Use of a Fundus Image-Based Titration Strategy for Selective Retina Therapy for Central Serous Chorioretinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. SRT Procedure
2.3. Clinical Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Munch, I.C.; Hasler, P.W.; Prünte, C.; Larsen, M. Central serous chorioretinopathy. Acta Ophthalmol. 2008, 86, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Kitzmann, A.S.; Pulido, J.S.; Diehl, N.N.; Hodge, D.O.; Burke, J.P. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology 2008, 115, 169–173. [Google Scholar] [CrossRef]
- Liu, B.; Deng, T.; Zhang, J. Risk Factors for Central Serous Chorioretinopathy: A Systematic Review and Meta-Analysis. Retina 2016, 36, 9–19. [Google Scholar] [CrossRef]
- Schellevis, R.L.; Altay, L.; Kalisingh, A.; Mulders, T.W.F.; Sitnilska, V.; Hoyng, C.B.; Boon, C.J.F.; Groenewoud, J.M.M.; de Jong, E.K.; den Hollander, A.I. Elevated Steroid Hormone Levels in Active Chronic Central Serous Chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3407–3413. [Google Scholar] [CrossRef]
- Spitznas, M. Pathogenesis of central serous retinopathy: A new working hypothesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 1986, 224, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Quin, G.; Gillies, M.; Fraser-Bell, S. Central serous chorioretinopathy: A review of epidemiology and pathophysiology. Clin. Exp. Ophthalmol. 2013, 41, 201–214. [Google Scholar] [CrossRef]
- Guyer, D.R.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.A.; Ho, A.; Orlock, D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch. Ophthalmol. 1994, 112, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Piccolino, F.C.; Borgia, L.; Zinicola, E.; Zingirian, M. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye 1995, 9, 324–332. [Google Scholar] [CrossRef]
- Klein, M.L.; Van Buskirk, E.M.; Friedman, E.; Gragoudas, E.; Chandra, S. Experience with nontreatment of central serous choroidopathy. Arch. Ophthalmol. 1974, 91, 247–250. [Google Scholar] [CrossRef]
- Gilbert, C.M.; Owens, S.L.; Smith, P.D.; Fine, S.L. Long-term follow-up of central serous chorioretinopathy. Br. J. Ophthalmol. 1984, 68, 815–820. [Google Scholar] [CrossRef]
- Arora, S.; Maltsev, D.S.; Sahoo, N.K.; Parameshwarappa, D.C.; Iovino, C.; Arora, T.; Kulikov, A.N.; Tatti, F.; Venkatesh, R.; Reddy, N.G.; et al. Visual acuity correlates with multimodal imaging-based categories of central serous chorioretinopathy. Eye 2022, 36, 517–523. [Google Scholar] [CrossRef]
- Koskela, P.; Laatikainen, L.; von Dickhoff, K. Contrast sensitivity after resolution of central serous retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1994, 232, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Loo, R.H.; Scott, I.U.; Flynn, H.W., Jr.; Gass, J.D.M.; Murray, T.G.; Lewis, M.L.; Rosenfeld, P.J.; Smiddy, W.E. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002, 22, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Ficker, L.; Vafidis, G.; While, A.; Leaver, P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br. J. Ophthalmol. 1988, 72, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Khosla, P.K.; Rana, S.S.; Tewari, H.K.; Azad, R.U.; Talwar, D. Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 1997, 28, 693–697. [Google Scholar] [CrossRef]
- Reibaldi, M.; Cardascia, N.; Longo, A.; Furino, C.; Avitabile, T.; Faro, S.; Sanfilippo, M.; Russo, A.; Uva, M.G.; Munno, F.; et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: A nonrandomized clinical trial. Am. J. Ophthalmol. 2010, 149, 307–315.e2. [Google Scholar] [CrossRef]
- Lai, T.Y.; Chan, W.M.; Li, H.; Lai, R.Y.; Liu, D.T.; Lam, D.S. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: A short term pilot study. Br. J. Ophthalmol. 2006, 90, 869–874. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Lugo, F.L.; Armadá, F.; Silva, R.; Montero, J.A.; Arevalo, J.F.; Arias, L.; Gómez-Ulla, F. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmol. 2010, 88, 371–376. [Google Scholar] [CrossRef]
- Lim, J.I.; Glassman, A.R.; Aiello, L.P.; Chakravarthy, U.; Flaxel, C.J.; Spaide, R.F.; Macula Society CSC Collaborative Study Group, Research and Education Committee and Website Committee. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014, 121, 1073–1078. [Google Scholar] [CrossRef]
- Brinkmann, R.; Roider, J.; Birngruber, R. Selective retina therapy (SRT): A review on methods, techniques, preclinical and first clinical results. Bull. Soc. Belge. Ophtalmol. 2006, 302, 51–69. [Google Scholar]
- Richert, E.; Koinzer, S.; Tode, J.; Schlott, K.; Brinkmann, R.; Hillenkamp, J.; Klettner, A.; Roider, J. Release of different cell mediators during retinal pigment epithelium regeneration following selective retina therapy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Treumer, F.; Klettner, A.; Baltz, J.; Hussain, A.A.; Miura, Y.; Brinkmann, R.; Roider, J.; Hillenkamp, J. Vectorial release of matrix metalloproteinases (MMPs) from porcine RPE-choroid explants following selective retina therapy (SRT): Towards slowing the macular ageing process. Exp. Eye Res. 2012, 97, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Klatt, C.; Saeger, M.; Oppermann, T.; Pörksen, E.; Treumer, F.; Hillenkamp, J.; Fritzer, E.; Brinkmann, R.; Birngruber, R.; Roider, J. Selective retina therapy for acute central serous chorioretinopathy. Br. J. Ophthalmol. 2011, 95, 83–88. [Google Scholar] [CrossRef]
- Yasui, A.; Yamamoto, M.; Hirayama, K.; Shiraki, K.; Theisen-Kunde, D.; Brinkmann, R.; Miura, Y.; Kohno, T. Retinal sensitivity after selective retina therapy (SRT) on patients with central serous chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 243–254. [Google Scholar] [CrossRef]
- Park, Y.G.; Kang, S.; Kim, M.; Yoo, N.; Roh, Y.J. Selective retina therapy with automatic real-time feedback-controlled dosimetry for chronic central serous chorioretinopathy in Korean patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1375–1383. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.H.; Jeon, S.H.; Lee, S.H.; Roh, Y.J. The effect of selective retina therapy with automatic real-time feedback-controlled dosimetry for chronic central serous chorioretinopathy: A randomized, open-label, controlled clinical trial. J. Clin. Med. 2021, 10, 4295. [Google Scholar] [CrossRef]
- Nam, S.W.; Lee, J.H.; Byun, Z.; Ham, D.I.; Kong, M. Evaluation of the microperimetry in eyes with cuticular drusen. Sci. Rep. 2022, 12, 17557. [Google Scholar] [CrossRef]
- Framme, C.; Brinkmann, R.; Birngruber, R.; Roider, J. Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: A pilot study. Br. J. Ophthalmol. 2002, 86, 1099–1106. [Google Scholar] [CrossRef]
- Kang, S.; Park, Y.G.; Kim, J.R.; Seifert, E.; Theisen-Kunde, D.; Brinkmann, R.; Roh, Y.J. Selective retina therapy in patients with chronic central serous chorioretinopathy: A pilot study. Medicine 2016, 95, e2524. [Google Scholar] [CrossRef]
- Park, Y.G.; Kim, J.R.; Kang, S.; Seifert, E.; Theisen-Kunde, D.; Brinkmann, R.; Roh, Y.J. Safety and efficacy of selective retina therapy (SRT) for the treatment of diabetic macular edema in Korean patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1703–1713. [Google Scholar] [CrossRef]
- Banerjee, R.K.; Zhu, L.; Gopalakrishnan, P.; Kazmierczak, M.J. Influence of laser parameters on selective retinal treatment using single-phase heat transfer analyses. Med. Phys. 2007, 34, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Framme, C.; Schuele, G.; Roider, J.; Birngruber, R.; Brinkmann, R. Influence of pulse duration and pulse number in selective RPE laser treatment. Lasers Surg. Med. 2004, 34, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging retinal laser therapy: Rationale and applications to the macula. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
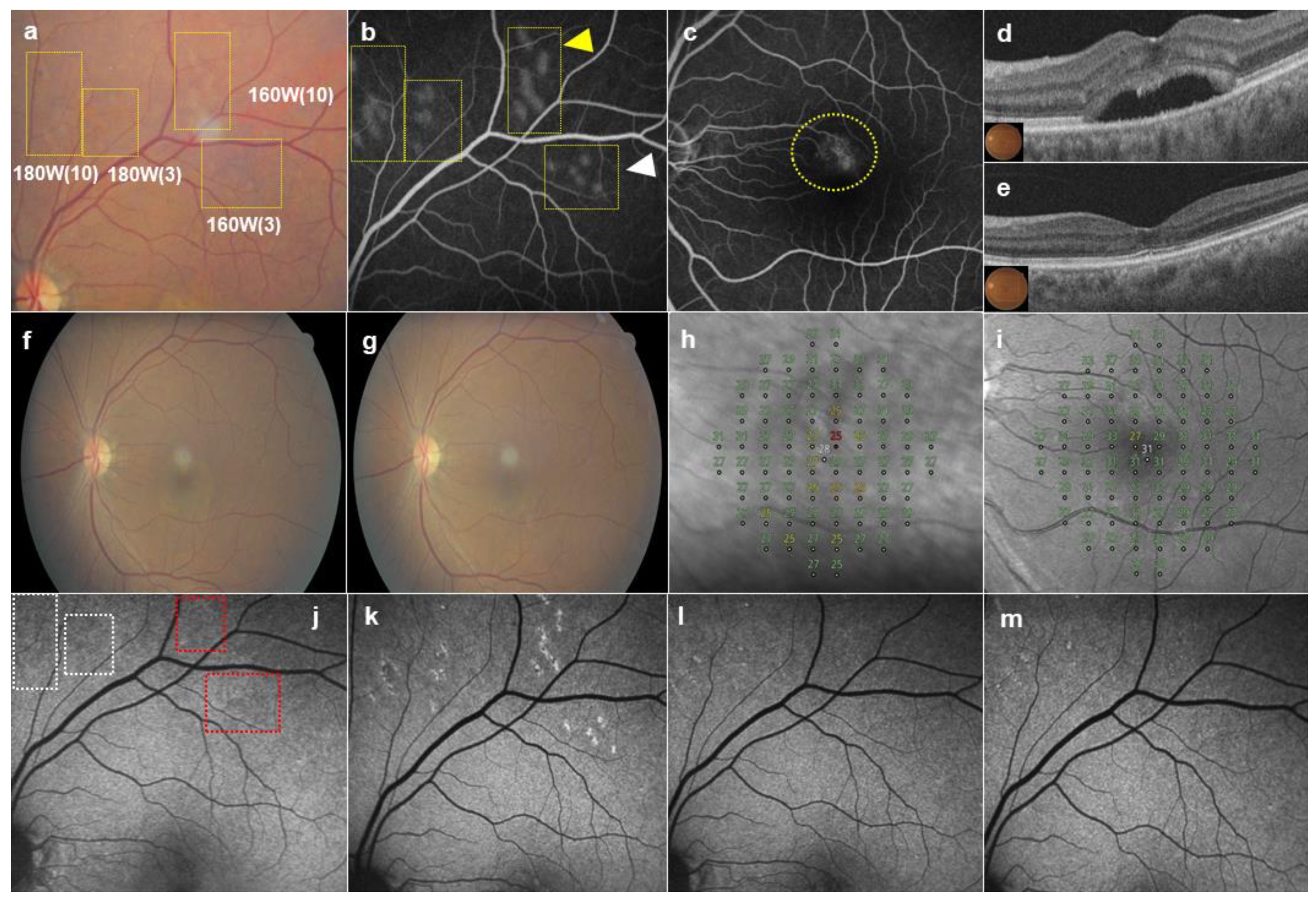

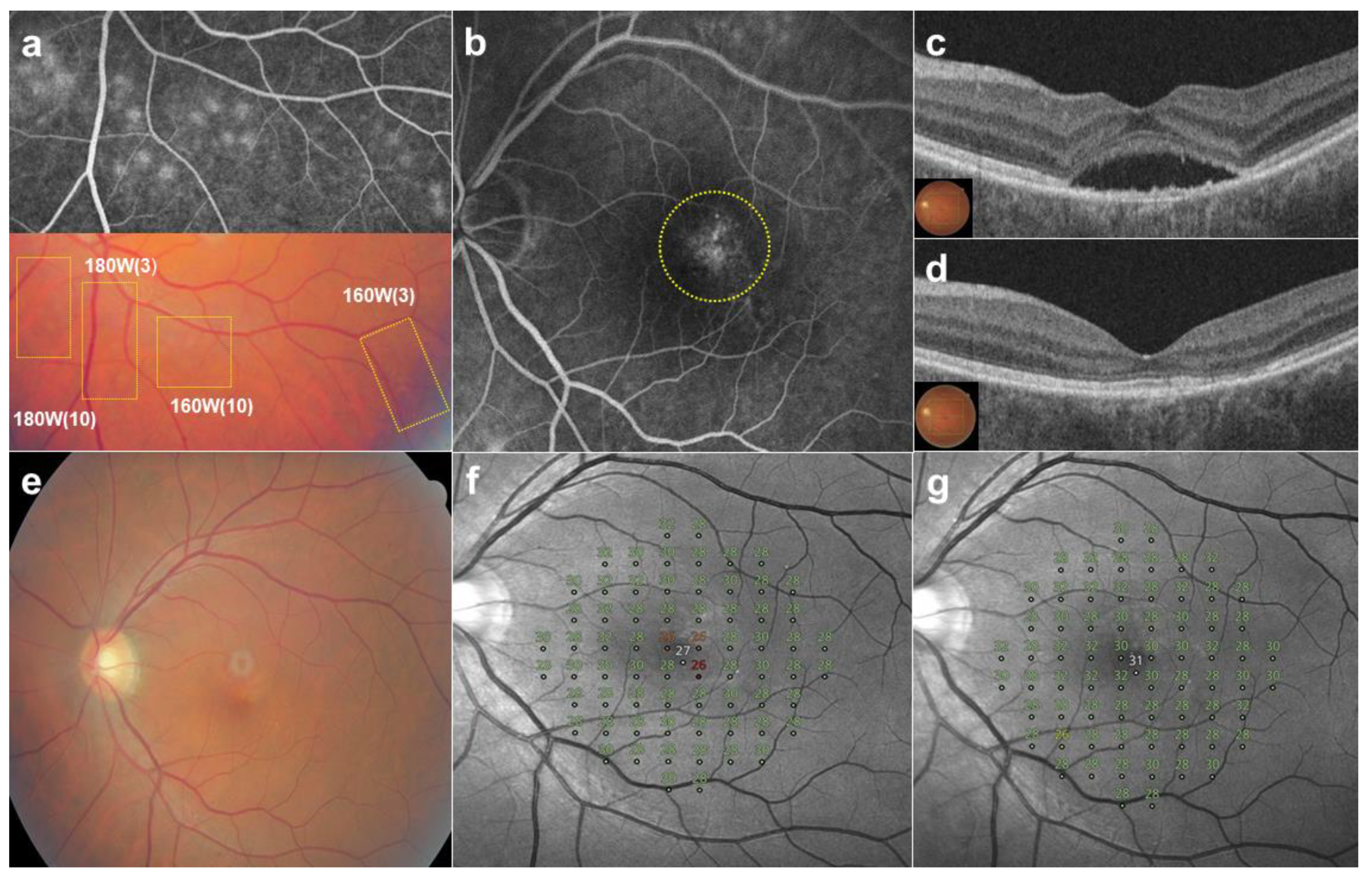
| Patients’ Characteristics | Values |
|---|---|
| Patients (eyes), n | 29 (29) |
| Age (years), mean ± SD | 50.5 ± 9.8 |
| Sex, n (%) | Male 24 (82.8%), Female 5 (17.2%) |
| Duration of current episode (months), mean ± SD | 34.21 ± 38.91 |
| Number of episodes (n), mean ± SD | 3.64 ± 3.38 |
| Number of patients who underwent previous intravitreal bavacizumab injection, n (%) | 19 (65.5%) |
| BCVA (logMAR), mean ± SD | 0.34 ± 0.31 |
| CFT (μm), mean ± SD | 309.31 ± 81.6 |
| SRF height (μm), mean ± SD | 138.36 ± 56.78 |
| MD (dB) of microperimetry, mean ± SD | −1.56 ± 1.47 |
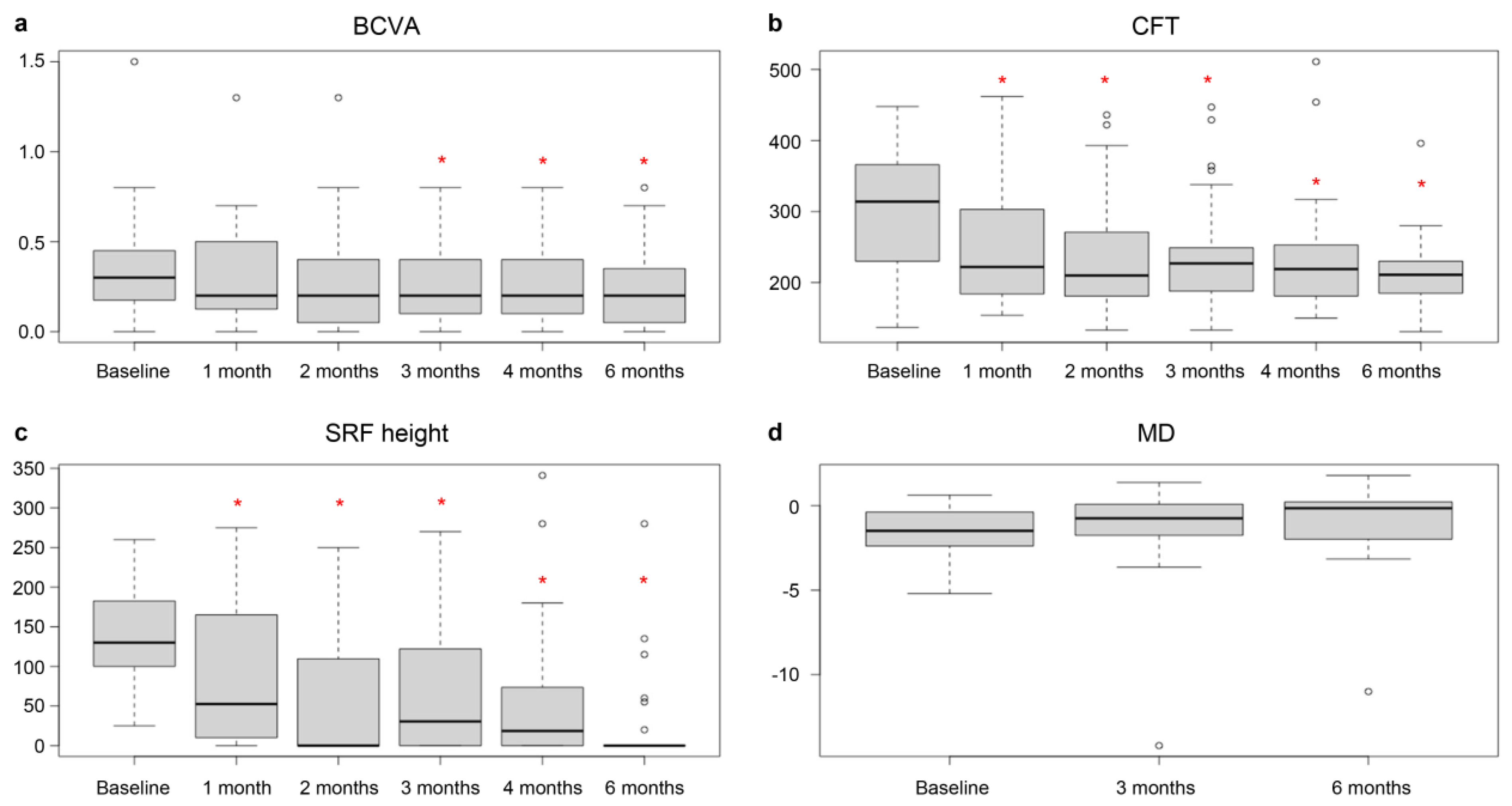
| Baseline | 1 Month | 2 Months | 3 Months | 4 Months | 6 Months | |
|---|---|---|---|---|---|---|
| BCVA (logMAR) | ||||||
| Mean | 0.34 | 0.33 | 0.31 | 0.26 | 0.25 | 0.24 |
| SD | 0.31 | 0.3 | 0.31 | 0.24 | 0.21 | 0.24 |
| p value | 0.499 | 0.177 | 0.026 * | 0.007 * | 0.009 * | |
| CFT (μm) | ||||||
| Mean | 309.31 | 257.79 | 244.62 | 239.41 | 234.38 | 211.07 |
| SD | 81.6 | 96.8 | 86.44 | 78.11 | 81.55 | 50.21 |
| p value | 0.003 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| SRF height (μm) | ||||||
| Mean | 138.36 | 85.93 | 63.5 | 64.11 | 51.68 | 23.75 |
| SD | 56.78 | 88.22 | 85.18 | 79.57 | 85.26 | 61.19 |
| p value | 0.002 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| MD (dB) | ||||||
| Mean | −1.56 | −1.20 | −1.03 | |||
| SD | 1.47 | 2.86 | 2.43 | |||
| p value | 0.374 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.H.; Kim, M.; Roh, Y.-J. Use of a Fundus Image-Based Titration Strategy for Selective Retina Therapy for Central Serous Chorioretinopathy. J. Clin. Med. 2024, 13, 5230. https://doi.org/10.3390/jcm13175230
Jeon SH, Kim M, Roh Y-J. Use of a Fundus Image-Based Titration Strategy for Selective Retina Therapy for Central Serous Chorioretinopathy. Journal of Clinical Medicine. 2024; 13(17):5230. https://doi.org/10.3390/jcm13175230
Chicago/Turabian StyleJeon, Seung Hee, Minhee Kim, and Young-Jung Roh. 2024. "Use of a Fundus Image-Based Titration Strategy for Selective Retina Therapy for Central Serous Chorioretinopathy" Journal of Clinical Medicine 13, no. 17: 5230. https://doi.org/10.3390/jcm13175230






