistically significant differences in the parameters of D, between cases characterized by histopathologically classified inactive or mild disease activity and those with moderate to severe disease activity (respectively, mean = 1.34 10−3 mm2 /s and mean = 1.07 × 10−3 mm2 /s, p = 0.0083; AUC = 0.735, sensitivity 0.91, specificity 0.54, accuracy 0.66 for cut-off value 1.24 × 10−3 mm2 /s and mean = 0.19 and mean = 0.28, p = 0.024; AUC = 0.723, sensitivity 0.82, specificity 0.59, accuracy 0.67 for a 0.185 cut-off value). However, for D* no significant difference was found. This study did not analyse ADC. |
| Kılıçkesmez et al. [23]. | 28 UC patients Prospective study | Colonoscopy 0, 500 and 1000 | No | The ADC values of the sigmoid colon did not significantly differ among patients in the active, subacute, and remission phases of UC (p = 0.472). The ADC values of the rectum were different between patients in the remission, subacute and active phases. The rectum ADC values of the patients in remission were higher than the rectum ADC values of patients in subacute (p = 0.007) and the active (p = 0.009) phases and were similar in patients in the subacute and active phases of the disease (p > 0.05). The study found that higher disease activity is associated with lower ADC values. |
6. Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Disease-A-Month 2019, 65, 100851. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Goldblum, J.R.; Fiocchi, C.; Rieder, F. Fibrosis in ulcerative colitis: Mechanisms, features, and consequences of a neglected problem. Inflamm. Bowel Dis. 2014, 20, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 2018, 47, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Sousa, H. ulcerative colitis submucosal fibrosis and inflammation: More than just strictures. Aliment. Pharmacol. Ther. 2018, 47, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, C.; Colucci, R.; Segnani, C.; Errede, M.; Girolamo, F.; Virgintino, D.; Dolfi, A.; Tirotta, E.; Buccianti, P.; Di Candio, G. Fibrotic and vascular remodelling of colonic wall in patients with active ulcerative colitis. J. Crohn’s Colitis 2016, 10, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Koletzko, S.; Kannengiesser, K.; Dignass, A. Ulcerative colitis—Diagnostic and therapeutic algorithms. Dtsch. Ärzteblatt Int. 2020, 117, 564. [Google Scholar] [CrossRef]
- Pandey, A.; Achrafie, L.; Kodjamanova, P.; Tencer, T.; Kumar, J. Endoscopic mucosal healing and histologic remission in ulcerative colitis: A systematic literature review of clinical, quality-of-life and economic outcomes. Curr. Med. Res. Opin. 2022, 38, 1531–1541. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Leighton, J.A.; Shen, B.; Baron, T.H.; Adler, D.G.; Davila, R.; Egan, J.V.; Faigel, D.O.; Gan, S.-I.; Hirota, W.K.; Lichtenstein, D. ASGE guideline: Endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest. Endosc. 2006, 63, 558–565. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P. Importance of mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 338–346. [Google Scholar] [CrossRef]
- Shah, S.C.; Colombel, J.-F.; Sands, B.E.; Narula, N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1245–1255.e1248. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W. STRIDE-II: An update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Rosenfeld, G.; Bressler, B.; Seidman, E. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 742–756. [Google Scholar] [CrossRef]
- Bartlett, D.J.; Ramos, G.P.; Fletcher, J.G.; Bruining, D.H. Imaging evaluation of inflammatory bowel disease complications. Gastrointest. Endosc. Clin. 2022, 32, 651–673. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Bicci, E.; Danti, G.; Flammia, F.; Chiti, G.; Palumbo, P.; Bruno, F.; Borgheresi, A.; Grassi, R.; Grassi, F. The role of magnetic resonance enterography in Crohn’s disease: A review of recent literature. Diagnostics 2022, 12, 1236. [Google Scholar] [CrossRef] [PubMed]
- Minordi, L.M.; Bevere, A.; Papa, A.; Larosa, L.; Manfredi, R. CT and MRI Evaluations in Crohn’s Complications: A Guide for the Radiologist. Acad. Radiol. 2022, 29, 1206–1227. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, A.; Rath, H.; Kikinis, R.; Völk, M.; Schölmerich, J.; Feuerbach, S.; Rogler, G.; Seitz, J.; Herfarth, H. Comparison of magnetic resonance imaging colonography with conventional colonoscopy for the assessment of intestinal inflammation in patients with inflammatory bowel disease: A feasibility study. Gut 2005, 54, 250. [Google Scholar] [CrossRef]
- Langhorst, J.; Kühle, C.A.; Ajaj, W.; Nüfer, M.; Barkhausen, J.; Michalsen, A.; Dobos, G.J.; Lauenstein, T.C. MR colonography without bowel purgation for the assessment of inflammatory bowel diseases: Diagnostic accuracy and patient acceptance. Inflamm. Bowel Dis. 2007, 13, 1001–1008. [Google Scholar] [CrossRef]
- Khachab, F.; Loundou, A.; Roman, C.; Colavolpe, N.; Aschero, A.; Bourlière-Najean, B.; Daidj, N.; Desvignes, C.; Pico, H.; Gorincour, G. Can diffusion weighting replace gadolinium enhancement in magnetic resonance enterography for inflammatory bowel disease in children? Pediatr. Radiol. 2018, 48, 1432–1440. [Google Scholar] [CrossRef]
- Dubron, C.; Avni, F.; Boutry, N.; Turck, D.; Duhamel, A.; Amzallag-Bellenger, E. Prospective evaluation of free-breathing diffusion-weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br. J. Radiol. 2016, 89, 20150840. [Google Scholar] [CrossRef]
- Florie, J.; Birnie, E.; van Gelder, R.E.; Jensch, S.; Haberkorn, B.; Bartelsman, J.F.; van der Sluys Veer, A.; Snel, P.; van der Hulst, V.P.; Bonsel, G.J. MR colonography with limited bowel preparation: Patient acceptance compared with that of full-preparation colonoscopy. Radiology 2007, 245, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Shuto, K.; Okazumi, S.; Miyauchi, H.; Kazama, T.; Matsubara, H. Evaluation of ulcerative colitis using diffusion-weighted imaging. Hepato-gastroenterology 2010, 57, 468–471. [Google Scholar] [PubMed]
- Kılıçkesmez, O.; Soylu, A.; Yaşar, N.; Demirbaş, T.; Dolapçıoğlu, C.; Poturoğlu, S.; Sevindir, İ.; Cimilli, T. Is quantitative diffusion-weighted MRI a reliable method in the assessment of the inflammatory activity in ulcerative colitis. Diagn. Interv. Radiol. 2010, 16, 293–298. [Google Scholar] [PubMed]
- Oussalah, A.; Laurent, V.; Bruot, O.; Bressenot, A.; Bigard, M.-A.; Régent, D.; Peyrin-Biroulet, L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 2010, 59, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Podgórska, J.; Pasicz, K.; Zagórowicz, E.; Mróz, A.; Gołębiewski, B.; Kuś, P.; Jasieniak, J.; Skrzyński, W.; Wieszczy, P.; Kukołowicz, P. Intravoxel incoherent motion MRI in evaluating inflammatory activity in ulcerative colitis: A pilot study. Acta Radiol. 2021, 62, 439–446. [Google Scholar] [CrossRef]
- Nicholson, C.; Phillips, J. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J. Physiol. 1981, 321, 225–257. [Google Scholar] [CrossRef]
- Szafer, A.; Zhong, J.; Anderson, A.W.; Gore, J.C. Diffusion-weighted imaging in tissues: Theoretical models. NMR Biomed. 1995, 8, 289–296. [Google Scholar] [CrossRef]
- Dohan, A.; Taylor, S.; Hoeffel, C.; Barret, M.; Allez, M.; Dautry, R.; Zappa, M.; Savoye-Collet, C.; Dray, X.; Boudiaf, M. Diffusion-weighted MRI in Crohn’s disease: Current status and recommendations. J. Magn. Reson. Imaging 2016, 44, 1381–1396. [Google Scholar] [CrossRef]
- Koh, D.-M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef]
- Padhani, A.R.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Iima, M.; Le Bihan, D. Clinical intravoxel incoherent motion and diffusion MR imaging: Past, present, and future. Radiology 2016, 278, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Bouhnik, Y.; Reinisch, W.; Stoker, J.; Taylor, S.; Baumgart, D.; Danese, S.; Halligan, S.; Marincek, B.; Matos, C. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohn’s Colitis 2013, 7, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Rimola, J.; García-Bosch, O.; Rodríguez, S.; Gallego, M.; Etchevers, M.J.; Pellisé, M.; Feu, F.; González-Suárez, B.; Ayuso, C. Diagnostic accuracy of magnetic resonance colonography for the evaluation of disease activity and severity in ulcerative colitis: A prospective study. Gut 2012, 62, 1566–1572. [Google Scholar] [CrossRef]
- Pouillon, L.; Laurent, V.; Pouillon, M.; Bossuyt, P.; Bonifacio, C.; Danese, S.; Deepak, P.; Loftus, E.V.; Bruining, D.H.; Peyrin-Biroulet, L. Diffusion-weighted MRI in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2018, 3, 433–443. [Google Scholar] [CrossRef]
- Baik, J.; Kim, S.H.; Lee, Y.; Yoon, J.-H. Comparison of T2-weighted imaging, diffusion-weighted imaging and contrast-enhanced T1-weighted MR imaging for evaluating perianal fistulas. Clin. Imaging 2017, 44, 16–21. [Google Scholar] [CrossRef]
- Dohan, A.; Eveno, C.; Oprea, R.; Pautrat, K.; Placé, V.; Pocard, M.; Hoeffel, C.; Boudiaf, M.; Soyer, P. Diffusion-weighted MR imaging for the diagnosis of abscess complicating fistula-in-ano: Preliminary experience. Eur. Radiol. 2014, 24, 2906–2915. [Google Scholar] [CrossRef]
- Seo, N.; Park, S.H.; Kim, K.-J.; Kang, B.-K.; Lee, Y.; Yang, S.-K.; Ye, B.D.; Park, S.H.; Kim, S.Y.; Baek, S. MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: A prospective noninferiority study. Radiology 2016, 278, 762–772. [Google Scholar] [CrossRef]
- Gee, M.S.; Harisinghani, M.G. MRI in patients with inflammatory bowel disease. J. Magn. Reson. Imaging 2011, 33, 527–534. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.M.; Loftus Jr, E.V.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A.; Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology 2018, 286, 776–799. [Google Scholar] [CrossRef]
- Chavoshi, M.; Mirshahvalad, S.A.; Kasaeian, A.; Djalalinia, S.; Kolahdoozan, S.; Radmard, A.R. Diagnostic accuracy of magnetic resonance enterography in the evaluation of colonic abnormalities in Crohn’s disease: A systematic review and meta-analysis. Acad. Radiol. 2021, 28, S192–S202. [Google Scholar] [CrossRef]
- Iacucci, M.; Cannatelli, R.; Tontini, G.E.; Panaccione, R.; Danese, S.; Fiorino, G.; Matsumoto, T.; Kochhar, G.S.; Shen, B.; Kiesslich, R. Improving the quality of surveillance colonoscopy in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2019, 4, 971–983. [Google Scholar] [CrossRef]
- Kaushal, P.; Somwaru, A.S.; Charabaty, A.; Levy, A.D. MR enterography of inflammatory bowel disease with endoscopic correlation. Radiographics 2017, 37, 116–131. [Google Scholar] [CrossRef]
- Langan, R.C.; Gotsch, P.B.; Krafczyk, M.A.; Skillinge, D.D. Ulcerative colitis: Diagnosis and treatment. Am. Fam. Physician 2007, 76, 1323–1330. [Google Scholar] [PubMed]
- Olpin, J.D.; Sjoberg, B.P.; Stilwill, S.E.; Jensen, L.E.; Rezvani, M.; Shaaban, A.M. Beyond the bowel: Extraintestinal manifestations of inflammatory bowel disease. Radiographics 2017, 37, 1135–1160. [Google Scholar] [CrossRef] [PubMed]
- Paine, E.R. Colonoscopic evaluation in ulcerative colitis. Gastroenterol. Rep. 2014, 2, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Radmard, A.R.; Amouei, M.; Torabi, A.; Sima, A.R.; Saffar, H.; Geahchan, A.; Davarpanah, A.H.; Taouli, B. MR Enterography in Ulcerative Colitis: Beyond Endoscopy. Radiographics 2023, 44, e230131. [Google Scholar] [CrossRef] [PubMed]
- Campari, A.; Napolitano, M.; Zuin, G.; Maestri, L.; Di Leo, G.; Sardanelli, F. Colonic inflammation in pediatric inflammatory bowel disease: Detection with magnetic resonance enterography. Pediatr. Radiol. 2017, 47, 850–859. [Google Scholar] [CrossRef]
- Maccioni, F.; Colaiacomo, M.; Parlanti, S. Ulcerative colitis: Value of MR imaging. Abdom. Imaging 2005, 30, 584–592. [Google Scholar] [CrossRef]
- Roggeveen, M.J.; Tismenetsky, M.; Shapiro, R. Best cases from the AFIP-Ulcerative colitis. Radiographics 2006, 26, 947–951. [Google Scholar] [CrossRef]
- Hafeez, R.; Wagner, C.; Smith, S.; Boulos, P.; Halligan, S.; Bloom, S.; Taylor, S. Patient experiences of MR colonography and colonoscopy: A qualitative study. Br. J. Radiol. 2012, 85, 765–769. [Google Scholar] [CrossRef]
- Khosravi, B.; Salehnia, A.; Pak, N.; Montazeri, S.A.; Sima, A.R.; Vahedi, H.; Malekzadeh, R.; Radmard, A.R. A Practical Index to Distinguish Backwash Ileitis From Crohn’s Terminal Ileitis in MR Enterography. Inflamm. Bowel Dis. 2023, 29, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bruining, D.H.; Loftus, E.V., Jr. Technology Insight: New techniques for imaging the gut in patients with IBD. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, A.Y.; Yang, S.-K.; Chung, J.-W.; Kim, S.Y.; Park, S.H.; Ha, H.K. Crohn disease of the small bowel: Comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology 2009, 251, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Masselli, G.; Gualdi, G. MR imaging of the small bowel. Radiology 2012, 264, 333–348. [Google Scholar] [CrossRef]
- Kilcoyne, A.; Kaplan, J.L.; Gee, M.S. Inflammatory bowel disease imaging: Current practice and future directions. World J. Gastroenterol. 2016, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabri, R.; Wetwittayakhlang, P.; Lakatos, P.L. Monitoring of Inflammatory Bowel Disease in Pregnancy: A Review of the Different Modalities. J. Clin. Med. 2023, 12, 7343. [Google Scholar] [CrossRef]
- Hordonneau, C.; Buisson, A.; Scanzi, J.; Goutorbe, F.; Pereira, B.; Borderon, C.; Da Ines, D.; Montoriol, P.; Garcier, J.; Boyer, L. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: Validation of quantitative index of activity. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 89–98. [Google Scholar] [CrossRef]
- Laurent, V.; Naudé, S.; Vuitton, L.; Zallot, C.; Baumann, C.; Girard-Gavanier, M.; Peyrin-Biroulet, L. Accuracy of diffusion-weighted magnetic resonance colonography in assessing mucosal healing and the treatment response in patients with ulcerative colitis. J. Crohn’s Colitis 2017, 11, 716–723. [Google Scholar] [CrossRef]
- Saleh, G.A.; Razek, A.A.K.A.; Awad, S.I.; Shokeir, M.A.E.R.; Megahed, A. The role of magnetic resonance enterography and diffusion-weighted imaging in pediatric inflammatory bowel disease compared to endoscopic and clinical activity scores: Pilot study. Egypt. J. Radiol. Nucl. Med. 2023, 54, 204. [Google Scholar] [CrossRef]
- Yu, L.-L.; Yang, H.-S.; Zhang, B.-T.; Lv, Z.-W.; Wang, F.-R.; Zhang, C.-Y.; Chen, W.-B.; Zhang, H.-M. Diffusion-weighted magnetic resonance imaging without bowel preparation for detection of ulcerative colitis. World J. Gastroenterol. WJG 2015, 21, 9785. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Danese, S.; Laurent, V.; Peyrin-Biroulet, L. Use of cross-sectional imaging for tight monitoring of inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1309–1323.e1304. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Off. J. Am. Coll. Gastroenterol. ACG 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Rimola, J.; Rodríguez, S.; Paredes, J.M.; Martínez-Pérez, M.J.; Blanc, E.; Arévalo, J.A.; Aduna, M.; Andreu, M.; Radosevic, A. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology 2014, 146, 374–382.e371. [Google Scholar] [CrossRef] [PubMed]
- Jesuratnam-Nielsen, K.; Løgager, V.B.; Rezanavaz-Gheshlagh, B.; Munkholm, P.; Thomsen, H.S. Plain magnetic resonance imaging as an alternative in evaluating inflammation and bowel damage in inflammatory bowel disease–a prospective comparison with conventional magnetic resonance follow-through. Scand. J. Gastroenterol. 2015, 50, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H. DWI at MR enterography for evaluating bowel inflammation in Crohn disease. Am. J. Roentgenol. 2016, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gordic, S.; Bane, O.; Kihira, S.; Peti, S.; Hectors, S.; Torres, J.; Cho, J.; Colombel, J.-F.; Taouli, B. Evaluation of ileal Crohn’s disease response to TNF antagonists: Validation of MR enterography for assessing response. Initial results. Eur. J. Radiol. Open 2020, 7, 100217. [Google Scholar] [CrossRef]
- Rimola, J.; Fernandez-Clotet, A.; Capozzi, N.; Caballol, B.; Rodríguez, S.; Gallego, M.; Masamunt, M.C.; Panés, J.; Ricart, E.; Ordás, I. ADC values for detecting bowel inflammation and biologic therapy response in patients with Crohn disease: A post hoc prospective trial analysis. Am. J. Roentgenol. 2024, 222, e2329639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyami, A.S. Imaging of Ulcerative Colitis: The Role of Diffusion-Weighted Magnetic Resonance Imaging. J. Clin. Med. 2024, 13, 5204. https://doi.org/10.3390/jcm13175204
Alyami AS. Imaging of Ulcerative Colitis: The Role of Diffusion-Weighted Magnetic Resonance Imaging. Journal of Clinical Medicine. 2024; 13(17):5204. https://doi.org/10.3390/jcm13175204
Chicago/Turabian StyleAlyami, Ali S. 2024. "Imaging of Ulcerative Colitis: The Role of Diffusion-Weighted Magnetic Resonance Imaging" Journal of Clinical Medicine 13, no. 17: 5204. https://doi.org/10.3390/jcm13175204





