Contextual Hospital Conditions and the Risk of Nosocomial SARS-CoV-2 Infection: A Matched Case-Control Study with Density Sampling in a Large Portuguese Hospital
Abstract
:1. Introduction
2. Materials and Methods
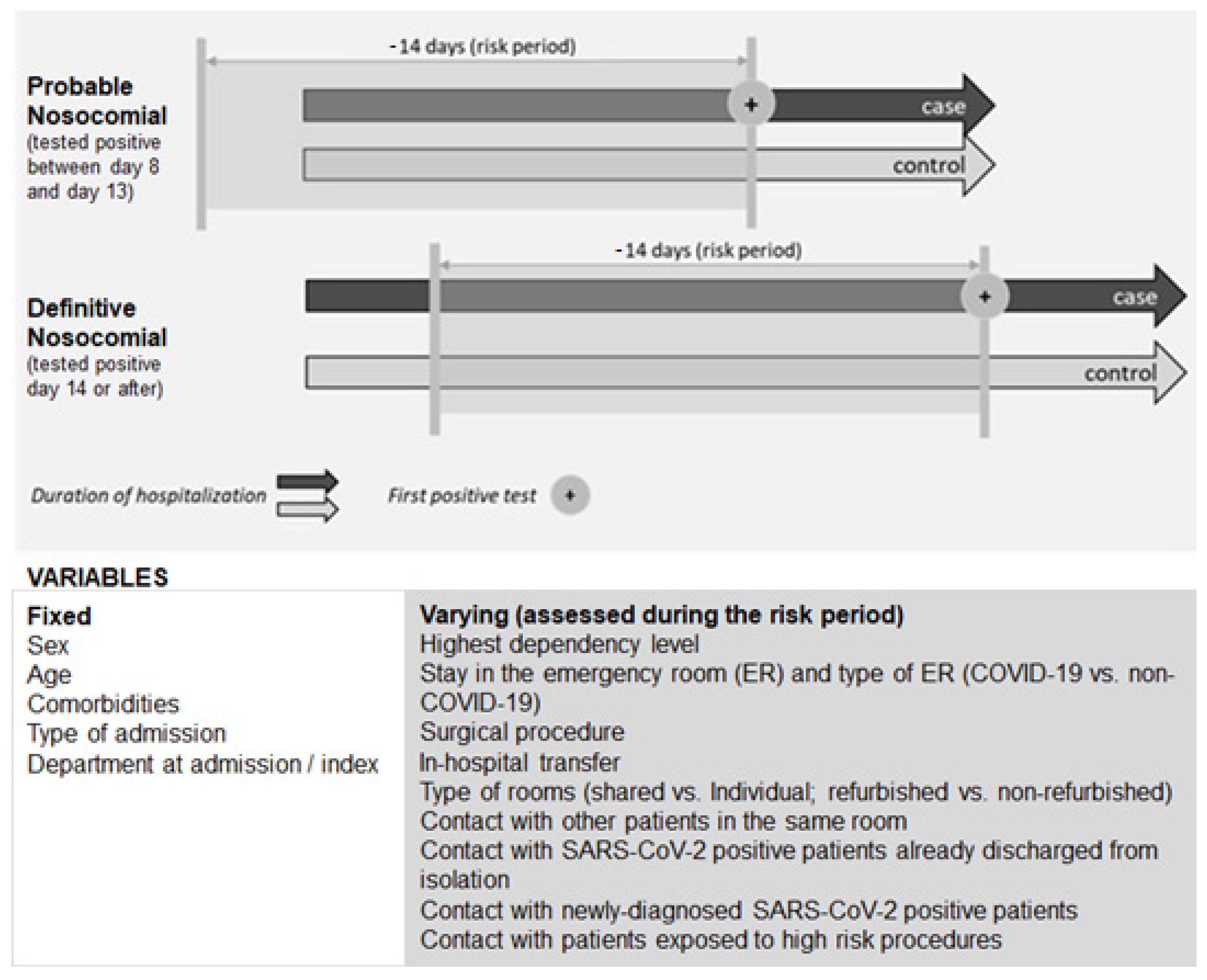
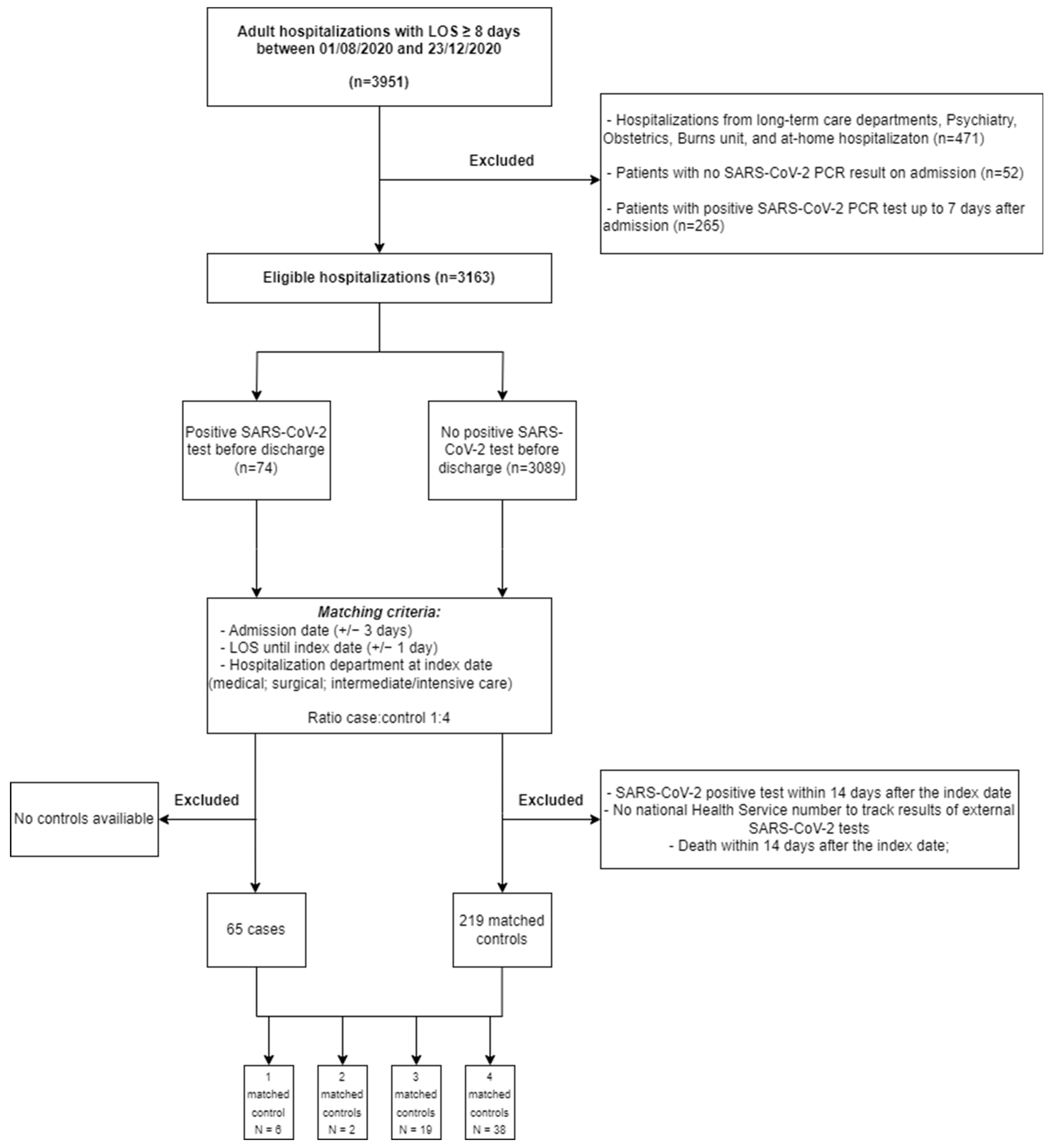
- Case-control eligibility criteria
- Cases, controls, and matching
- Risk factors
- Statistical analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhee, C.; Baker, M.; Vaidya, V.; Tucker, R.; Resnick, A.; Morris, C.A.; Klompas, M.; The CDC Prevention Epicenters Program. Incidence of Nosocomial COVID-19 in Patients Hospitalized at a Large US Academic Medical Center. JAMA Netw. Open 2020, 3, e2020498. [Google Scholar] [CrossRef] [PubMed]
- Rickman, H.M.; Rampling, T.; Shaw, K.; Martinez-Garcia, G.; Hail, L.; Coen, P.; Shahmanesh, M.; Shin, G.Y.; Nastouli, E.; Houlihan, C.F. Nosocomial Transmission of Coronavirus Disease 2019: A Retrospective Study of 66 Hospital-acquired Cases in a London Teaching Hospital. Clin. Infect. Dis. 2021, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Rangaiah, J.; Narasimhan, S.; Clark, J.; Alexander, Z.; Manuel, R.; Balasegaram, S. Nosocomial COVID-19: Experience from a large acute NHS Trust in South-West London. J. Hosp. Infect. 2020, 106, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.S.; Evans, S.; Jafari, Y.; Pham, T.M.; Mo, Y.; Lim, C.; Pritchard, M.G.; Pople, D.; Hall, V.; Stimson, J.; et al. The burden and dynamics of hospital-acquired SARS-CoV-2 in England. Nature 2023, 623, 132–138. [Google Scholar] [CrossRef]
- Yu, I.T.; Xie, Z.H.; Tsoi, K.K.; Chiu, Y.L.; Lok, S.W.; Tang, X.P.; Hui, D.S.; Lee, N.; Li, Y.M.; Huang, Z.T.; et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin. Infect. Dis. 2007, 44, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Nunes, T.R.; Martischang, R.; Zingg, W.; Iten, A.; Pittet, D.; Harbarth, S. Nosocomial transmission and outbreaks of coronavirus disease 2019: The need to protect both patients and healthcare workers. Antimicrob. Resist. Infect. Control 2021, 10, 7. [Google Scholar] [CrossRef]
- Hawkins, L.P.A.; Pallett, S.J.C.; Mazzella, A.; Anton-Vazquez, V.; Rosas, L.; Jawad, S.M.; Shakespeare, D.; Breathnach, A.S. Transmission dynamics and associated mortality of nosocomial COVID-19 throughout 2021: A retrospective study at a large teaching hospital in London. J. Hosp. Infect. 2023, 133, 62–69. [Google Scholar] [CrossRef]
- Wolfensberger, A.; Kufner, V.; Zaheri, M.; Zeeb, M.; Nortes, I.; Schreiber, P.W.; Vazquez, M.; Schärer, V.; Scheier, T.; Schmutz, S.; et al. Nosocomial COVID-19 Incidence and Secondary Attack Rates among Patients of Tertiary Care Center, Zurich, Switzerland. Emerg. Infect. Dis. 2022, 28, 2087–2090. [Google Scholar] [CrossRef]
- Yek, C. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series—465 Health Care Facilities, United States, December 2020–October 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 19–25. [Google Scholar] [CrossRef]
- Huang, Q.; Mondal, A.; Jiang, X.; Horn, M.A.; Fan, F.; Fu, P.; Wang, X.; Zhao, H.; Ndeffo-Mbah, M.; Gurarie, D. SARS-CoV-2 transmission and control in a hospital setting: An individual-based modelling study. R. Soc. Open Sci. 2021, 8, 201895. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Wang, B.; Lu, H. The COVID-19-designated hospitals in China: Preparing for public health emergencies. Emerg. Microbes Infect. 2021, 10, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Kaito, D.; Matsumura, K.; Yamamoto, R. Hospital Preparedness for COVID-19: The Known and The Known Unknown. Keio J. Med. 2021, 70, 25–34. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.; Tong, D.W.; Chuang, V.W.; Chen, J.H.; Lee, L.L.; To, K.K.; Hung, I.F.; Ho, P.; Yeung, D.T.; et al. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID-19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect. Control Hosp. Epidemiol. 2022, 43, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Karan, A.; Klompas, M.; Tucker, R.; Baker, M.; Vaidya, V.; Rhee, C. The Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission from Patients With Undiagnosed Coronavirus Disease 2019 (COVID-19) to Roommates in a Large Academic Medical Center. Clin. Infect. Dis. 2022, 74, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wong, J.Y.; Murphy, C.; Yeung, A.; Ali, S.T.; Wu, P.; Cowling, B.J. The Incubation Period Distribution of Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2021, 73, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.S.; Maini, P.K.; Thompson, R.N. High infectiousness immediately before COVID-19 symptom onset highlights the importance of continued contact tracing. Elife 2021, 10, e65534. [Google Scholar] [CrossRef]
- Grassly, N.C.; Pons-Salort, M.; Parker, E.P.K.; White, P.J.; Ferguson, N.M.; Imperial College COVID-19 Response Team. Comparison of molecular testing strategies for COVID-19 control: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 1381–1389. [Google Scholar] [CrossRef]
- Aghalari, Z.; Dahms, H.U.; Sosa-Hernandez, J.E.; Oyervides-Munoz, M.A.; Parra-Saldivar, R. Evaluation of SARS-COV-2 transmission through indoor air in hospitals and prevention methods: A systematic review. Environ. Res. 2021, 195, 110841. [Google Scholar] [CrossRef]
- Stiller, A.; Salm, F.; Bischoff, P.; Gastmeier, P. Relationship between hospital ward design and healthcare-associated infection rates: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2016, 5, 51. [Google Scholar] [CrossRef]
- Evans, S.; Agnew, E.; Vynnycky, E.; Stimson, J.; Bhattacharya, A.; Rooney, C.; Warne, B.; Robotham, J. The impact of testing and infection prevention and control strategies on within-hospital transmission dynamics of COVID-19 in English hospitals. Philos. Trans. R. Soc. B 2021, 376, 20200268. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- van Kampen, J.J.; van de Vijver, D.A.; Fraaij, P.L.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.; Endeman, H.; Gommers, D.A.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. New Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.F.; Chorlton, S.; Tyson, J.; Al-Rawahi, G.N.; Jassem, A.N.; Prystajecky, N.; Masud, S.; Deans, G.D.; Chapman, M.G.; Mirzanejad, Y.; et al. COVID-19 in an immunocompromised host: Persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: A case report. Int. J. Infect. Dis. 2022, 114, 178–182. [Google Scholar] [CrossRef]
- Hensley, M.K.; Bain, W.G.; Jacobs, J.; Nambulli, S.; Parikh, U.; Cillo, A.; Staines, B.; Heaps, A.; Sobolewski, M.D.; Rennick, L.J.; et al. Intractable Coronavirus Disease 2019 (COVID-19) and Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Replication in a Chimeric Antigen Receptor-Modified T-Cell Therapy Recipient: A Case Study. Clin. Infect. Dis. 2021, 73, e815–e821. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control Guidance on Ending the Isolation Period for People with COVID-19, Third Update. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation (accessed on 16 January 2024).
- Aghdassi, S.J.S.; Schwab, F.; Peña Diaz, L.A.; Brodzinski, A.; Fucini, G.B.; Hansen, S.; Kohlmorgen, B.; Piening, B.; Schlosser, B.; Schneider, S.; et al. Risk factors for nosocomial SARS-CoV-2 infections in patients: Results from a retrospective matched case-control study in a tertiary care university center. Antimicrob. Resist. Infect. Control 2022, 11, 9. [Google Scholar] [CrossRef]
- Dave, N.; Sjöholm, D.; Hedberg, P.; Ternhag, A.; Granath, F.; Verberk, J.D.; Johansson, A.F.; van der Werff, S.D.; Nauclér, P. Nosocomial SARS-CoV-2 Infections and Mortality During Unique COVID-19 Epidemic Waves. JAMA Netw. Open 2023, 6, e2341936. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.M.; Duval, A.; Grant, R.; Abbas, M.; Harbarth, S.; Opatowski, L.; Temime, L. Predicting consequences of COVID-19 control measure de-escalation on nosocomial transmission and mortality: A modelling study in a French rehabilitation hospital. J. Hosp. Infect. 2024, 147, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Pak, T.R.; Rhee, C.; Wang, R.; Klompas, M. Discontinuation of Universal Admission Testing for SARS-CoV-2 and Hospital-Onset COVID-19 Infections in England and Scotland. JAMA Intern. Med. 2023, 183, 877–880. [Google Scholar] [CrossRef]
| Nosocomial SARS-CoV-2 Cases (n = 65) | Controls (n = 219) | |
|---|---|---|
| Week of the admission date a,b | 44 (41; 46) | 44 (41; 46) |
| Length of stay until index date a | 14.0 (10.0; 23.4) | 14.0 (9.0; 21.0) |
| Type of department at index date % (n) | ||
| Surgical | 17 (25.2) | 56 (25.6) |
| Medical | 45 (69.2) | 156 (71.2) |
| Intensive care | 3 (4.6) | 7 (3.2) |
| SARS-CoV-2 Nosocomial Transmission | ||||
|---|---|---|---|---|
| Probable | Definitive | |||
| Controls | Cases | Controls | Cases | |
| (n = 114) | (n = 31) | (n = 105) | (n = 34) | |
| Age (years) | ||||
| 18–54 | 30 (26.3) | 6 (19.4) | 29 (27.6) | 7 (20.6) |
| 55–64 | 17 (14.9) | 6 (19.4) | 20 (19.0) | 4 (11.8) |
| 65–74 | 27 (23.7) | 5 (16.1) | 30 (28.6) | 11 (32.4) |
| ≥75 | 40 (35.1) | 14 (45.2) | 26 (24.8) | 12 (35.3) |
| Male | 63 (55.3) | 17 (54.8) | 64 (61.0) | 19 (55.9) |
| Urgent admission | 87 (76.3) | 28 (90.3) | 80 (76.2) | 29 (85.3) |
| Type of admission department | ||||
| Surgical | 21 (18.4) | 5 (16.1) | 23 (21.9) | 10 (29.4) |
| Medical | 74 (64.9) | 19 (61.3) | 53 (50.5) | 20 (58.8) |
| Intensive care | 19 (16.7) | 7 (22.6) | 29 (27.6) | 4 (11.8) |
| Dependent patient | 78 (68.4) | 24 (77.4) | 45 (42.9) | 13 (38.2) |
| Comorbidities | 95 (83.3) | 22 (71.0) | 84 (80.0) | 29 (85.3) |
| Arterial hypertension | 57 (50.0) | 15 (48.4) | 53 (50.5) | 19 (55.9) |
| Chronic obstructive pulmonary disease | 15 (13.2) | 6 (19.4) | 9 (8.6) | 3 (8.8) |
| Heart failure | 30 (26.3) | 6 (19.4) | 13 (12.4) | 9 (26.5) |
| Ischemic heart disease | 19 (16.7) | 5 (16.1) | 14 (13.3) | 4 (11.8) |
| Diabetes mellitus | 21 (18.4) | 8 (25.8) | 34 (32.4) | 14 (41.2) |
| Active neoplasm | 34 (29.8) | 5 (16.1) | 29 (27.6) | 6 (17.6) |
| Transplant | 4 (3.5) | 0 (0.0) | 5 (4.8) | 0 (0.0) |
| Renal replacement therapy | 4 (3.5) | 0 (0.0) | 2 (1.9) | 1 (2.9) |
| Contextual characteristics (14 days prior to index date) | ||||
| Emergency room visit a | 82 (71.9) | 28 (90.3) | ||
| Surgeries | 23 (20.2) | 3 (9.7) | 31 (29.5) | 10 (29.4) |
| Stay in a non-refurbished room | 54 (47.4) | 24 (77.4) | 54 (51.4) | 20 (58.8) |
| Number of different rooms | ||||
| 1 | 71 (62.3) | 15 (48.4) | 59 (56.2) | 21 (61.8) |
| 2 | 34 (29.8) | 11 (35.5) | 26 (24.8) | 9 (26.5) |
| 3 | 9 (7.9) | 5 (16.1) | 20 (19.0) | 4 (11.8) |
| Stay in a shared ward | 106 (93.0) | 31 (100.0) | 100 (95.2) | 34 (100.0) |
| Maximum number of beds in room | ||||
| 1 | 8 (7.0) | 0 (0.0) | 5 (4.8) | 0 (0.0) |
| 2–4 | 39 (34.2) | 8 (25.8) | 42 (40) | 16 (47.1) |
| 5–9 | 51 (44.7) | 17 (54.8) | 32 (30.5) | 15 (44.1) |
| ≥10 | 16 (14) | 6 (19.4) | 26 (24.8) | 3 (8.8) |
| Contact with other patients | 106 (93.0) | 31 (100.0) | 100 (95.2) | 33 (97.1) |
| Duration of contact (hours) | ||||
| ≤750 | 60 (56.6) | 16 (51.6) | 39 (39.0) | 13 (39.4) |
| 751–1500 | 34 (32.1) | 12 (38.7) | 33 (33.0) | 14 (42.4) |
| >1500 | 12 (11.3) | 3 (9.7) | 28 (28.0) | 6 (18.2) |
| Contact with patients exposed to high-risk procedures | 64 (56.1) | 21 (67.7) | 51 (48.6) | 18 (52.9) |
| Contact with SARS-CoV-2-positive patients already discharged from isolation | 30 (26.3) | 14 (45.2) | 9 (8.6) | 6 (17.6) |
| Contact with newly diagnosed SARS-CoV-2-positive patients | 12 (10.5) | 9 (29.0) | 12 (11.4) | 12 (35.3) |
| Probable (n = 145) | Definitive (n = 139) | |||||
|---|---|---|---|---|---|---|
| Crude Analysis | M2 a | M3 b | Crude Analysis | M2 a | M3 b | |
| Stay in non-refurbished wards | 4.16 (1.59–10.85) | 4.78 (1.65–13.83) | 3.58 (1.18–10.87) | 1.27 (0.57–2.81) | 1.14 (0.49–2.66) | 0.72 (0.27–1.91) |
| Sharing room with SARS-CoV-2-positive patients already discharged from isolation | 2.73 (1–7.48) | 3.2 (1.03–9.92) | 2.51 (0.78–8.03) | 2.44 (0.75–7.94) | 2.23 (0.68–7.38) | 2.27 (0.65–7.93) |
| Sharing room with newly diagnosed SARS-CoV-2-positive patients | 3.84 (1.37–10.72) | 3.85 (1.35–11.02) | 3.35 (1.09–10.3) | 10.17 (2.2–46.97) | 9.91 (2.13–46.2) | 9.92 (2.11–46.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, F.; Correia, S.; Leal, C.; Guedes, M.; Duro, R.; Andrade, P.; Pedrosa, A.; Rocha-Pereira, N.; Lima-Alves, C.; Azevedo, A. Contextual Hospital Conditions and the Risk of Nosocomial SARS-CoV-2 Infection: A Matched Case-Control Study with Density Sampling in a Large Portuguese Hospital. J. Clin. Med. 2024, 13, 5251. https://doi.org/10.3390/jcm13175251
Almeida F, Correia S, Leal C, Guedes M, Duro R, Andrade P, Pedrosa A, Rocha-Pereira N, Lima-Alves C, Azevedo A. Contextual Hospital Conditions and the Risk of Nosocomial SARS-CoV-2 Infection: A Matched Case-Control Study with Density Sampling in a Large Portuguese Hospital. Journal of Clinical Medicine. 2024; 13(17):5251. https://doi.org/10.3390/jcm13175251
Chicago/Turabian StyleAlmeida, Francisco, Sofia Correia, Cátia Leal, Mariana Guedes, Raquel Duro, Paulo Andrade, Afonso Pedrosa, Nuno Rocha-Pereira, Carlos Lima-Alves, and Ana Azevedo. 2024. "Contextual Hospital Conditions and the Risk of Nosocomial SARS-CoV-2 Infection: A Matched Case-Control Study with Density Sampling in a Large Portuguese Hospital" Journal of Clinical Medicine 13, no. 17: 5251. https://doi.org/10.3390/jcm13175251






