Left Atrial Appendage Thrombus as a Marker of Disease Severity in 500 Patients with Atrial Fibrillation on Oral Anticoagulation: A 13-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Anticoagulation Therapy
2.3. Echocardiographic Examination
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turek, L.; Sadowski, M.; Janion-Sadowska, A.; Kurzawski, J.; Jaroszynski, A. Left atrial appendage thrombus in patients referred for electrical cardioversion for atrial fibrillation: A prospective single-center study. Pol. Arch. Intern. Med. 2022, 132, 16214. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 2016, 50, e1–e88. [Google Scholar] [CrossRef]
- Tomaszuk-Kazberuk, A.; Kozinski, M.; Kuzma, L.; Bujno, E.; Lopatowska, P.; Rogalska, E.; Dobrzycki, S.; Sobkowicz, B.; Lip, G.Y.H. Atrial fibrillation is more frequently associated with nonobstructive coronary lesions: The Bialystok Coronary Project. Pol. Arch. Intern. Med. 2020, 130, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Tracz, J.; Gorczyca-Głowacka, I.; Wałek, P.; Rosołowska, A.; Wożakowska-Kapłon, B. Risk factors of ischaemic stroke in patients with atrial fibrillation. Med. Stud. 2023, 39, 8–13. [Google Scholar] [CrossRef]
- Bielecka, B.; Gorczyca-Głowacka, I.; Ciba-Stemplewska, A.; Wożakowska-Kapłon, B. Secondary prevention of thromboembolic complications in patients with nonvalvular atrial fibrillation—Clinical practice in relation to guidelines. Med. Stud. 2023, 39, 159–171. [Google Scholar] [CrossRef]
- Hamatani, Y.; Iguchi, M.; Okamoto, K.; Nakanishi, Y.; Minami, K.; Ishigami, K.; Ikeda, S.; Doi, K.; Yoshizawa, T.; Ide, Y.; et al. Association of left atrial enlargement with heart failure events in non-valvular atrial fibrillation patients with preserved left ventricular ejection fraction. Eur. Heart J. Open 2024, 4, oeae015. [Google Scholar] [CrossRef]
- Taniguchi, N.; Miyasaka, Y.; Suwa, Y.; Harada, S.; Nakai, E.; Kawazoe, K.; Shiojima, I. Usefulness of Left Atrial Volume as an Independent Predictor of Development of Heart Failure in Patients With Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 1430–1435. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.; Seward, J.B.; Iwasaka, T.; Tsang, T.S. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: A community-based study over two decades. Eur. Heart J. 2006, 27, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Al-Saady, N.M.; Obel, O.A.; Camm, A.J. Left atrial appendage: Structure, function, and role in thromboembolism. Heart 1999, 82, 547–554. [Google Scholar] [CrossRef]
- European Heart Rhythm, A.; European Association for Cardio-Thoracic, S.; Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [CrossRef]
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P.; Guidelines, E.S.C.C.f.P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012, 33, 2719–2747. [Google Scholar] [CrossRef] [PubMed]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013, 15, 625–651. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Diener, H.C.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015, 17, 1467–1507. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology, C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Kasprzak, J.D.; Hoffman, P.; Płońska, E.; Szyszka, A.; Braksator, W.; Gackowski, A.; Plewka, M.; Drożdż, J.; Gąsior, Z.; Pruszczyk, P. In Poland Echokardiografia w praktyce klinicznej–Standardy Sekcji Echokardiografii Polskiego Towarzystwa Kardiologicznego 2007. Kardiol. Pol. 2007, 65, 1142–1162. [Google Scholar]
- Anselmino, M.; Garberoglio, L.; Gili, S.; Bertaglia, E.; Stabile, G.; Marazzi, R.; Themistoclakis, S.; Solimene, F.; Frea, S.; Grosso Marra, W.; et al. Left atrial appendage thrombi relate to easily accessible clinical parameters in patients undergoing atrial fibrillation transcatheter ablation: A multicenter study. Int. J. Cardiol. 2017, 241, 218–222. [Google Scholar] [CrossRef]
- Seidl, K.; Rameken, M.; Drogemuller, A.; Vater, M.; Brandt, A.; Schwacke, H.; Bergmeier, C.; Zahn, R.; Senges, J. Embolic events in patients with atrial fibrillation and effective anticoagulation: Value of transesophageal echocardiography to guide direct-current cardioversion. Final results of the Ludwigshafen Observational Cardioversion Study. J. Am. Coll. Cardiol. 2002, 39, 1436–1442. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- Tsiachris, D.; Papakonstantinou, P.E.; Doundoulakis, I.; Tsioufis, P.; Botis, M.; Dimitriadis, K.; Leontsinis, I.; Kordalis, A.; Antoniou, C.K.; Mantzouranis, E.; et al. Anticoagulation Status and Left Atrial Appendage Occlusion Indications in Hospitalized Cardiology Patients with Atrial Fibrillation: A Hellenic Cardiorenal Morbidity Snapshot (HECMOS) Sub-Study. Medicina 2023, 59, 1881. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Baines, O.; Hayes, A.; Tompkins, K.; Kalla, M.; Holmes, A.P.; O’Shea, C.; Pavlovic, D. Impact of Obesity on Atrial Fibrillation Pathogenesis and Treatment Options. J. Am. Heart Assoc. 2024, 13, e032277. [Google Scholar] [CrossRef] [PubMed]
- Nteli, M.; Nteli, D.; Moysidis, D.V.; Foka, A.; Zymaris, P.; Grantza, T.; Kazarli, O.; Vagianos, A.; Papazoglou, A.S.; Kartas, A.; et al. Prognostic Impact of Body Mass Index in Atrial Fibrillation. J. Clin. Med. 2024, 13, 3294. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, A.S.; Albertsen, A.E.; Christesen, A.M.S.; Vinter, N.; Frost, L.; Moller, D.S. Cardioversion of atrial fibrillation in a real-world setting: Non-vitamin K antagonist oral anticoagulants ensure a fast and safe strategy compared to warfarin. Europace 2018, 20, 1078–1085. [Google Scholar] [CrossRef]
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.A.; et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420. [Google Scholar] [CrossRef]
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Scardi, S.; Mazzone, C.; Pandullo, C.; Goldstein, D.; Perkan, A. A longitudinal study on left atrial thrombosis in patients with non-rheumatic atrial fibrillation treated with anticoagulants. G. Ital. Cardiol. 1997, 27, 1036–1043. [Google Scholar]
- Janion-Sadowska, A.; Turek, Ł.; Dudek, A.; Andrychowski, J.; Sadowski, M. Atrial fibrillation and flutter—The state of the art. Part 2. Med. Stud. 2021, 37, 239–249. [Google Scholar] [CrossRef]
- Schaeffer, B.; Ruden, L.; Salzbrunn, T.; Pinnschmidt, H.O.; Akbulak, R.O.; Moser, J.M.; Jularic, M.; Meyer, C.; Eickholt, C.; Sultan, A.; et al. Incidence of intracardiac thrombus formation prior to electrical cardioversion in respect to the mode of oral anticoagulation. J. Cardiovasc. Electrophysiol. 2018, 29, 537–547. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, J.I.; Kim, M.N.; Cho, D.H.; Oh, S.K.; Kook, H.; Park, H.S.; Lee, K.N.; Baek, Y.S.; Roh, S.Y.; et al. Non-vitamin K antagonist oral anticoagulants versus warfarin for the prevention of spontaneous echo-contrast and thrombus in patients with atrial fibrillation or flutter undergoing cardioversion: A trans-esophageal echocardiography study. PLoS ONE 2018, 13, e0191648. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Rago, A.; Papa, A.A.; D’Onofrio, A.; Golino, P.; Nigro, G. Efficacy and safety of dabigatran in patients with atrial fibrillation scheduled for transoesophageal echocardiogram-guided direct electrical current cardioversion: A prospective propensity score-matched cohort study. J. Thromb. Thrombolysis 2018, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; D’Amato, S.A.; Al-Kazaz, M.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; Sharma, S.K.; Yang, H.; Singh, P.; et al. Prevalence of Left Atrial Thrombus Detection by Transesophageal Echocardiography: A Comparison of Continuous Non-Vitamin K Antagonist Oral Anticoagulant Versus Warfarin Therapy in Patients Undergoing Catheter Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2016, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, I.; Chrapek, M.; Jelonek, O.; Michalska, A.; Kaplon-Cieslicka, A.; Uzieblo-Zyczkowska, B.; Budnik, M.; Gawalko, M.; Krzesinski, P.; Jurek, A.; et al. Left Atrial Appendage Thrombus Formation Despite Continuous Non-Vitamin K Antagonist Oral Anticoagulant Therapy in Atrial Fibrillation Patients Undergoing Electrical Cardioversion or Catheter Ablation: A Comparison of Dabigatran and Rivaroxaban. Cardiol. Res. Pract. 2020, 2020, 1206402. [Google Scholar] [CrossRef]
- Angelini, F.; Bocchino, P.P.; Peyracchia, M.; Saglietto, A.; Magnano, M.; Patane, N.; D’Ascenzo, F.; Giustetto, C.; Anselmino, M.; Gaita, F.; et al. Prevalence and predictors of left atrial thrombosis in atrial fibrillation patients treated with non-vitamin K antagonist oral anticoagulants. Acta Cardiol. 2023, 78, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Karpuz, M.H.; Bilgehan, K.; Ikitimur, B.; Ozmen, E.; Ebren, C.; Polat, F.; Koca, D.; Tokdil, K.O.; Kandemirli, S.G.; et al. Left atrial thrombus in patients with atrial fibrillation and under oral anticoagulant therapy; 3-D transesophageal echocardiographic study. Int. J. Cardiovasc. Imaging 2020, 36, 1097–1103. [Google Scholar] [CrossRef]
- Nair, C.K.; Holmberg, M.J.; Aronow, W.S.; Shen, X.; Li, H.; Lakkireddy, D. Thromboembolism in patients with atrial fibrillation with and without left atrial thrombus documented by transesophageal echocardiography. Am. J. Ther. 2009, 16, 385–392. [Google Scholar] [CrossRef]
- Vinereanu, D.; Lopes, R.D.; Mulder, H.; Gersh, B.J.; Hanna, M.; de Barros, E.S.P.G.M.; Atar, D.; Wallentin, L.; Granger, C.B.; Alexander, J.H.; et al. Echocardiographic Risk Factors for Stroke and Outcomes in Patients with Atrial Fibrillation Anticoagulated With Apixaban or Warfarin. Stroke 2017, 48, 3266–3273. [Google Scholar] [CrossRef]
- Kosmalska, K.; Gilis-Malinowska, N.; Rzyman, M.; Danilowicz-Szymanowicz, L.; Fijalkowski, M. Risk of Death and Ischemic Stroke in Patients with Atrial Arrhythmia and Thrombus or Sludge in Left Atrial Appendage at One-Year Follow-Up. J. Clin. Med. 2022, 11, 1128. [Google Scholar] [CrossRef]
- Hamatani, Y.; Iguchi, M.; Minami, K.; Ishigami, K.; Esato, M.; Tsuji, H.; Wada, H.; Hasegawa, K.; Ogawa, H.; Abe, M.; et al. Utility of left ventricular ejection fraction in atrial fibrillation patients without pre-existing heart failure. ESC Heart Fail. 2023, 10, 3091–3101. [Google Scholar] [CrossRef]
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.T.; Cheng, S.; Vasan, R.S.; Lee, D.S.; et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation 2016, 133, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Chudasama, R.; Lane, D.A.; Kirchhof, P.; Lip, G.Y. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta-analysis of death and adverse outcomes. Int. J. Cardiol. 2016, 203, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sagara, K.; Otsuka, T.; Matsuno, S.; Funada, R.; Uejima, T.; Oikawa, Y.; Yajima, J.; Koike, A.; Nagashima, K.; et al. A new scoring system for evaluating the risk of heart failure events in Japanese patients with atrial fibrillation. Am. J. Cardiol. 2012, 110, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Potpara, T.S.; Polovina, M.M.; Licina, M.M.; Marinkovic, J.M.; Lip, G.Y. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: The Belgrade Atrial Fibrillation Study. Eur. J. Heart Fail. 2013, 15, 415–424. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
| Variable | Patients with LAAT (n = 65) | Patients without LAAT (n = 435) | p Value |
|---|---|---|---|
| Female sex | 26 (40) | 172 (39.5) | 0.94 |
| Age, years | 68.4 (8.9) | 64.0 (9.8) | 0.001 |
| BMI, kg/m2 | 28.2 (26.0–32.4) | 29.4 (27.1–32.9) | 0.045 |
| CHA2DS2-VASc score | 4 (3–4) | 3 (2–4) | <0.001 |
| Heart rate, 1/min | 100 (85–115) | 90 (80–110) | 0.1 |
| SBP, mmHg | 130 (120–130) | 125 (120–130) | 0.67 |
| DBP, mmHg | 80 (70–80) | 80 (70–80) | 0.4 |
| eGFR, mL/min/1.73 m2 | 66.1 (50.3–75.3) | 66.9 (57.9–76.4) | 0.09 |
| LA diameter, mm | 47 (44–52) | 44 (42–48) | <0.001 |
| LVEF, % | 46 (30–56) | 59 (50–60) | <0.001 |
| Previous stroke/TIA/systemic thromboembolism | 5 (7.7) | 33 (7.6) | >0.99 |
| Arterial hypertension | 50 (76.9) | 338 (77.7) | 0.89 |
| HF | 49 (75.4) | 223 (51.3) | <0.001 |
| Diabetes mellitus | 14 (21.5) | 92 (21.1) | 0.94 |
| COPD | 6 (9.2) | 18 (4.1) | 0.11 |
| Previous MI | 10 (15.4) | 28 (6.4) | 0.02 |
| PAD or aortic plaque | 9 (13.8) | 30 (6.9) | 0.051 |
| Anticoagulation on admission | |||
| VKA | 14 (21.5) | 50 (11.5) | 0.02 |
| Rivaroxaban | 18 (27.7) | 127 (29.2) | 0.8 |
| Apixaban | 6 (9.2) | 67 (15.4) | 0.19 |
| Dabigatran | 27 (41.5) | 191 (43.9) | 0.72 |
| Medication at discharge | |||
| VKA | 17 (26.2) | 46 (10.6) | <0.001 |
| Rivaroxaban | 10 (15.4) | 129 (29.7) | 0.02 |
| Apixaban | 13 (20.0) | 69 (15.9) | 0.4 |
| Dabigatran | 25 (38.5) | 191 (43.9) | 0.41 |
| ACEI or ARB | 49 (75.4) | 316 (72.6) | 0.64 |
| Beta-blocker | 62 (95.4) | 386 (88.7) | 0.1 |
| Statin | 40 (61.5) | 275 (63.2) | 0.79 |
| Variable | Patients with LAAT (n = 65) | Patients without LAAT (n = 435) | p Value |
|---|---|---|---|
| HF hospitalization | 23 (35.4) | 49 (11.3) | <0.001 |
| MI | 3 (4.6) | 8 (1.8) | 0.16 |
| Systemic thromboembolism | 1 (1.5) | 2 (0.5) | 0.34 |
| TIA | 1 (1.5) | 4 (0.9) | 0.503 |
| Stroke | 3 (4.6) | 10 (2.3) | 0.23 |
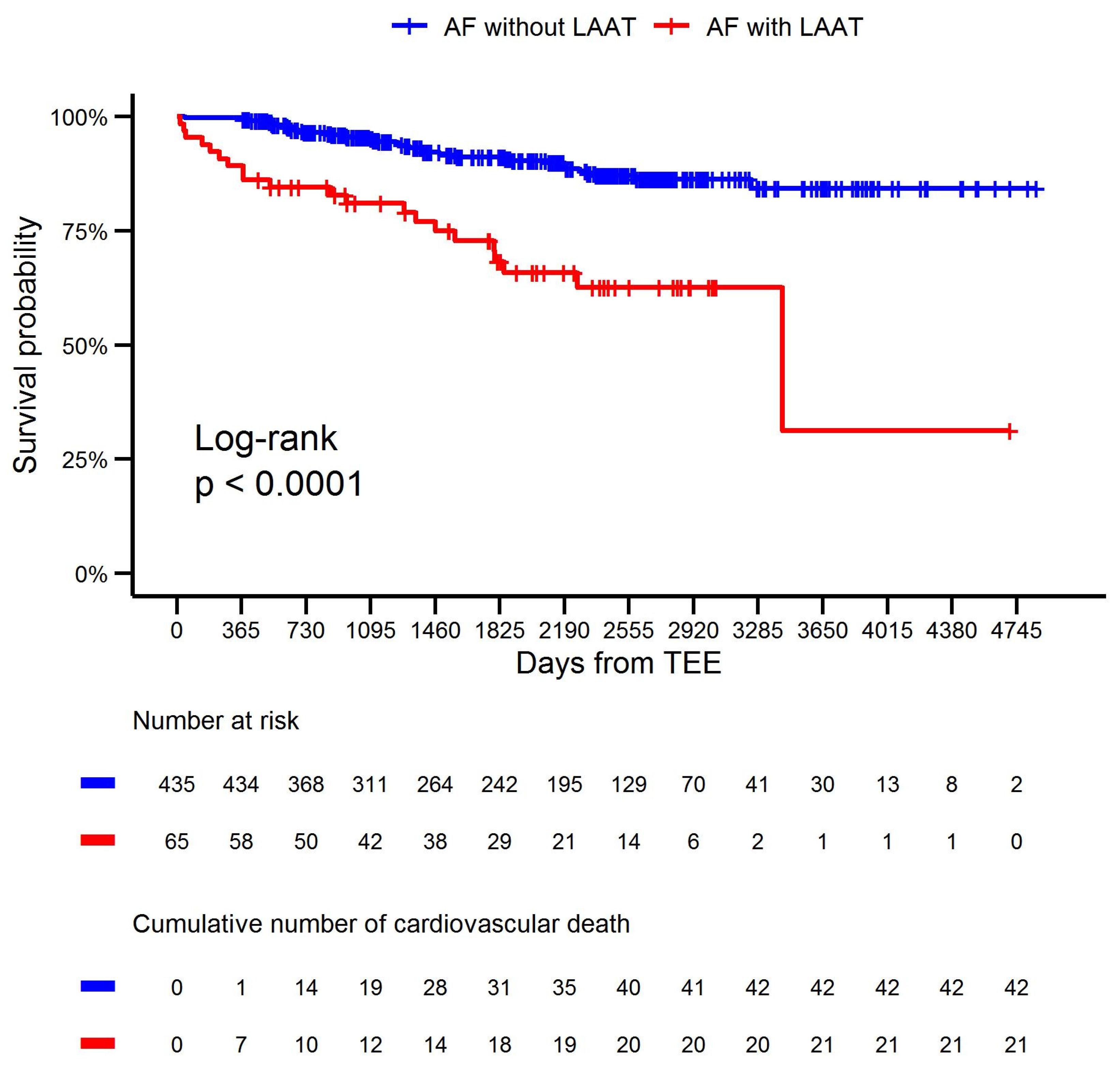
| Cardiovascular death | 21 (32.3) | 42 (9.7) | <0.001 |
| All-cause death | 23 (35.4) | 48 (11) | <0.001 |
| Variable | Univariable HR (95% CI) | p Value | Multivariable HR (95% CI) | p Value |
|---|---|---|---|---|
| LAAT | 3.9 (2.31–6.59) | <0.001 | 2.03 (1.13–3.65) | 0.02 |
| Male sex | 1.2 (0.71–2.01) | 0.49 | ||
| Age, year | 1.05 (1.02–1.09) | <0.001 | 1.02 (0.98–1.06) | 0.41 |
| BMI, kg/m2 | 0.97 (0.92–1.03) | 0.29 | ||
| CHA2DS2-VASc score per 1 | 1.37 (1.17–1.6) | <0.001 | 1.09 (0.86–1.38) | 0.47 |
| Heart rate, 1/min | 1 (0.99–1.01) | 0.98 | ||
| SBP, mmHg | 0.98 (0.96–1.01) | 0.07 | ||
| DBP, mmHg | 0.98 (0.95–1.01) | 0.22 | ||
| eGFR, mL/min/1.73 m2 | 0.97 (0.95–0.98) | <0.001 | 0.98 (0.96–1) | 0.051 |
| LA diameter, mm | 1.13 (1.08–1.18) | <0.001 | 1.09 (1.03–1.14) | 0.003 |
| LVEF, % | 0.95 (0.94–0.97) | <0.001 | 0.99 (0.97–1.01) | 0.29 |
| Previous stroke, TIA, systemic thromboembolism | 1.58 (0.72–3.48) | 0.25 | ||
| Arterial hypertension | 0.91 (0.51–1.63) | 0.76 | ||
| HF | 2.56 (1.5–4.25) | <0.001 | 1.15 (0.6–2.21) | 0.67 |
| Diabetes mellitus | 1.38 (0.77–2.46) | 0.28 | ||
| COPD | 1.48 (0.54–4.08) | 0.45 | ||
| Previous MI | 2.54 (1.29–4.99) | 0.01 | 1.72 (0.85–3.47) | 0.13 |
| PAD or aortic plaque | 1.84 (0.87–3.86) | 0.11 | ||
| Anticoagulation on admission | ||||
| VKA | 1.84 (1.05–3.24) | 0.03 | ||
| Rivaroxaban | 0.76 (0.42–1.39) | 0.37 | ||
| Apixaban | 0.66 (0.24–1.85) | 0.43 | ||
| Dabigatran | 0.91 (0.55–1.5) | 0.72 | ||
| Medication at discharge | ||||
| VKA | 1.85 (1.05–3.25) | 0.03 | 1.45 (0.81–2.58) | 0.21 |
| Rivaroxaban | 0.64 (0.34–1.21) | 0.17 | ||
| Apixaban | 0.71 (0.28–1.76) | 0.47 | ||
| Dabigatran | 1.01 (0.62–1.66) | 0.96 | ||
| ACEI or ARB | 1.09 (0.62–1.93) | 0.76 | ||
| Beta-blocker | 2.45 (0.77–7.81) | 0.13 | ||
| Statin | 0.79 (0.48–1.32) | 0.37 | ||
| Variable | Univariable HR (95% CI) | p Value | Multivariable HR (95% CI) | p Value |
|---|---|---|---|---|
| LAAT | 3.99 (2.42–6.58) | <0.001 | 1.67 (0.94–2.98) | 0.08 |
| Male sex | 1.14 (0.7–1.84) | 0.6 | ||
| Age, year | 1.05 (1.02–1.08) | 0.001 | 1.01 (0.97–1.05) | 0.62 |
| BMI, kg/m2 | 1.01 (0.96–1.06) | 0.81 | ||
| CHA2DS2-VASc score per 1 | 1.38 (1.19–1.59) | <0.001 | 1.15 (0.91–1.45) | 0.23 |
| Heart rate, 1/min | 1.02 (1.01–1.03) | 0.002 | 1.01 (0.99–1.02) | 0.07 |
| SBP, mmHg | 0.99 (0.98–1.02) | 0.89 | ||
| DBP, mmHg | 1.01 (0.98–1.05) | 0.4 | ||
| eGFR, mL/min/1.73 m2 | 0.96 (0.94–0.97) | <0.001 | 0.97(0.96–0.99) | 0.007 |
| LA diameter, mm | 1.16 (1.12–1.21) | <0.001 | 1.11 (1.06–1.16) | <0.001 |
| LVEF, % | 0.94 (0.93–0.95) | <0.001 | 0.97 (0.95–0.99) | 0.005 |
| Previous stroke, TIA, systemic thromboembolism | 1.59 (0.76–3.33) | 0.21 | ||
| Arterial hypertension | 1.36 (0.75–2.49) | 0.31 | ||
| HF | 2.43 (1.48–3.99) | <0.001 | 0.79 (0.42–1.51) | 0.48 |
| Diabetes mellitus | 1.54 (0.91–2.62) | 0.1 | ||
| COPD | 1.25 (0.46–3.43) | 0.66 | ||
| Previous MI | 1.85 (0.92–3.72) | 0.08 | ||
| PAD or aortic plaque | 2.02 (1.01–4.08) | 0.048 | 1.23 (0.56–2.71) | 0.61 |
| Anticoagulation on admission | ||||
| VKA | 2.18 (1.28–3.7) | 0.004 | ||
| Rivaroxaban | 0.89 0.52–1.52) | 0.66 | ||
| Apixaban | 0.85 (0.38–1.86) | 0.68 | ||
| Dabigatran | 0.71 (0.44–1.14) | 0.15 | ||
| Medication at discharge | ||||
| VKA | 1.86 (1.07–3.22) | 0.03 | 1.34 (0.76–2.35) | 0.31 |
| Rivaroxaban | 1.01 (0.59–1.7) | 0.99 | ||
| Apixaban | 1.11 (0.56–2.18) | 0.77 | ||
| Dabigatran | 0.64 (0.39–1.05) | 0.07 | ||
| ACEI or ARB | 1.23 (0.71–2.12) | 0.45 | ||
| Beta-blocker | 3.02 (0.95–9.64) | 0.06 | ||
| Statin | 0.95 (0.59–1.53) | 0.83 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turek, Ł.; Sadowski, M.; Kurzawski, J.; Janion, M. Left Atrial Appendage Thrombus as a Marker of Disease Severity in 500 Patients with Atrial Fibrillation on Oral Anticoagulation: A 13-Year Follow-Up Study. J. Clin. Med. 2024, 13, 5258. https://doi.org/10.3390/jcm13175258
Turek Ł, Sadowski M, Kurzawski J, Janion M. Left Atrial Appendage Thrombus as a Marker of Disease Severity in 500 Patients with Atrial Fibrillation on Oral Anticoagulation: A 13-Year Follow-Up Study. Journal of Clinical Medicine. 2024; 13(17):5258. https://doi.org/10.3390/jcm13175258
Chicago/Turabian StyleTurek, Łukasz, Marcin Sadowski, Jacek Kurzawski, and Marianna Janion. 2024. "Left Atrial Appendage Thrombus as a Marker of Disease Severity in 500 Patients with Atrial Fibrillation on Oral Anticoagulation: A 13-Year Follow-Up Study" Journal of Clinical Medicine 13, no. 17: 5258. https://doi.org/10.3390/jcm13175258
APA StyleTurek, Ł., Sadowski, M., Kurzawski, J., & Janion, M. (2024). Left Atrial Appendage Thrombus as a Marker of Disease Severity in 500 Patients with Atrial Fibrillation on Oral Anticoagulation: A 13-Year Follow-Up Study. Journal of Clinical Medicine, 13(17), 5258. https://doi.org/10.3390/jcm13175258





