Impact of Negative Pressure Wound Therapy on Perfusion Dynamics in Free Latissimus Dorsi Muscle Flaps
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Patient Sample
2.3. Flap Microcirculation Analysis
2.4. Statistical Analysis
3. Results
3.1. General Results
3.2. Flap Microcirculation
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dolan, R.T.; Butler, J.S.; Murphy, S.M.; Cronin, K.J. Health-related quality of life, surgical and aesthetic outcomes following microvascular free flap reconstructions: An 8-year institutional review. Ann. R. Coll. Surg. Engl. 2012, 94, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.C.; Agarwal, J.P. An analysis of free flap failure using the ACS NSQIP database. Does flap site and flap type matter? Microsurgery 2017, 37, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Pohlenz, P.; Klatt, J.; Schon, G.; Blessmann, M.; Li, L.; Schmelzle, R. Microvascular free flaps in head and neck surgery: Complications and outcome of 1000 flaps. Int. J. Oral Maxillofac. Surg. 2012, 41, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Genden, E.M.; Rinaldo, A.; Suarez, C.; Wei, W.I.; Bradley, P.J.; Ferlito, A. Complications of free flap transfers for head and neck reconstruction following cancer resection. Oral Oncol. 2004, 40, 979–984. [Google Scholar] [CrossRef]
- Disa, J.J.; Pusic, A.L.; Hidalgo, D.H.; Cordeiro, P.G. Simplifying microvascular head and neck reconstruction: A rational approach to donor site selection. Ann. Plast. Surg. 2001, 47, 385–389. [Google Scholar] [CrossRef]
- Haughey, B.H.; Wilson, E.; Kluwe, L.; Piccirillo, J.; Fredrickson, J.; Sessions, D.; Spector, G. Free flap reconstruction of the head and neck: Analysis of 241 cases. Otolaryngol. Head Neck Surg. 2001, 125, 10–17. [Google Scholar] [CrossRef]
- Harashina, T. Analysis of 200 free flaps. Br. J. Plast. Surg. 1988, 41, 33–36. [Google Scholar] [CrossRef]
- Krag, C. Experience with transplantation of composite tissues by means of microsurgical vascular anastomoses. I. Indications, techniques and early results. Scand. J. Plast. Reconstr. Surg. 1985, 19, 135–156. [Google Scholar] [CrossRef]
- Krag, C. Experience with transplantation of composite tissues by means of microsurgical vascular anastomoses. II. Late results and comments. Scand. J. Plast. Reconstr. Surg. 1985, 19, 157–173. [Google Scholar] [CrossRef]
- Chen, K.T.; Mardini, S.; Chuang, D.C.; Lin, C.H.; Cheng, M.H.; Lin, Y.T.; Huang, W.C.; Tsao, C.K.; Wei, F.C. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast. Reconstr. Surg. 2007, 120, 187–195. [Google Scholar] [CrossRef]
- Blume, P.A.; Key, J.J.; Thakor, P.; Thakor, S.; Sumpio, B. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: Comparing V.A.C.(R) therapy and conventional therapy in foot and ankle reconstructive surgeries. Int. Wound J. 2010, 7, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.H., 2nd; Boemi, L.; Hall, W.W.; Jeffords, K.; Hauck, R.M.; Banducci, D.R.; Graham, W.P., 3rd. Negative-pressure dressings as a bolster for skin grafts. Ann. Plast. Surg. 1998, 40, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gabriel, A.; Shores, J. The perioperative use of negative pressure wound therapy in skin grafting. Ostomy Wound Manag. 2004, 50 (Suppl. 11A), 32–34. [Google Scholar]
- Sakamoto, Y.; Takahara, T.; Ota, Y.; Aoki, T.; Yamazaki, H.; Otsuru, M.; Takahashi, M.; Aoyama, K.; Kaneko, A.; Kawada, S.; et al. MRI analysis of chronological changes in free-flap volume in head and neck reconstruction by volumetry. Tokai J. Exp. Clin. Med. 2014, 39, 44–50. [Google Scholar]
- Oksman, D.; de Almeida, O.M.; de Arruda, R.G.; de Almeida, M.L.M.; do Carmo, F.S. Comparative study between fasciocutaneous and myocutaneous flaps in the surgical treatment of pressure ulcers of the sacral region. JPRAS Open 2018, 16, 50–60. [Google Scholar] [CrossRef]
- Hofman, D. Oedema and its treatment. J. Wound Care 1998, 7 (Suppl. S7), 10–13. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Incandela, L.; De Sanctis, M.T.; Belcaro, G.; Geroulakos, G.; Griffin, M.; Lennox, A.; Di Renzo, A.D.; Cacchio, M.; Bucci, M. Flight microangiopathy in medium-to long-distance flights: Prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology 2001, 52 (Suppl. S2), S33–S37. [Google Scholar] [CrossRef]
- Marouf, A.; Mortada, H.; Khedr, B.; Halawani, L.; Zino Alarki, S.M.K.; Alghamdi, H. Effectiveness and safety of immediate application of negative pressure wound therapy in head and neck free flap reconstruction: A systematic review. Br. J. Oral Maxillofac. Surg. 2022, 60, 1005–1011. [Google Scholar] [CrossRef]
- Opoku-Agyeman, J.L.; Matera, D.V.; Simone, J.E.; Behnam, A.B. Flap Viability after Direct Immediate Application of Negative Pressure Wound Therapy on Free Flaps: A Systematic Review and Pooled Analysis of Reported Outcomes. J. Reconstr. Microsurg. Open 2019, 4, e77–e82. [Google Scholar] [CrossRef]
- Kumbla, P.A.; Henry, S.L.; Boyd, C.J.; Kelley, P.K.; Thorburn, A.Q.; Myers, R.P. Negative Pressure Dressings over Free Muscle Flaps with Immediate Split-Thickness Skin Grafting: A 9-Year Experience. J. Reconstr. Microsurg. Open 2020, 5, e27–e31. [Google Scholar] [CrossRef]
- Berner, J.E.; Will, P.; Geoghegan, L.; Troisi, L.; Nanchahal, J.; Jain, A. Safety and effectiveness of negative pressure therapy on free flaps following lower limb reconstruction: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 407–447. [Google Scholar] [CrossRef]
- Dornseifer, U.; Pfeiler, P.P.; Kargl, L.; Moog, P.; Schilling, A.F.; Ninkovic, M. Negative Pressure Wound Therapy in Free Muscle Flaps-Risk or Benefit? J. Reconstr. Microsurg. 2023, 40, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Moellhoff, N.; Heidekrueger, P.I.; Frank, K.; Pistek, S.; Alt, V.; Giunta, R.E.; Ehrl, D. Comparing the Time-Dependent Evolution of Microcirculation in Gracilis vs. ALT Flaps Using Laser-Doppler Flowmetry and Tissue-Spectrometry. J. Clin. Med. 2022, 11, 2425. [Google Scholar] [CrossRef] [PubMed]
- Moellhoff, N.; Gernert, C.; Frank, K.; Giunta, R.E.; Ehrl, D. The 72-Hour Microcirculation Dynamics in Viable Free Flap Reconstructions. J. Reconstr. Microsurg. 2022, 38, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Moellhoff, N.; Taha, S.; Wachtel, N.; Hirschmann, M.; Hellweg, M.; Giunta, R.E.; Ehrl, D. Analysis of Factors Determining Patient Survival after Receiving Free-Flap Reconstruction at a Single Center-A Retrospective Cohort Study. Diagnostics 2022, 12, 2877. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Tenland, T.; Oberg, P.A. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans. Biomed. Eng. 1980, 27, 597–604. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Tenland, T.; Obert, P.A. A new instrument for continuous measurement of tissue blood flow by light beating spectroscopy. IEEE Trans. Biomed. Eng. 1980, 27, 12–19. [Google Scholar] [CrossRef]
- Abdel-Galil, K.; Mitchell, D. Postoperative monitoring of microsurgical free tissue transfers for head and neck reconstruction: A systematic review of current techniques—Part I. Non-invasive techniques. Br. J. Oral Maxillofac. Surg. 2009, 47, 351–355. [Google Scholar] [CrossRef]
- Chen, S.Z.; Li, J.; Li, X.Y.; Xu, L.S. Effects of vacuum-assisted closure on wound microcirculation: An experimental study. Asian J. Surg. 2005, 28, 211–217. [Google Scholar] [CrossRef]
- Timmers, M.S.; Le Cessie, S.; Banwell, P.; Jukema, G.N. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann. Plast. Surg. 2005, 55, 665–671. [Google Scholar] [CrossRef]
- Silverman, R.P.; Apostolides, J.; Chatterjee, A.; Dardano, A.N.; Fearmonti, R.M.; Gabriel, A.; Grant, R.T.; Johnson, O.N., 3rd; Koneru, S.; Kuang, A.A.; et al. The use of closed incision negative pressure therapy for incision and surrounding soft tissue management: Expert panel consensus recommendations. Int. Wound J. 2022, 19, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Sogorski, A.; Becker, A.; Dadras, M.; Wallner, C.; Wagner, J.M.; Glinski, M.V.; Lehnhardt, M.; Behr, B. Superior Enhancement of Cutaneous Microcirculation Due to “Cyclic” Application of a Negative Pressure Wound Therapy Device in Humans-Local and Remote Effects. Front. Surg. 2022, 9, 822122. [Google Scholar] [CrossRef] [PubMed]
- Taeger, C.D.; Muehle, C.; Kruppa, P.; Prantl, L.; Biermann, N. Negative Pressure Wound Therapy-A Vacuum-Mediated Positive Pressure Wound Therapy and a Closer Look at the Role of the Laser Doppler. J. Clin. Med. 2024, 13, 2351. [Google Scholar] [CrossRef] [PubMed]
- Blood and Oxygen Supply of Tissue; LEA Medizintechnik GmbH: Gießen, Germany, 2023.
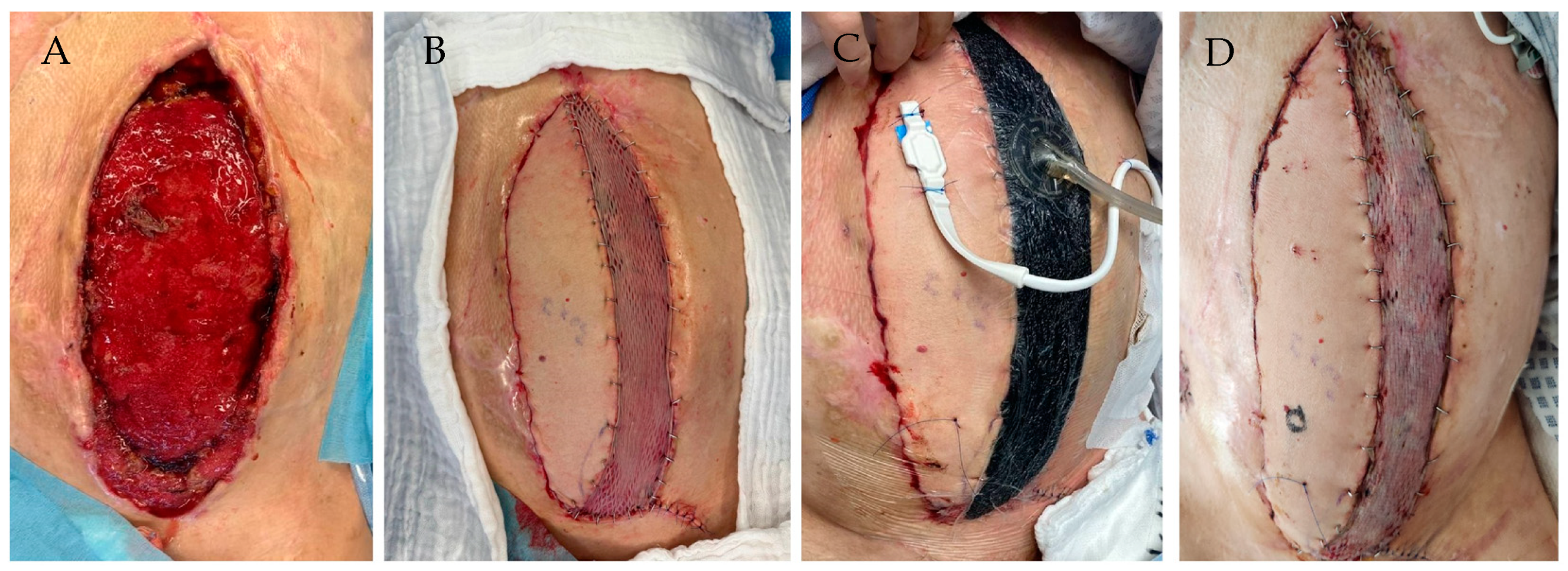
| Demographic Data | Total | With NPWT | Without NPWT | p-Value |
|---|---|---|---|---|
| Number | 61 | 12 | 49 | |
| Patient age [years] | 56.90 (17.4) | 58.75 (20.4) | 56.45 (16.8) | 0.685 |
| Operation time [minutes] | 363.40 (124.5) | 367.88 (94.7) | 362.59 (130.1) | 0.913 |
| Sex | 0.532 | |||
| Female | 26 | 4 | 22 | |
| Male | 35 | 8 | 27 | |
| Localization | 0.012 | |||
| Head | 25 | 0 | 25 | |
| Torso | 8 | 2 | 6 | |
| Upper Extremity | 5 | 2 | 3 | |
| Lower Extremity | 23 | 8 | 15 | |
| Arterial Anastomosis | 0.677 | |||
| End–End | 50 | 9 | 41 | |
| End–Side | 11 | 3 | 8 | |
| Complications | 0.689 | |||
| Major | 6 | 1 | 5 | |
| Minor | 7 | 2 | 5 | |
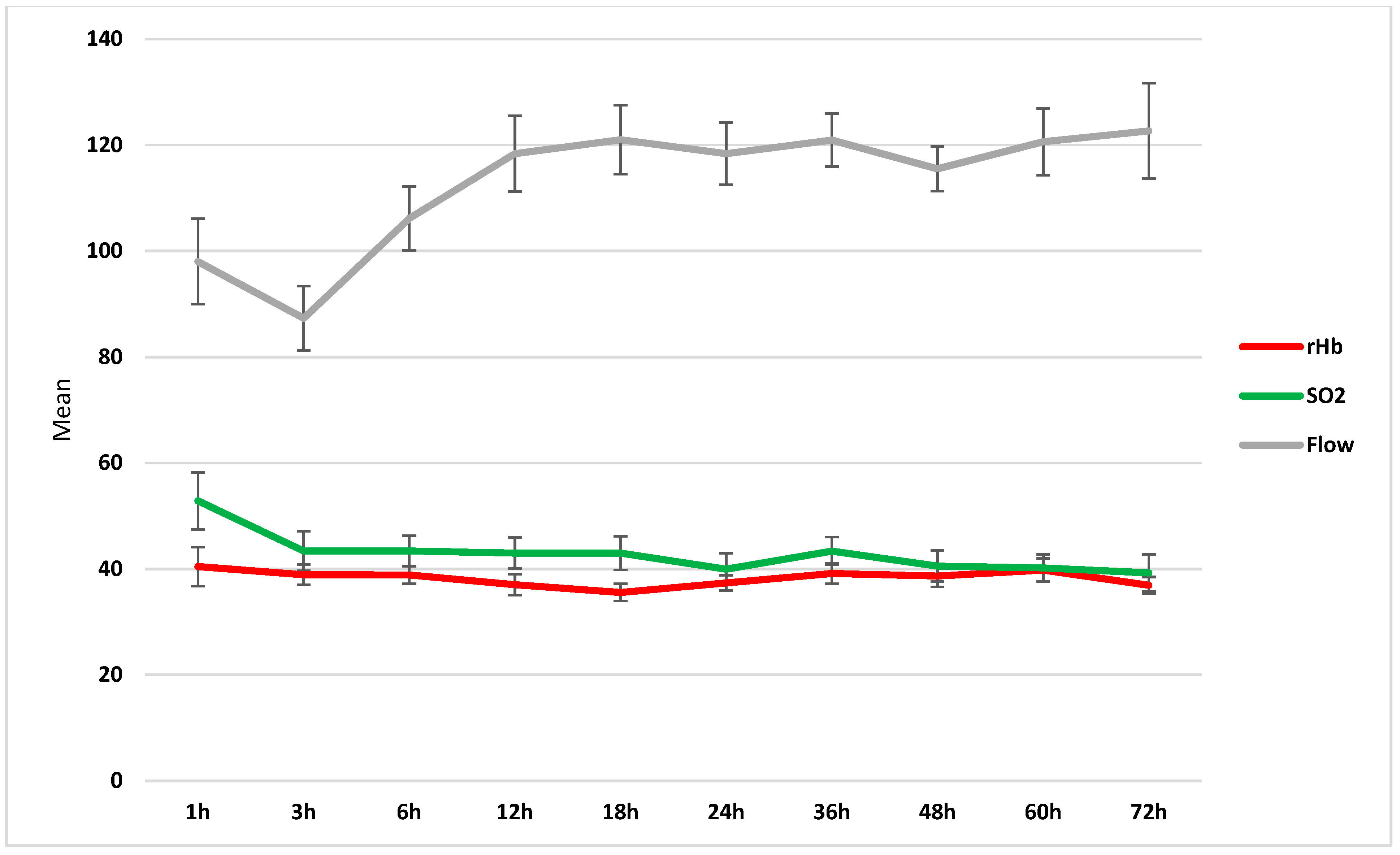
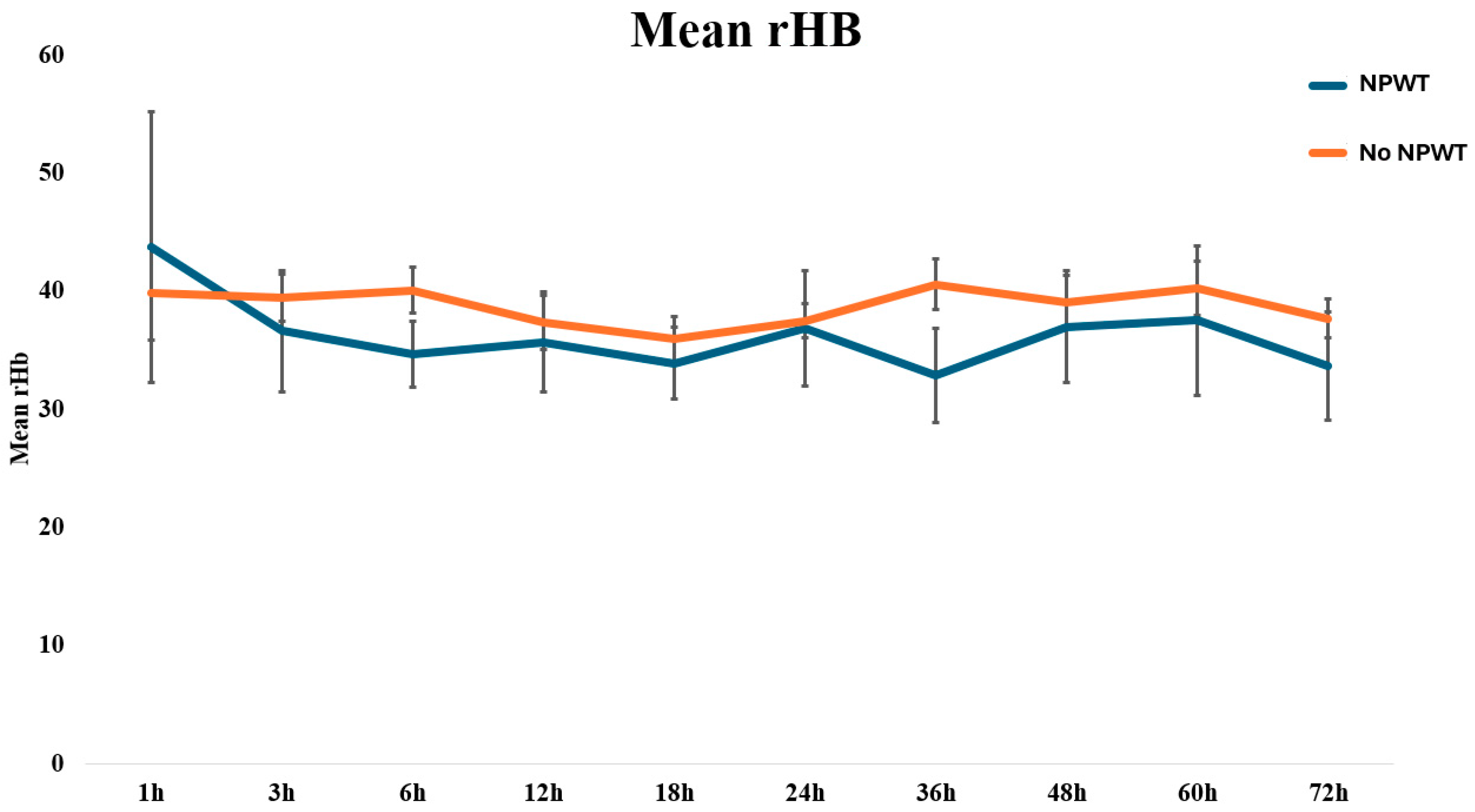
| Time (h) | rHb (A.U.) | SD | rHb (A.U.) | SD | p-Value |
|---|---|---|---|---|---|
| 1 | 42 | 21 | 41 | 21 | 0.889 |
| 3 | 42 | 17 | 36 | 14 | 0.279 |
| 6 | 41 | 13 | 34 | 9 | 0.085 |
| 12 | 38 | 15 | 35 | 13 | 0.498 |
| 18 | 37 | 13 | 34 | 9 | 0.450 |
| 24 | 38 | 10 | 35 | 14 | 0.497 |
| 36 | 42 | 14 | 32 | 11 | 0.051 |
| 48 | 40 | 15 | 36 | 14 | 0.448 |
| 60 | 41 | 15 | 37 | 17 | 0.461 |
| 72 | 39 | 10 | 32 | 11 | 0.109 |
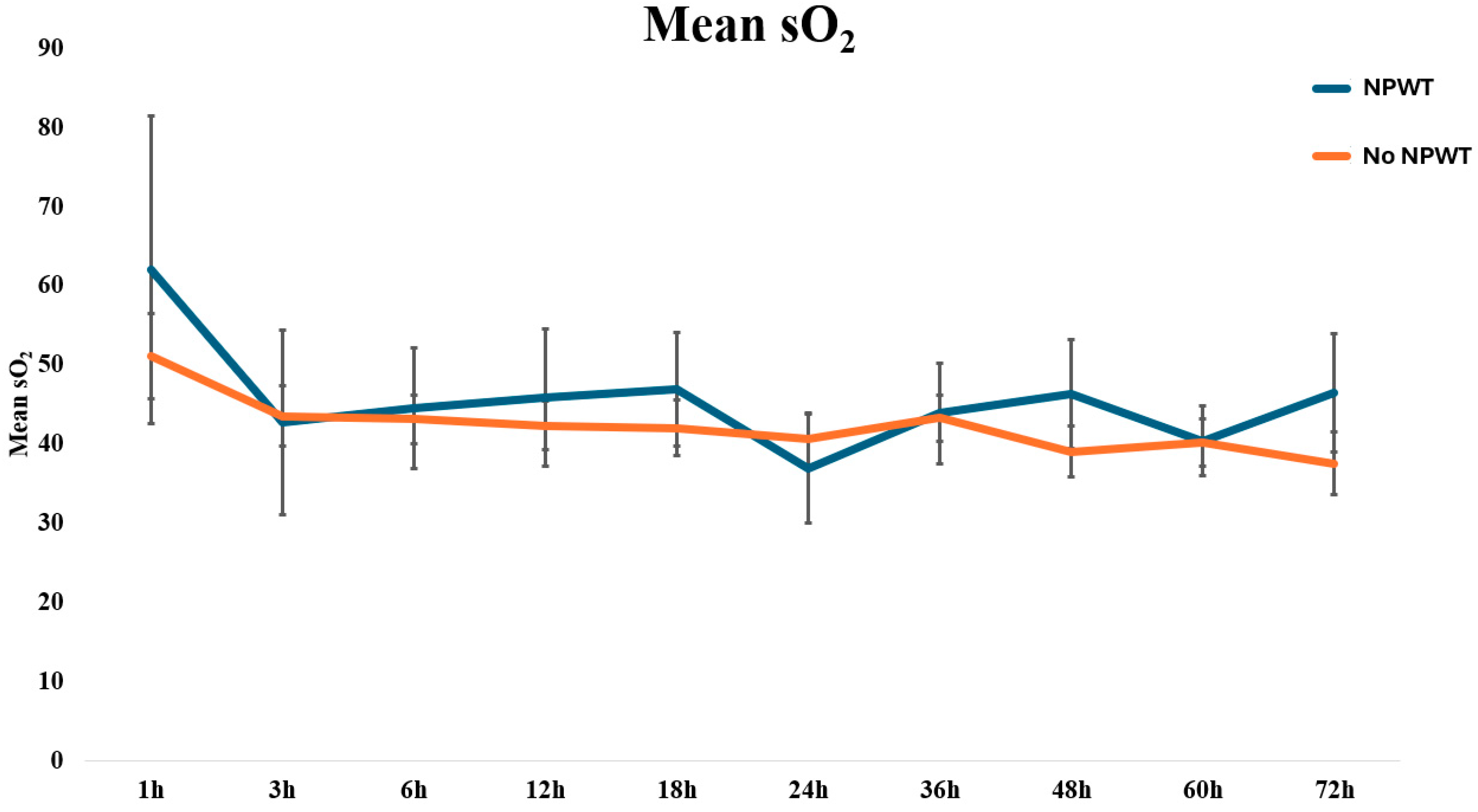
| Time (h) | SO2 (A.U.) | SD | SO2 (A.U.) | SD | p-Value |
| 1 | 50 | 25 | 55 | 37 | 0.701 |
| 3 | 43 | 22 | 41 | 31 | 0.849 |
| 6 | 43 | 18 | 42 | 26 | 0.904 |
| 12 | 42 | 19 | 44 | 27 | 0.811 |
| 18 | 41 | 22 | 45 | 22 | 0.608 |
| 24 | 40 | 21 | 38 | 19 | 0.891 |
| 36 | 42 | 19 | 46 | 19 | 0.605 |
| 48 | 39 | 20 | 48 | 21 | 0.217 |
| 60 | 40 | 18 | 42 | 12 | 0.776 |
| 72 | 38 | 19 | 48 | 17 | 0.184 |
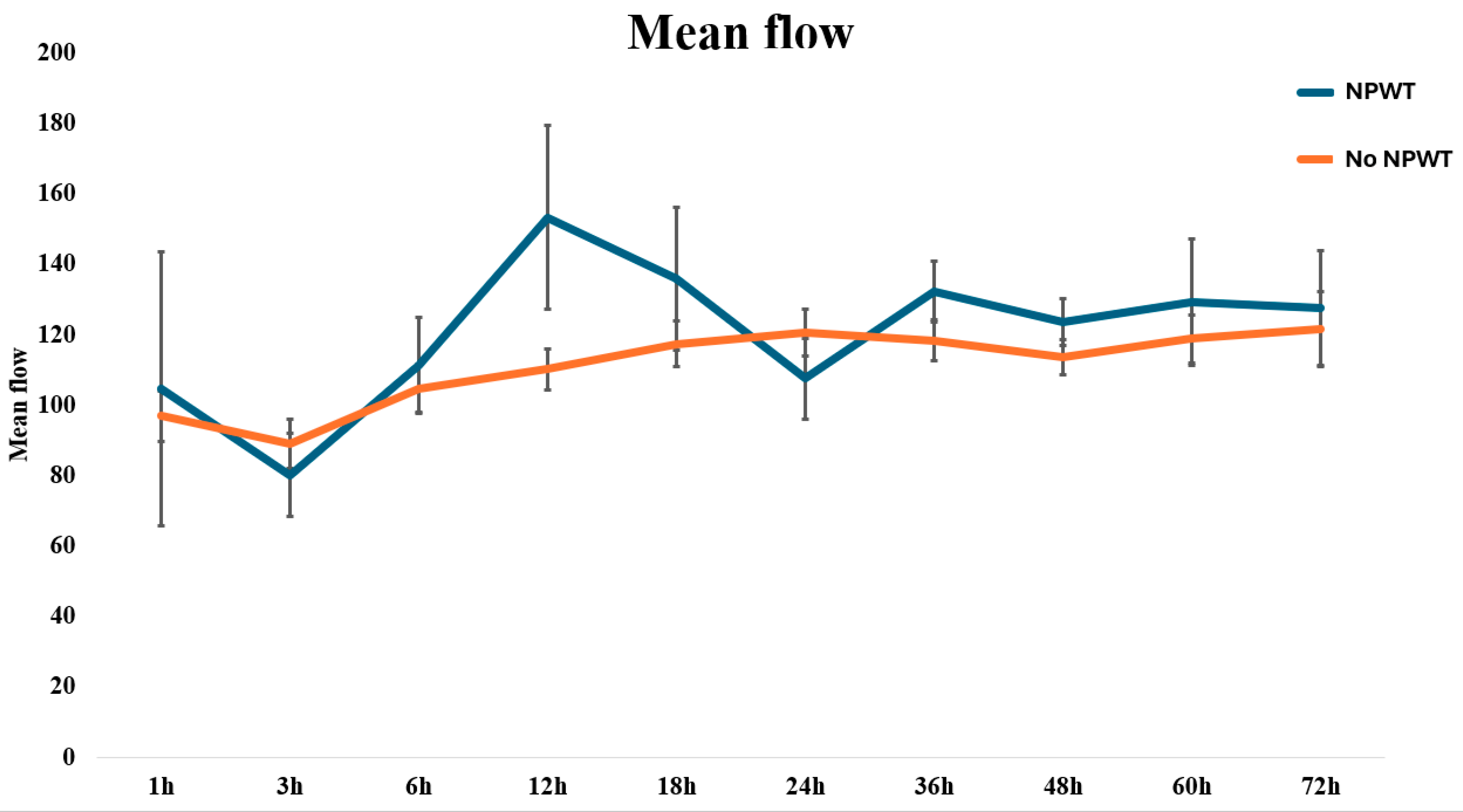
| Time (h) | No NPWT | NPWT | p-Value | ||
| Flow (A.U.) | SD | Flow (A.U.) | SD | ||
| 1 | 95 | 32 | 95 | 71 | 0.998 |
| 3 | 86 | 40 | 78 | 32 | 0.561 |
| 6 | 102 | 42 | 105 | 48 | 0.839 |
| 12 | 107 | 39 | 143 | 85 | 0.038 |
| 18 | 114 | 41 | 127 | 67 | 0.419 |
| 24 | 119 | 42 | 103 | 33 | 0.295 |
| 36 | 115 | 38 | 133 | 24 | 0.174 |
| 48 | 112 | 32 | 123 | 20 | 0.291 |
| 60 | 117 | 42 | 126 | 49 | 0.565 |
| 72 | 121 | 53 | 128 | 36 | 0.750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moellhoff, N.; Demmer, W.; Pistek, S.; Wachtel, N.; Bodenschatz, K.; Lui, L.; Alfertshofer, M.; Frank, K.; Giunta, R.E.; Ehrl, D. Impact of Negative Pressure Wound Therapy on Perfusion Dynamics in Free Latissimus Dorsi Muscle Flaps. J. Clin. Med. 2024, 13, 5261. https://doi.org/10.3390/jcm13175261
Moellhoff N, Demmer W, Pistek S, Wachtel N, Bodenschatz K, Lui L, Alfertshofer M, Frank K, Giunta RE, Ehrl D. Impact of Negative Pressure Wound Therapy on Perfusion Dynamics in Free Latissimus Dorsi Muscle Flaps. Journal of Clinical Medicine. 2024; 13(17):5261. https://doi.org/10.3390/jcm13175261
Chicago/Turabian StyleMoellhoff, Nicholas, Wolfram Demmer, Svenja Pistek, Nikolaus Wachtel, Karl Bodenschatz, Lulin Lui, Michael Alfertshofer, Konstantin Frank, Riccardo E. Giunta, and Denis Ehrl. 2024. "Impact of Negative Pressure Wound Therapy on Perfusion Dynamics in Free Latissimus Dorsi Muscle Flaps" Journal of Clinical Medicine 13, no. 17: 5261. https://doi.org/10.3390/jcm13175261






