Comparative Prognostic Value of Coronary Calcium Score and Perivascular Fat Attenuation Index in Coronary Artery Disease
Abstract
:1. Introduction
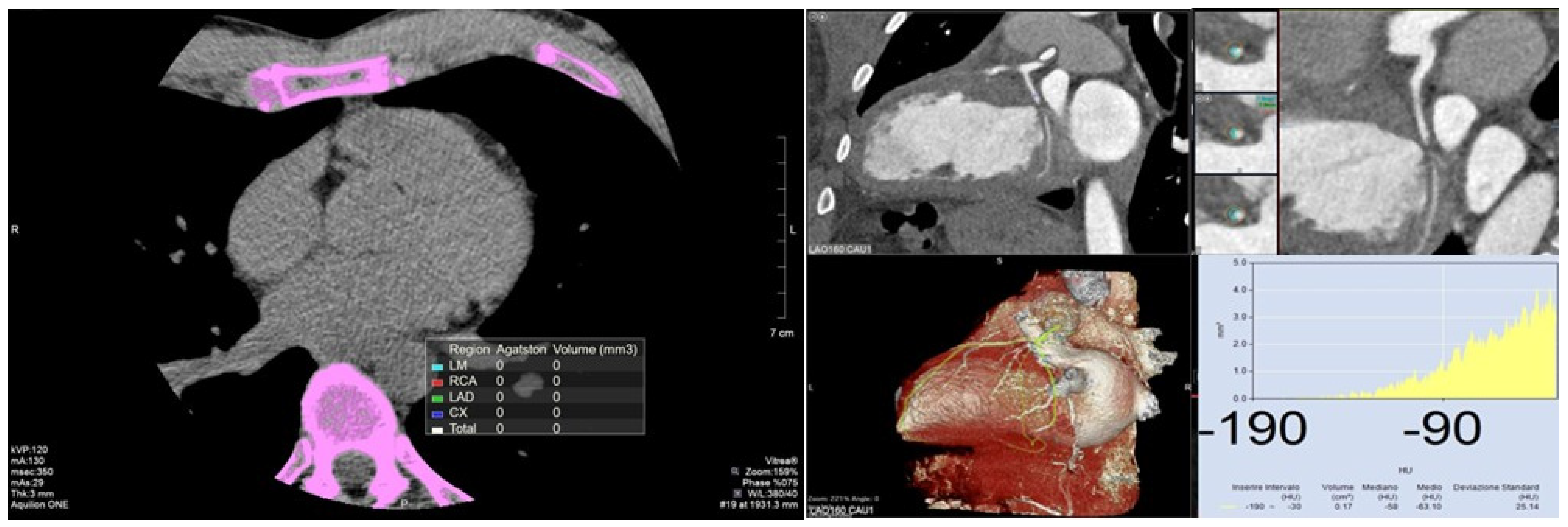
2. Coronary Calcium Score
2.1. Definition
2.2. Measurement
2.3. Prognostic Value
| Study/Work | Year | Country | n. Patient | Age | FU | Main Findings | Journal |
|---|---|---|---|---|---|---|---|
| South Bay Heart Watch [26] | 2004 | USA | 1312 | 65.7 | 8.5 years | CAC score significantly modifies risk prediction in all patients with moderate CV risk | Journal of American Medical Association |
| Coronary Artery Calcium Score and Coronary Heart Disease Events in a Large Cohort of Asymptomatic Men and Women [27] | 2005 | Dallas | 16,097 | 53.8 years | 3.5 years | CAC score predicts subclinical atherosclerosis and CAD | American Journal of Epidemiology |
| PACC Project [28] | 2005 | Washington | 1983 | 42.8 | 3 years | CAC score to better stratify patients at moderate CV risk | Journal of the American College of Cardiology |
| MESA sub-analysis [16] | 2008 | USA | 6722 | 45–84 years | 3.8 years | CAC score in multi-ethnic populations predicts CAD over standard coronary risk factors | New England Journal of Medicine |
| All-cause mortality by age and gender based on coronary artery calcium scores [29] | 2016 | USA | 13,092 | 58 ± 11 years | 15 years | CAC score in patients >75 years has a low predictive power of mortality risk for higher rate of non-cardiac death. CAC = 0, regardless of risk factors, correlates with very low long-term mortality | European Heart Journal—Cardiovascular Imaging |
| Incidental coronary artery calcification on non-gated CT thorax correlates with risk of cardiovascular events and death [30] | 2022 | England | 717 | 40–70 years | 41.6 months | Incidental CAC correlates short-term CVD and deaths | European Radiology |
| Heinz Nixdorf Recall [31] | 2023 | Essen, Germany | 4154 | 20 years | CAC score stratifies in 20 years FU better than assessing CV risk factor, especially in patients initially at intermediate risk | Deutsches Ärzteblatt International |
2.4. Clinical Guidelines
2.5. Limitations
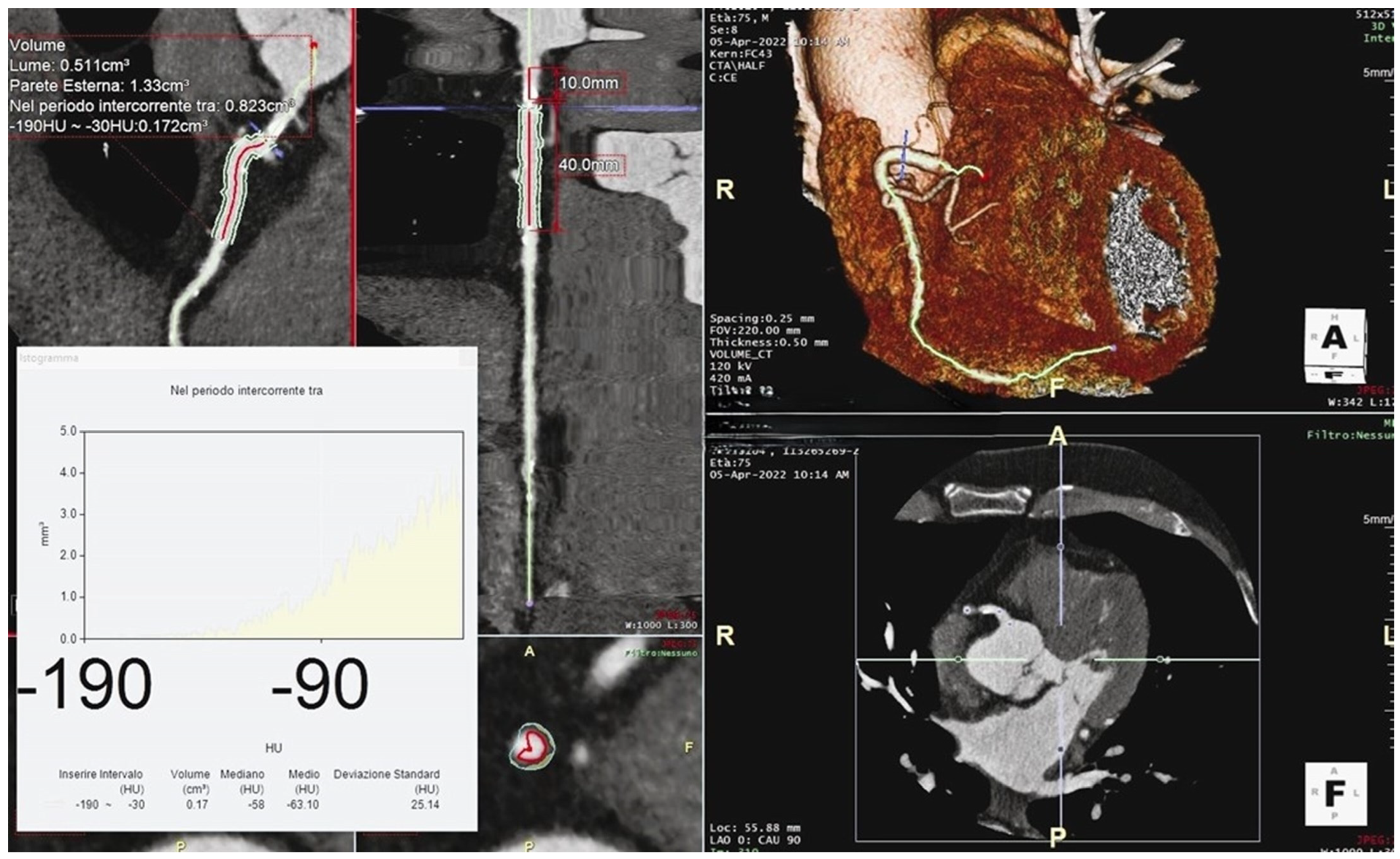
3. Perivascular Fat Attenuation Index
3.1. Definition
3.2. Measurement
3.3. Prognostic Value
3.4. Limitations
4. Comparative Summary
4.1. Clinical Implications
4.2. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mallika, V.; Goswami, B.; Rajappa, M. Atherosclerosis Pathophysiology and the Role of Novel Risk Factors: A Clinicobiochemical Perspective. Angiology 2007, 58, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic Review of Patients Presenting with Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Pergola, V.; Continisio, S.; Mantovani, F.; Motta, R.; Mattesi, G.; Marrazzo, G.; Dellino, C.M.; Montonati, C.; De Conti, G.; Galzerano, D.; et al. Spontaneous coronary artery dissection: The emerging role of coronary computed tomography. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 839–850. [Google Scholar] [CrossRef]
- Pergola, V.; Cabrelle, G.; Barbiero, G.; Dellino, C.M.; Reffo, E.; Di Salvo, G.; Motta, R. Single coronary artery originating from right sinus. Role of MDCT and a review of literature. Monaldi Arch. Chest Dis. 2022, 92. [Google Scholar] [CrossRef]
- Tassetti, L.; Sfriso, E.; Torlone, F.; Baggiano, A.; Mushtaq, S.; Cannata, F.; Del Torto, A.; Fazzari, F.; Fusini, L.; Junod, D.; et al. The Role of Multimodality Imaging (CT & MR) as a Guide to the Management of Chronic Coronary Syndromes. J. Clin. Med. 2024, 13, 3450. [Google Scholar] [CrossRef]
- Pergola, V.; Pradegan, N.; Cozza, E.; Cozac, D.A.; Cao, I.; Tessari, C.; Savo, M.T.; Toscano, G.; Angelini, A.; Tarzia, V.; et al. Redefining CAV surveillance strategies: Benefits of CCTA vs. ICA. J. Cardiovasc. Comput. Tomogr. 2024, 20, 1–7. [Google Scholar] [CrossRef]
- Pergola, V.; Cabrelle, G.; Mattesi, G.; Cattarin, S.; Furlan, A.; Dellino, C.M.; Continisio, S.; Montonati, C.; Giorgino, A.; Giraudo, C.; et al. Added Value of CCTA-Derived Features to Predict MACEs in Stable Patients Undergoing Coronary Computed Tomography. Diagnostics 2022, 12, 1446. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed Tomographic Angiography Characteristics of Atherosclerotic Plaques Subsequently Resulting in Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, J.A.; Simons, D.B.; Fitzpatrick, L.A.; Sheedy, P.F.; Schwartz, R.S. Coronary Artery Calcium Area by Electron-Beam Computed Tomography and Coronary Atherosclerotic Plaque Area. Circulation 1995, 92, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Budoff, M.J.; Nasir, K.; McClelland, R.L.; Detrano, R.; Wong, N.; Blumenthal, R.S.; Kondos, G.; Kronmal, R.A. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2009, 53, 345–352. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.L.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Hoffmann, U.; Massaro, J.M.; D’Agostino, R.B.; Kathiresan, S.; Fox, C.S.; O’Donnell, C.J. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J. Am. Heart Assoc. 2016, 5, e003144. [Google Scholar] [CrossRef]
- Paixao, A.R.M.; Ayers, C.R.; El Sabbagh, A.; Sanghavi, M.; Berry, J.D.; Rohatgi, A.; Kumbhani, D.J.; McGuire, D.K.; Das, S.R.; de Lemos, J.A.; et al. Coronary Artery Calcium Improves Risk Classification in Younger Populations. JACC Cardiovasc. Imaging 2015, 8, 1285–1293. [Google Scholar] [CrossRef]
- Baber, U.; Mehran, R.; Sartori, S.; Schoos, M.M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, Impact, and Predictive Value of Detecting Subclinical Coronary and Carotid Atherosclerosis in Asymptomatic Adults: The BioImage Study. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef]
- Erbel, R.; Möhlenkamp, S.; Moebus, S.; Schmermund, A.; Lehmann, N.; Stang, A.; Dragano, N.; Grönemeyer, D.; Seibel, R.; Kälsch, H.; et al. Coronary Risk Stratification, Discrimination, and Reclassification Improvement Based on Quantification of Subclinical Coronary Atherosclerosis: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2010, 56, 1397–1406. [Google Scholar] [CrossRef]
- Mattesi, G.; Savo, M.T.; De Amicis, M.; Amato, F.; Cozza, E.; Corradin, S.; Da Pozzo, S.; Previtero, M.; Bariani, R.; De Conti, G.; et al. Coronary artery calcium score: We know where we are but not where we may be. Monaldi Arch. Chest Dis. 2023, 94. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Blaha, M.J.; Berman, D.S.; Nasir, K.; Budoff, M.; Leipsic, J.; Blankstein, R.; Narula, J.; Rumberger, J.; Shaw, L.J. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Cox, A.J.; Herrington, D.M.; Jorgensen, N.W.; Xu, J.; Freedman, B.I.; Carr, J.J.; Bowden, D.W. Coronary Calcium Score Predicts Cardiovascular Mortality in Diabetes: Diabetes Heart Study. Diabetes Care 2013, 36, 972–977. [Google Scholar] [CrossRef] [PubMed]
- van der Aalst, C.M.; Denissen, S.J.A.M.; Vonder, M.; Gratama, J.W.C.; Adriaansen, H.J.; Kuijpers, D.; Vliegenthart, R.; Lennep, J.E.R.v.; van der Harst, P.; Braam, R.L.; et al. Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: The ROBINSCA trial. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Knowlton, K.U.; May, H.T.; Le, V.T.; Lappe’, D.L.; Cripps, S.T.; Schwab, L.H.; Winslow, T.; Bair, T.L.; Muhlestein, J.B. Impact of Active vs Passive Statin Selection for Primary Prevention. JACC Adv. 2023, 2, 100676. [Google Scholar] [CrossRef]
- Greenland, P.; LaBree, L.; Azen, S.P.; Doherty, T.M.; Detrano, R.C. Coronary Artery Calcium Score Combined with Framingham Score for Risk Prediction in Asymptomatic Individuals. JAMA 2004, 291, 210–215. [Google Scholar] [CrossRef]
- LaMonte, M.J.; FitzGerald, S.J.; Church, T.S.; Barlow, C.E.; Radford, N.B.; Levine, B.D.; Pippin, J.J.; Gibbons, L.W.; Blair, S.N.; Nichaman, M.Z. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am. J. Epidemiol. 2005, 162, 421–429. [Google Scholar] [CrossRef]
- Taylor, A.J.; Bindeman, J.; Feuerstein, I.; Cao, F.; Brazaitis, M.; O’Malley, P.G. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: Mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J. Am. Coll. Cardiol. 2005, 46, 807–814. [Google Scholar] [CrossRef]
- Nakanishi, R.; Li, D.; Blaha, M.J.; Whelton, S.P.; Darabian, S.; Flores, F.R.; Dailing, C.; Blumenthal, R.S.; Nasir, K.; Berman, D.S.; et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1305–1314. [Google Scholar] [CrossRef]
- Wetscherek, M.T.A.; McNaughton, E.; Majcher, V.; Wetscherek, A.; Sadler, T.J.; Alsinbili, A.; Teh, W.H.; Moore, S.D.; Patel, N.; Smith, W.P.W.; et al. Incidental coronary artery calcification on non-gated CT thorax correlates with risk of cardiovascular events and death. Eur. Radiol. 2023, 33, 4723–4733. [Google Scholar] [CrossRef]
- Erbel, R.; Lehmann, N.; Schramm, S.; Schmidt, B.; Hüsing, A.; Kowall, B.; Hermann, D.M.; Gronewold, J.; Schmermund, A.; Möhlenkamp, S.; et al. Diagnostic cardiac CT for the improvement of cardiovascular event prediction. Dtsch. Arztebl. Int. 2023, 120, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Golub, I.S.; Termeie, O.G.; Kristo, S.; Schroeder, L.P.; Lakshmanan, S.; Shafter, A.M.; Hussein, L.; Verghese, D.; Aldana-Bitar, J.; Manubolu, V.S.; et al. Major Global Coronary Artery Calcium Guidelines. JACC Cardiovasc. Imaging 2023, 16, 98–117. [Google Scholar] [CrossRef]
- Cainzos-Achirica, M.; Miedema, M.D.; McEvoy, J.W.; Al Rifai, M.; Greenland, P.; Dardari, Z.; Budoff, M.; Blumenthal, R.S.; Yeboah, J.; Duprez, D.A.; et al. Coronary Artery Calcium for Personalized Allocation of Aspirin in Primary Prevention of Cardiovascular Disease in 2019: The MESA Study (Multi-Ethnic Study of Atherosclerosis). Circulation 2020, 141, 1541–1553. [Google Scholar] [CrossRef]
- Miedema, M.D.; Duprez, D.A.; Misialek, J.R.; Blaha, M.J.; Nasir, K.; Silverman, M.G.; Blankstein, R.; Budoff, M.J.; Greenland, P.; Folsom, A.R. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: Estimates from the multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 453–460. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction. Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef]
- Wang, X.; Le, E.P.V.; Rajani, N.K.; Hudson-Peacock, N.; Pavey, H.; Tarkin, J.M.; Babar, J.; Williams, M.C.; Gopalan, D.; Rudd, J.H.F. A zero coronary artery calcium score in patients with stable chest pain is associated with a good prognosis, despite risk of non-calcified plaques. Open Heart 2019, 6, e000945. [Google Scholar] [CrossRef]
- Pergola, V.; Cabrelle, G.; Previtero, M.; Fiorencis, A.; Lorenzoni, G.; Dellino, C.M.; Montonati, C.; Continisio, S.; Masetto, E.; Mele, D.; et al. Impact of the ‘atherosclerotic pabulum’ on in-hospital mortality for SARS-CoV-2 infection. Is calcium score able to identify at-risk patients? Clin. Cardiol. 2022, 45, 629–640. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- West, H.W.; Dangas, K.; Antoniades, C. Advances in Clinical Imaging of Vascular Inflammation. JACC Basic Transl. Sci. 2024, 9, 710–732. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [PubMed]
- Guaricci, A.I.; Neglia, D.; Acampa, W.; Andreini, D.; Baggiano, A.; Bianco, F.; Carrabba, N.; Conte, E.; Gaudieri, V.; Mushtaq, S.; et al. Computed tomography and nuclear medicine for the assessment of coronary inflammation: Clinical applications and perspectives. J. Cardiovasc. Med. 2023, 24 (Suppl. 1), e67–e76. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Antonopoulos, A.S.; Schottlander, D.; Marwan, M.; Mathers, C.; Tomlins, P.; Siddique, M.; Klüner, L.V.; Shirodaria, C.; Mavrogiannis, M.C.; et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: Integration in clinical care as a prognostic medical device. Cardiovasc. Res. 2021, 117, 2677–2690. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Nerlekar, N.; Yuvaraj, J.; Fernandes, K.; Jiang, C.; Nicholls, S.J.; Dey, D.; Wong, D.T.L. Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: A cross-sectional study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared with Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef]
- Baritussio, A.; Vacirca, F.; Ocagli, H.; Tona, F.; Pergola, V.; Motta, R.; Marcolongo, R.; Lorenzoni, G.; Gregori, D.; Iliceto, S.; et al. Assessment of Coronary Inflammation by Pericoronary Fat Attenuation Index in Clinically Suspected Myocarditis with Infarct-Like Presentation. J. Clin. Med. 2021, 10, 4200. [Google Scholar] [CrossRef]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 636–643. [Google Scholar] [CrossRef]
- Mátyás, B.B.; Benedek, I.; Raț, N.; Blîndu, E.; Parajkó, Z.; Mihăilă, T.; Benedek, T. Assessing the Impact of Long-Term High-Dose Statin Treatment on Pericoronary Inflammation and Plaque Distribution-A Comprehensive Coronary CTA Follow-Up Study. Int. J. Mol. Sci. 2024, 25, 1700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, R.R.; You, H.R.; Geng, Y.Y.; Li, X.G.; Sun, Y.; Hou, J.; Ji, L.C.; Shi, J.L.; Zhang, L.B.; Yang, B.Q. Predicting major adverse cardiovascular events within 3 years by optimization of radiomics model derived from pericoronary adipose tissue on coronary computed tomography angiography: A case-control study. BMC Med. Imaging 2024, 24, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Napoli, G.; Pergola, V.; Basile, P.; De Feo, D.; Bertrandino, F.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Carrabba, N.; et al. Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and AcuteCoronary Syndromes. J. Clin. Med. 2023, 12, 7212. [Google Scholar] [CrossRef]
- Sperlongano, S.; D’andrea, A.; Mele, D.; Russo, V.; Pergola, V.; Carbone, A.; Ilardi, F.; Di Maio, M.; Bottino, R.; Giallauria, F.; et al. Left Ventricular Deformation and Vortex Analysis in Heart Failure: From Ultrasound Technique to Current Clinical Application. Diagnostics 2021, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- West, H.W.; Antoniades, C. Imaging and Targeting Coronary Artery Inflammation. Antioxid. Redox Signal. 2021, 34, 1217–1243. [Google Scholar] [CrossRef] [PubMed]
- Elnabawi, Y.A.; Oikonomou, E.K.; Dey, A.K.; Mancio, J.; Rodante, J.A.; Aksentijevich, M.; Choi, H.; Keel, A.; Erb-Alvarez, J.; Teague, H.L.; et al. Association of Biologic Therapy with Coronary Inflammation in Patients with Psoriasis as Assessed by Perivascular Fat Attenuation Index. JAMA Cardiol. 2019, 4, 885–891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| CAC Score | Risk Category | Probability of Severe CAD |
|---|---|---|
| 0 | Very low risk | <5% |
| 1–99 | Mildly increased | 5–10% |
| 100–299 | Moderately increased | 10–20% |
| 300–1000 | Moderate to severely increased | 20–40% |
| >1000 | Severely increased | >40% |
| Role | Details |
|---|---|
| Risk Stratification | Used in primary prevention, especially in asymptomatic individuals Crucial for intermediate-risk individuals to guide treatment decisions Reclassifies CVD risk upwards or downwards in addition to conventional risk factors |
| Impact on Therapy | Low CACs in low ASCVD-risk individuals can reassure clinicians High CACs may prompt a therapy reassessment Not indicated for patients already high ASCVD risk by definition |
| Guidelines for Lipid-Lowering Therapy | CACs = 0: possible discontinuation or avoidance of lipid-lowering therapy, unless other risk factors are present CACs = 1–99: consider initiating lipid-lowering therapy, particularly in patients aged 55+ CACs > 100: likely indication for therapy |
| Guidelines for Aspirin Therapy | CACs > 100: aspirin may provide a net benefit, especially with scores > 400 CACs = 0: bleeding risk outweighs benefits, suggesting aspirin may not be indicated |
| Feature | CAC Score | pFAI |
|---|---|---|
| Pros | Widely used and accepted in clinical practice | Provides insights into local coronary inflammation |
| Simple to measure and interpret | Can detect residual inflammatory coronary risk | |
| Strong correlation with incident vascular events | Correlates with CAD independently of CACs, age, gender, risk factors | |
| Cons | Does not detect non-calcified plaques | Requires advanced imaging techniques and specialized expertise |
| Limited in secondary prevention | Influenced by technical, anatomical, and biological factors | |
| Clinical utility still being explored |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savo, M.T.; De Amicis, M.; Cozac, D.A.; Cordoni, G.; Corradin, S.; Cozza, E.; Amato, F.; Lassandro, E.; Da Pozzo, S.; Tansella, D.; et al. Comparative Prognostic Value of Coronary Calcium Score and Perivascular Fat Attenuation Index in Coronary Artery Disease. J. Clin. Med. 2024, 13, 5205. https://doi.org/10.3390/jcm13175205
Savo MT, De Amicis M, Cozac DA, Cordoni G, Corradin S, Cozza E, Amato F, Lassandro E, Da Pozzo S, Tansella D, et al. Comparative Prognostic Value of Coronary Calcium Score and Perivascular Fat Attenuation Index in Coronary Artery Disease. Journal of Clinical Medicine. 2024; 13(17):5205. https://doi.org/10.3390/jcm13175205
Chicago/Turabian StyleSavo, Maria Teresa, Morena De Amicis, Dan Alexandru Cozac, Gabriele Cordoni, Simone Corradin, Elena Cozza, Filippo Amato, Eleonora Lassandro, Stefano Da Pozzo, Donatella Tansella, and et al. 2024. "Comparative Prognostic Value of Coronary Calcium Score and Perivascular Fat Attenuation Index in Coronary Artery Disease" Journal of Clinical Medicine 13, no. 17: 5205. https://doi.org/10.3390/jcm13175205





