Memory in Spina Bifida, from Childhood to Adulthood: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
- -
- The authors and year of publication;
- -
- Sample (experimental and control);
- -
- Demographic variables (age and sex);
- -
- Outcomes on the neuropsychological memory assessments;
- -
- Cognitive tests and/or tasks used to measure memory;
- -
- Subtests applied;
- -
- Type of memory assessed.
2.4. Risk of Bias
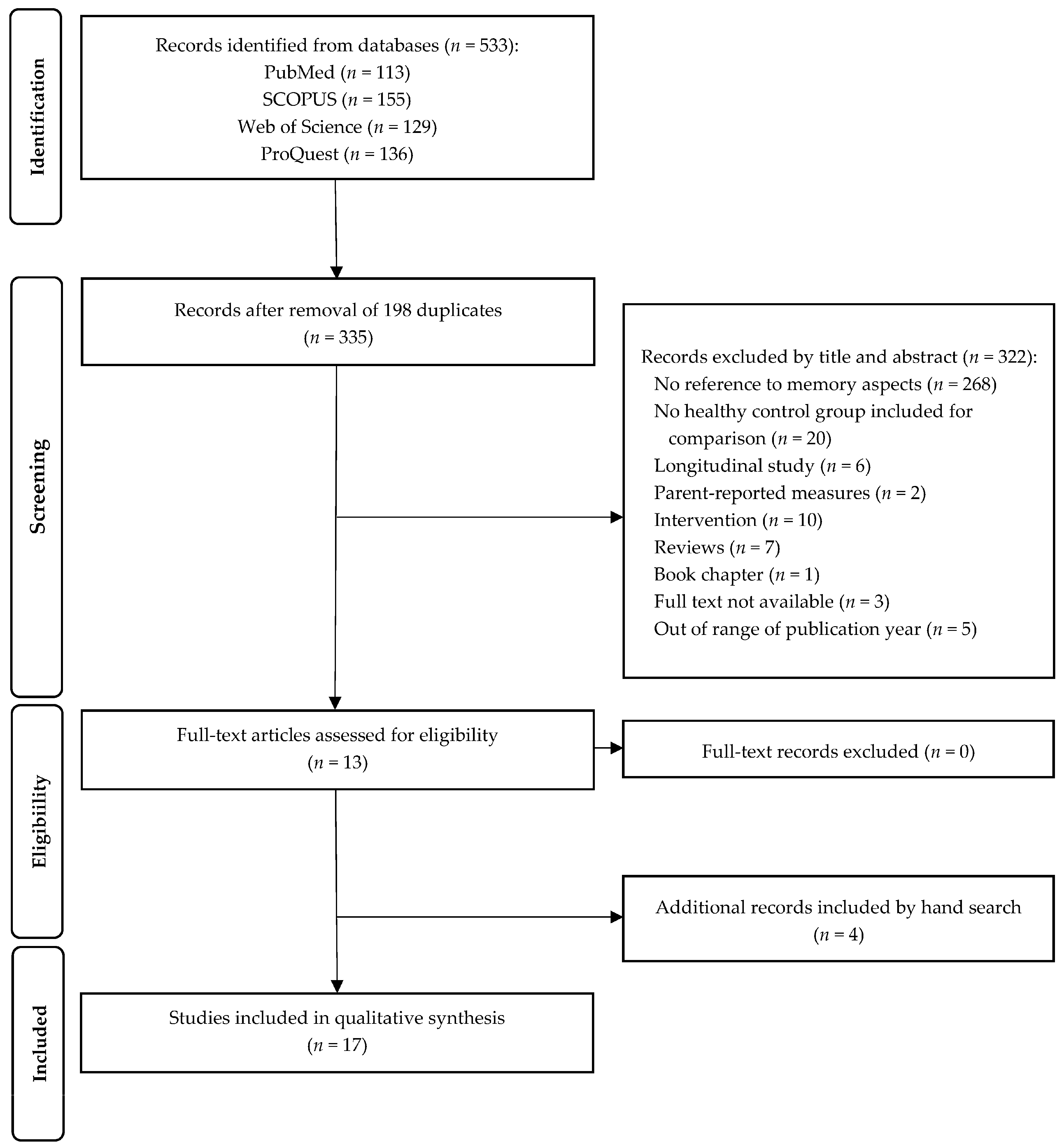
3. Results
3.1. General Overview
3.2. Memory Functioning in Children and Adolescents with SB
3.3. Memory Functioning in Adults with SB
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Botto, L.D.; Lisi, A.; Bower, C.; Canfield, M.A.; Dattani, N.; De Vigan, C.; De Walle, H.; Erickson, D.J.; Halliday, J.; Irgens, L.M.; et al. Trends of selected malformations in relation to folic acid recommendations and fortification: An international assessment. Birth Defects Res. 2006, 76, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Yang, Q.; Mai, C.; Kirby, R.S.; Collins, J.S.; Robbins, J.M.; Meyer, R.; Canfield, M.A.; Mulinare, J.; National Birth Defects Prevention Network. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res. 2008, 82, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Tilford, J.M.; Grosse, S.D.; Robbins, J.M.; Pyne, J.M.; Cleves, M.A.; Hobbs, C.A. Health state preference scores of children with spina bifida and their caregivers. Qual. Life Res. 2005, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Vinck, A.; Nijhuis-van der Sanden, M.W.; Roeleveld, N.J.; Mullaart, R.A.; Rotteveel, J.J.; Maassen, B.A. Motor profile and cognitive functioning in children with spina bifida. Eur. J. Paediatr. Neurol. 2010, 14, 86–92. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Campbell, S.; Hayhurst, C.; Clark, S.; Kandasamy, J.; Lee, M.K.; Flynn, A.; Murphy, P.; Mallucci, C.L. Cognitive and functional outcome in spina bifida-Chiari II malformation. Childs. Nerv. Syst. 2011, 27, 967–974. [Google Scholar] [CrossRef]
- Mitchell, L.E.; Adzick, N.S.; Melchionne, J.; Pasquariello, P.S.; Sutton, L.N.; Whitehead, A.S. Spina bifida. Lancet 2004, 364, 1885–1895. [Google Scholar] [CrossRef]
- Rendeli, C.; Ausili, E.; Moroni, R.; Capriati, M.; Massimi, L.; Zanetti, C. Neuropsychological profiles in children and young adults with spina bifida. Childs. Nerv. Syst. 2021, 37, 2033–2038. [Google Scholar] [CrossRef]
- Alriksson-Schmidt, A.I.; Wallander, J.; Biasini, F. Quality of life and resilience in adolescents with a mobility disability. J. Pediatr. Psychol. 2007, 32, 370–379. [Google Scholar] [CrossRef]
- Holmbeck, G.N.; Devine, K.A. Psychosocial and family functioning in spina bifida. Dev. Disabil. Res. Rev. 2010, 16, 40–46. [Google Scholar] [CrossRef]
- Sarwark, J.F. Spina bifida. Pediat. Clin. N. Am. 1996, 43, 1151–1158. [Google Scholar] [CrossRef]
- Sandler, A.D. Children with spina bifida: Key clinical issues. Pediat. Clin. N. Am. 2010, 57, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Mohr, N.; Aliatakis, N.; Seidel, U.; John, R.; Promnitz, G.; Spors, B.; Kaindl, A.M. Brain malformations and cognitive performance in spina bifida. Dev. Med. Child Neurol. 2021, 63, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rofail, D.; Maguire, L.; Kissner, M.; Colligs, A.; Abetz-Webb, L. Health-related quality of life is compromised in individuals with spina bifida: Results from qualitative and quantitative studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bannink, F.; Stroeken, K.; Idro, R.; van Hove, G. Community knowledge, beliefs, attitudes, and practices towards children with spina bifida and hydrocephalus in Uganda. Int. J Disabil. Dev. Educ. 2015, 62, 182–201. [Google Scholar] [CrossRef]
- Lim, S.W.; Yi, M. Illness Experiences of Adults with Spina Bifida: Protecting the Whole Self. Asian Nurs. Res. 2021, 15, 67–75. [Google Scholar] [CrossRef]
- Nahal, M.S.; Axelsson, Å.B.; Imam, A.; Wigert, H. Palestinian children’s narratives about living with spina bifida: Stigma, vulnerability, and social exclusion. Child Care Health Dev. 2019, 45, 54–62. [Google Scholar] [CrossRef]
- Mukherjee, S. Transition to adulthood in spina bifida: Changing roles and expectations. Sci. World J. 2007, 7, 1890–1895. [Google Scholar] [CrossRef]
- Roux, G.; Sawin, K.J.; Bellin, M.H.; Buran, C.F.; Brei, T.J. The experience of adolescent women living with spina bifida. Part II: Peer relationships. Rehabil. Nurs. 2007, 32, 112–119. [Google Scholar] [CrossRef]
- Barf, H.A. Spina Bifida: Implications for Cognitive Functioning, Disability and Health in Young Adults; Utrecht University: Utrecht, The Netherlands, 2008. [Google Scholar]
- Bowman, R.M.; McLone, D.G.; Grant, J.A.; Tomita, T.; Ito, J.A. Spina bifida outcome: A 25-year prospective. Pediatr. Neurosurg. 2001, 34, 114–120. [Google Scholar] [CrossRef]
- Zukerman, J.M.; Devine, K.A.; Holmbeck, G.N. Adolescent predictors of emerging adulthood milestones in youth with spina bifida. J. Pediatr. Psychol. 2011, 36, 265–276. [Google Scholar] [CrossRef]
- McDonell, G.V.; McCann, J.P. Issues of medical management in adults with spina bifida. Childs Nerv. Syst. 2000, 16, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.H.; Zabel, T.A.; Dicianno, B.E.; Levey, E.; Garver, K.; Linroth, R.; Braun, P. Correlates of depressive and anxiety symptoms in young adults with spina bifida. J. Pediatr. Psychol. 2010, 35, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.H.; Dosa, N.; Zabel, T.A.; Aparicio, E.; Dicianno, B.E.; Osteen, P. Self-management, satisfaction with family functioning, and the course of psychological symptoms in emerging adults with spina bifida. J. Pediatr. Psychol. 2013, 38, 50–62. [Google Scholar] [CrossRef]
- Dicianno, B.E.; Kinback, N.; Bellin, M.H.; Chaikind, L.; Buhari, A.M.; Holmbeck, G.N.; Zabel, T.A.; Donlan, R.M.; Collins, D.M. Depressive symptoms in adults with spina bifida. Rehabil. Psychol. 2015, 60, 246–253. [Google Scholar] [CrossRef]
- Hayter, M.R.; Dorstyn, D.S. Resilience, self-esteem and self-compassion in adults with spina bifida. Spinal Cord 2014, 52, 167–171. [Google Scholar] [CrossRef]
- Lidal, I.B.; Lundberg Larsen, K. Anxiety, depression, and fatigue in middle-aged and older persons with spina bifida: A cross-sectional study. Disabil. Rehabil. 2022, 44, 7936–7946. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Lin, P.; Kamdar, N.; Mahmoudi, E.; Marsack-Topolewski, C.N.; Haapala, H.; Muraszko, K.; Hurvitz, E.A. Psychological morbidity among adults with cerebral palsy and spina bifida. Psychol. Med. 2021, 51, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Zurmöhle, U.M.; Homann, T.; Schroeter, C.; Rothgerber, H.; Hommel, G.; Ermert, J.A. Psychosocial adjustment of children with spina bifida. J. Child Neurol. 1998, 13, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Buran, C.F.; Sawin, K.J.; Brei, T.J.; Fastenau, P.S. Adolescents with myelomeningocele: Activities, beliefs, expectations, and perceptions. Dev. Med. Child Neurol. 2004, 46, 244–252. [Google Scholar] [CrossRef]
- Copp, A.J.; Adzick, N.S.; Chitty, L.S.; Fletcher, J.M.; Holmbeck, G.N.; Shaw, G.M. Spina bifida. Nat. Rev. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Dicianno, B.E.; Wilson, R. Hospitalizations of adults with spina bifida and congenital spinal cord anomalies. Arch. Phys. Med. Rehabil. 2010, 91, 529–535. [Google Scholar] [CrossRef]
- Holmbeck, G.N.; DeLucia, C.; Essner, B.; Kelly, L.; Zebracki, K.; Friedman, D.; Jandasek, B. Trajectories of psychosocial adjustment in adolescents with spina bifida: A 6-year, four-wave longitudinal follow-up. J. Consult. Clin. Psychol. 2010, 78, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.M.; Collins, N.; Bryan, D.; Brown, D.; Hope, M.A.; Bowes, G. Young people with spina bifida: Transfer from paediatric to adult health care. J. Paediatr. Child Health 1998, 34, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, C.R.J. Myelomeningocele in young adults. BJU Int. 2005, 95, 223. [Google Scholar] [CrossRef] [PubMed]
- Lemelle, J.L.; Guillemin, F.; Aubert, D.; Guys, J.M.; Lottmann, H.; Lortat-Jacob, S.; Mouriquand, P.; Ruffion, A.; Moscovici, J.; Schmitt, M. Quality of life and continence in patients with spina bifida. Qual. Life Res. 2006, 15, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Holmbeck, G.N.; Ros, A.M.; Flores, D.M.; Mir, S.A.; Varni, J.W. A longitudinal examination of health-related quality of life in children and adolescents with spina bifida. J. Pediatr. Psychol. 2015, 40, 419–430. [Google Scholar] [CrossRef]
- Cate, I.M.; Kennedy, C.; Stevenson, J. Disability and quality of life in spina bifida and hydrocephalus. Dev. Med. Child. Neuro. 2002, 44, 317–322. [Google Scholar] [CrossRef]
- Rocque, B.G.; Bishop, E.R.; Scogin, M.A.; Hopson, B.D.; Arynchyna, A.A.; Boddiford, C.J.; Shannon, C.N.; Blount, J.P. Assessing health-related quality of life in children with spina bifida. J. Neurosurg. Pediatr. 2015, 15, 144–149. [Google Scholar] [CrossRef]
- Bendt, M.; Gabrielsson, H.; Riedel, D.; Hagman, G.; Hultling, C.; Franzén, E.; Eriksson, M.; Seiger, Å. Adults with spina bifida: A cross-sectional study of health issues and living conditions. Brain Behav. 2020, 10, e01736. [Google Scholar] [CrossRef]
- Bendt, M.; Seiger, Å.; Hagman, G.; Hultling, C.; Franzén, E.; Forslund, E.B. Adults with spina bifida: Ambulatory performance and cognitive capacity in relation to muscle function. Spinal Cord 2022, 60, 122–128. [Google Scholar] [CrossRef]
- Kalfoss, M.H.; Merkens, M.J. A comparative study of quality of life among adults with spina bifida. Cerebrospinal Fluid Res. 2006, 3, S31. [Google Scholar] [CrossRef]
- Dennis, M.; Landry, S.H.; Barnes, M.; Fletcher, J.M. A model of neurocognitive function in spina bifida over the life span. J. Int. Neuropsychol. 2006, 12, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.W.; Short, B.F.; Saltzman, H.M. Adult consequences of spina bifida: A cohort study. Clin. Orthop. Relat. Res. 2011, 469, 1246–1252. [Google Scholar] [CrossRef]
- Hurley, A.D.; Dorman, C.; Laatsch, L.; Bell, S.; D’Avignon, J. Cognitive functioning in patients with spina bifida, hydrocephalus, and the “cocktail party” syndrome. Dev. Neuropsychol. 1990, 6, 151–172. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Copeland, K.; Frederick, J.A.; Blaser, S.E.; Kramer, L.A.; Northrup, H.; Hannay, H.J.; Brandt, M.E.; Francis, D.J.; Villarreal, G.; et al. Spinal lesion level in spina bifida: A source of neural and cognitive heterogeneity. J. Neurosurg. 2005, 102, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Lomax-Bream, L.E.; Barnes, M.; Copeland, K.; Taylor, H.B.; Landry, S.H. The impact of spina bifida on development across the first 3 years. Dev. Neuropsychol. 2007, 31, 1–20. [Google Scholar] [CrossRef]
- Mohd-Zin, S.W.; Marwan, A.I.; Abou Chaar, M.K.; Ahmad-Annuar, A.; Abdul-Aziz, N.M. Spina Bifida: Pathogenesis, Mechanisms, and Genes in Mice and Humans. Scientifica 2017, 2017, 5364827. [Google Scholar] [CrossRef]
- Burro, F.; Cama, A.; Lertora, V.; Veneselli, E.; Rossetti, S.; Pezzuti, L. Intellectual efficiency in children and adolescents with spina bifida myelomeningocele and shunted hydrocephalus. Dev. Neuropsychol. 2018, 43, 198–206. [Google Scholar] [CrossRef]
- Fagereng, E.; Lidal, I.B.; Larsen, K.L.; Løvstad, M.; Rekand, T.; Hauger, S.L. Cognition and emotional distress in middle-aged and older adults with spina bifida myelomeningocele. PLoS ONE 2024, 19, e0298891. [Google Scholar] [CrossRef]
- Juranek, J.; Salman, M.S. Anomalous development of brain structure and function in spina bifida myelomeningocele. Dev. Disabil. Res. Rev. 2010, 16, 23–30. [Google Scholar] [CrossRef]
- Koch, V.H.; Lopes, M.; Furusawa, E.; Vaz, K.; Barroso, U. Multidisciplinary management of people with spina bifida across the lifespan. Pediatr. Nephrol. 2024, 39, 681–697. [Google Scholar] [CrossRef]
- Matozinho, H.H.S.; Rosa, J.M.; Ferreira, J.A.; Borges, S.; Costa, V.F.P.; Garrote, M.S.; Souza, W.S.S.; Morais, L.C.; Silva, F.H.R.; Cavalcante, J.E.S. Case report of a newborn with Arnold-Chiari malformation type II associated with hydrocephalus and myelomeningocele. J. Neurol. Sci. 2015, 357, E204. [Google Scholar] [CrossRef][Green Version]
- Vinck, A.; Maassen, B.; Mullaart, R.; Rotteveel, J. Arnold-Chiari-II malformation and cognitive functioning in spina bifida. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1083–1086. [Google Scholar] [CrossRef]
- Vachha, B.; Adams, R. Language differences in young children with myelomeningocele and shunted hydrocephalus. Pediatr. Neurosurg. 2003, 39, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M. Language disorders in children with central nervous system injury. J. Clin. Exp. Neuropsychol. 2010, 32, 417–432. [Google Scholar] [CrossRef]
- Dennis, M.; Barnes, M. Math and numeracy in young adults with spina bifida and hydrocephalus. Dev. Neuropsychol. 2002, 21, 141–155. [Google Scholar] [CrossRef]
- English, L.H.; Barnes, M.A.; Taylor, H.B.; Landry, S.H. Mathematical development in spina bifida. Dev. Disabil. Res. Rev. 2009, 15, 28–34. [Google Scholar] [CrossRef]
- Hetherington, R.; Dennis, M.; Barnes, M.; Drake, J.; Gentili, F. Functional outcome in young adults with spina bifida and hydrocephalus. Childs Nerv. Syst. 2006, 22, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Raghubar, K.P.; Barnes, M.A.; Dennis, M.; Cirino, P.T.; Taylor, H.; Landry, S. Neurocognitive predictors of mathematical processing in school-aged children with spina bifida and their typically developing peers: Attention, working memory, and fine motor skills. Neuropsychology 2015, 29, 861–873. [Google Scholar] [CrossRef]
- Barf, H.A.; Verhoef, M.; Jennekens-Schinkel, A.; Post, M.W.; Gooskens, R.H.; Prevo, A.J. Cognitive status of young adults with spina bifida. Dev. Med. Child. Neurol. 2003, 45, 813–820. [Google Scholar] [CrossRef]
- Brown, T.M.; Ris, M.D.; Beebe, D.; Ammerman, R.T.; Oppenheimer, S.G.; Yeates, K.O.; Enrile, B.G. Factors of biological risk and reserve associated with executive behaviors in children and adolescents with spina bifida myelomeningocele. Child Neuropsychol. 2008, 14, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, R.; Hannay, H.J.; Copeland, K.; Fletcher, J.M.; Boudousquie, A.; Dennis, M. Attention problems and executive functions in children with spina bifida and hydrocephalus. Child Neuropsychol. 2005, 11, 265–283. [Google Scholar] [CrossRef]
- Kelly, N.C.; Ammerman, R.T.; Rausch, J.R.; Ris, M.D.; Yeates, K.O.; Oppenheimer, S.G.; Enrile, B.G. Executive functioning and psychological adjustment in children and youth with spina bifida. Child Neuropsychol. 2012, 18, 417–431. [Google Scholar] [CrossRef]
- Landry, S.H.; Taylor, H.B.; Swank, P.R.; Barnes, M.; Juranek, J. Longitudinal mediators of social problem solving in spina bifida and typical development. Rehabil. Psychol. 2013, 58, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.M.; Holmbeck, G.N. Attention and executive functions in adolescents with spina bifida. J. Pediatr. Psychol. 2007, 32, 983–994. [Google Scholar] [CrossRef]
- Tarazi, R.A.; Zabel, T.A.; Mahone, E.M. Age-related differences in executive function among children with spina bifida/hydrocephalus based on parent behavior ratings. Clin. Neuropsychol. 2008, 22, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Brewer, V.R.; Fletcher, J.M.; Hiscock, M.; Davidson, K.C. Attention processes in children with shunted hydrocephalus versus attention deficit-hyperactivity disorder. Neuropsychology 2001, 15, 185–198. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, G.G.; Martin, A.; Cervantes, E.; Guil, R.; Mestre, J.M. Attention lapses in children with spina bifida and hydrocephalus and children with attention-deficit/hyperactivity disorder. J. Clin. Exp. Neuropsychol. 2017, 39, 563–573. [Google Scholar] [CrossRef]
- Dennis, M.; Edelstein, K.; Frederick, J.; Copeland, K.; Francis, D.; Blaser, S.E.; Kramer, L.A.; Drake, J.M.; Brandt, M.; Hetherington, R.; et al. Peripersonal spatial attention in children with spina bifida: Associations between horizontal and vertical line bisection and congenital malformations of the corpus callosum, midbrain, and posterior cortex. Neuropsychologia 2005, 43, 2000–2010. [Google Scholar] [CrossRef]
- Kulesz, P.A.; Treble-Barna, A.; Williams, V.J.; Juranek, J.; Cirino, P.T.; Dennis, M.; Fletcher, J.M. Attention in spina bifida myelomeningocele: Relations with brain volume and integrity. NeuroImage Clin. 2015, 8, 72–78. [Google Scholar] [CrossRef]
- Swartwout, M.D.; Cirino, P.T.; Hampson, A.W.; Fletcher, J.M.; Brandt, M.E.; Dennis, M. Sustained attention in children with two etiologies of early hydrocephalus. Neuropsychology 2008, 22, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Vinck, A.; Mullaart, R.; Rotteveel, J.; Maassen, B. Neuropsychological assessment of attention in children with spina bifida. Cerebrospinal Fluid Res. 2009, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Craik, F.I.M.; Anderson, N.D.; Kerr, S.A.; Li, K.Z.H. Memory changes in normal ageing. In Handbook of Memory Disorders; Baddeley, A.D., Wilson, B.A., Watts, F.N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1995; pp. 211–241. [Google Scholar]
- Vachha, B.; Adams, R.C. Memory and selective learning in children with spina bifida-myelomeningocele and shunted hydrocephalus: A preliminary study. Cerebrospinal Fluid Res. 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Mammarella, N.; Cornoldi, C.; Donadello, E. Visual but not spatial working memory deficit in children with spina bifida. Brain Cog. 2003, 53, 311–314. [Google Scholar] [CrossRef]
- Dennis, M.; Jewell, D.; Drake, J.; Misakyan, T.; Spiegler, B.; Hetherington, R.; Gentili, F.; Barnes, M. Prospective, declarative, and nondeclarative memory in young adults with spina bifida. J. Int. Neuropsychol. 2007, 13, 312–323. [Google Scholar] [CrossRef]
- Hering, A.; Kliegel, M.; Rendell, P.G.; Craik, F.I.M.; Rose, N.S. Prospective Memory Is a Key Predictor of Functional Independence in Older Adults. J. Int. Neuropsychol. 2018, 24, 640–645. [Google Scholar] [CrossRef]
- Woods, S.P.; Weinborn, M.; Velnoweth, A.; Rooney, A.; Bucks, R.S. Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. J. Int. Neuropsychol. 2012, 18, 134–138. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Alloway, T.P.; Kirkwood, H.J.; Elliott, J.G.; Holmes, J.; Hilton, K.A. Attentional and executive function behaviours in children with poor working memory. Learn. Individ. Differ. 2008, 18, 214–223. [Google Scholar] [CrossRef]
- Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 1996, 153, 321–330. [Google Scholar] [CrossRef]
- Kolakowska, T.; Williams, A.O.; Jambor, K.; Ardern, M. Schizophrenia with good and poor outcome. III: Neurological ‘soft’ signs, cognitive impairment and their clinical significance. Br. J. Psychiatry 1985, 146, 348–357. [Google Scholar] [CrossRef]
- Raghubar, K.P.; Barnes, M.A.; Hecht, S.A. Working memory and mathematics: A review of developmental, individual difference, and cognitive approaches. Learn. Individ. Differ. 2010, 20, 110–122. [Google Scholar] [CrossRef]
- Sachdeva, S.; Kolarova, M.Z.; Foreman, B.E.; Kaplan, S.J.; Jasien, J.M. A Systematic Review of Cognitive Function in Adults with Spina Bifida. Dev. Neurorehabil. 2021, 24, 569–582. [Google Scholar] [CrossRef]
- Juranek, J.; Fletcher, J.M.; Hasan, K.M.; Breier, J.I.; Cirino, P.T.; Pazo-Alvarez, P.; Diaz, J.D.; Ewing-Cobbs, L.; Dennis, M.; Papanicolaou, A.C. Neocortical reorganization in spina bifida. NeuroImage 2008, 40, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Treble-Barna, A.; Juranek, J.; Stuebing, K.K.; Cirino, P.T.; Dennis, M.; Fletcher, J.M. Prospective and episodic memory in relation to hippocampal volume in adults with spina bifida myelomeningocele. Neuropsychology 2015, 29, 92–101. [Google Scholar] [CrossRef]
- Hasan, K.M.; Eluvathingal, T.J.; Kramer, L.A.; Ewing-Cobbs, L.; Dennis, M.; Fletcher, J.M. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: A diffusion tensor tractography study of the association pathways. J. Magn. Reson. Imaging 2008, 27, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Linroth, R.; Gangl, C.; Mitchell, N.; Hall, M.; Cady, R.; Christenson, M. Perception of secondary conditions in adults with spina bifida and impact on daily life. Disabil. Health J. 2015, 8, 492–498. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Bawor, M.; Dennis, B.B.; Bhalerao, A.; Plater, C.; Worster, A.; Varenbut, M.; Daiter, J.; Marsh, D.C.; Desai, D.; Steiner, M.; et al. Sex differences in outcomes of methadone maintenance treatment for opioid use disorder: A systematic review and meta-analysis. CMAJ Open 2015, 3, E344–E351. [Google Scholar] [CrossRef]
- Boyer, K.M.; Yeates, K.O.; Enrile, B.G. Working memory and information processing speed in children with myelomeningocele and shunted hydrocephalus: Analysis of the children’s paced auditory serial addition test. J. Int. Neuropsychol. Soc. 2006, 12, 305–313. [Google Scholar] [CrossRef]
- Jansen-Osmann, P.; Wiedenbauer, G.; Heil, M. Spatial cognition and motor development: A study of children with spina bifida. Percept. Mot. Skills 2008, 106, 436–446. [Google Scholar] [CrossRef]
- Lindquist, B.; Persson, E.K.; Uvebrant, P.; Carlsson, G. Learning, memory and executive functions in children with hydrocephalus. Acta Paediatr. 2008, 97, 596–601. [Google Scholar] [CrossRef] [PubMed]
- English, L.; Barnes, M.A.; Fletcher, J.M.; Dennis, M.; Raghubar, K.P. Effects of reading goals on reading comprehension, reading rate, and allocation of working memory in children and adolescents with spina bifida meningomyelocele. J. Int. Neuropsychol. Soc. 2010, 16, 517–525. [Google Scholar] [CrossRef][Green Version]
- Hampton, L.E.; Fletcher, J.M.; Cirino, P.; Blaser, S.; Kramer, L.A.; Dennis, M. Neuropsychological profiles of children with aqueductal stenosis and Spina Bifida myelomeningocele. J. Int. Neuropsychol. Soc 2013, 19, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Attout, L.; Noël, M.P.; Rousselle, L. Magnitude processing in populations with spina-bifida: The role of visuospatial and working memory processes. Res. Dev. Disabil. 2020, 102, 103655. [Google Scholar] [CrossRef] [PubMed]
- Bartonek, Å.; Guariglia, C.; Piccardi, L. Locomotion and Topographical Working Memory in Children with Myelomeningocele and Arthrogryposis Multiplex Congenita. Front. Psychiatry 2021, 12, 729859. [Google Scholar] [CrossRef]
- Iddon, J.L.; Morgan, D.J.; Loveday, C.; Sahakian, B.J.; Pickard, J.D. Neuropsychological profile of young adults with spina bifida with or without hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1112–1118. [Google Scholar] [CrossRef]
- Dennis, M.; Nelson, R.; Jewell, D.; Fletcher, J.M. Prospective memory in adults with spina bifida. Childs Nerv. Syst. 2010, 26, 1749–1755. [Google Scholar] [CrossRef]
- Ware, A.L.; Kulesz, P.A.; Juranek, J.; Cirino, P.T.; Fletcher, J.M. Cognitive control and associated neural correlates in adults with spina bifida myelomeningocele. Neuropsychology 2017, 31, 411–423. [Google Scholar] [CrossRef]
- Lindquist, B.; Jacobsson, H.; Strinnholm, M.; Peny-Dahlstrand, M. A scoping review of cognition in spina bifida and its consequences for activity and participation throughout life. Acta Paediatr. 2022, 111, 1682–1694. [Google Scholar] [CrossRef]
- Crawley, J.T.; Hasan, K.; Hannay, H.J.; Dennis, M.; Jockell, C.; Fletcher, J.M. Structure, integrity, and function of the hypoplastic corpus callosum in spina bifida myelomeningocele. Brain Connect. 2014, 4, 608–618. [Google Scholar] [CrossRef]
- Miller, E.; Widjaja, E.; Blaser, S.; Dennis, M.; Raybaud, C. The old and the new: Supratentorial MR findings in Chiari II malformation. Childs Nerv. Syst. 2008, 24, 563–575. [Google Scholar] [CrossRef]
- Salman, M.S.; Blaser, S.E.; Sharpe, J.A.; Dennis, M. Cerebellar vermis morphology in children with spina bifida and Chiari type II malformation. Childs Nerv. Syst. 2006, 22, 385–393. [Google Scholar] [CrossRef]
- Ammerman, R.T.; Kane, V.R.; Slomka, G.T.; Reigel, D.H.; Franzen, M.D.; Gadow, K.D. Psychiatric symptomatology and family functioning in children and adolescents with spina bifida. J. Clin. Psychol. Med. Settings 1998, 5, 449–465. [Google Scholar] [CrossRef]
- Appleton, P.L.; Ellis, N.C.; Minchom, P.E.; Lawson, V.; Böll, V.; Jones, P. Depressive symptoms and self-concept in young people with spina bifida. J. Pediatr. Psychol. 1997, 22, 707–722. [Google Scholar] [CrossRef]
- Oddson, B.E.; Clancy, C.A.; McGrath, P.J. The role of pain in reduced quality of life and depressive symptomology in children with spina bifida. Clin. J. Pain 2006, 22, 784–789. [Google Scholar] [CrossRef]
- Showen, A.; Copp, H.L.; Allen, I.E.; Baradaran, N.; Liaw, A.; Hampson, L.A. Characteristics Associated With Depression, Anxiety, and Social Isolation in Adults With Spina Bifida. Urology 2021, 149, 255–262. [Google Scholar] [CrossRef]
- Clancy, C.A.; McGrath, P.J.; Oddson, B.E. Pain in children and adolescents with spina bifida. Dev. Med. Child. Neurol. 2005, 47, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Richardson, E.J.; Cowan, R. Pain interference, pain type, and quality of life among adults with spina bifida. PM R 2024, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Palermo, T.M.; Holmbeck, G.N. A Multimethod, Case-Controlled Study of Sleep-Wake Disturbances in Adolescents with Spina Bifida. J. Pediatr. Psychol. 2018, 43, 601–612. [Google Scholar] [CrossRef]
- Gunnett, M.; Rocque, B.G.; Nourani, A.; Beltran-Ale, G. Impact of Spina Bifida on Sleep Quality: Current Insights. Nat. Sci. Sleep 2023, 15, 967–978. [Google Scholar] [CrossRef]
| P | People with SB diagnosis. No restriction on age, cultlure. |
| I | Any cognitive or neuropsychological assessment on memory. |
| C | Any group comparison or studies using normative data for comparison. |
| O | Memory performance (cross-sectional studies). |
| S | Empirical studies (any sample size). |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| PubMed ((spina bifida[Title/Abstract]) OR (myelomeningocele[Title/Abstract])) AND (memory[Title/Abstract]) |
| SCOPUS TITLE-ABS (spina AND bifida OR myelomeningocele AND memory) |
| Web of Science ((TI=(spina bifida)) OR TI=(myelomeningocele)) AND TI=(memory) ((AB=(spina bifida)) OR AB=(myelomeningocele)) AND AB=(memory) |
| ProQuest title(spina bifida) OR title(myelomeningocele) AND title(memory) abstract(spina bifida) OR abstract(myelomeningocele) AND abstract(memory) |
| Methods to Control Confounding | Statistical Methods | Methods for Measuring Outcomes | |||||
|---|---|---|---|---|---|---|---|
| Authors, Year | Method for Selecting Sample | Sample Size | Identification of Confounders | Appropiate Analyses | Missing Data | Outcome Measures | Objective Assessment |
| SB pediatric and adolescent population | |||||||
| Mammarella et al., 2003 [76] | Moderate | Moderate | Low | Low | Low | Low | Low |
| Burmeister et al., 2005 [63] | Low | Low | Moderate | Low | Low | Low | Low |
| Vachha and Adams, 2005 [75] | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Boyer et al., 2006 [91] | Moderate | Moderate | Low | Low | Low | Low | Low |
| Jansen-Osmann et al., 2008 [92] | High | High | Low | Low | Low | Low | Low |
| Lindquist et al., 2008 [93] | High | High | Moderate | Low | Low | Low | Low |
| English et al., 2010 [94] | Low | Low | Low | Low | Low | Low | Low |
| Hampton et al., 2013 [95] | Low | Low | Moderate | Low | Moderate | Low | Low |
| Raghubar et al., 2015 [60] | Low | Low | Low | Low | Low | Low | Low |
| Burro et al., 2018 [49] | High | High | Moderate | Low | Low | Low | Low |
| Attout et al., 2020 [96] | High | High | Low | Low | Low | Low | Low |
| Bartonek et al., 2021 [97] | Low | Low | Low | Low | Low | Low | Low |
| SB adult population | |||||||
| Iddon et al., 2004 [98] | High | Moderate | Moderate | Low | Moderate | Low | Low |
| Dennis et al., 2007 [77] | Moderate | Moderate | Low | Low | Moderate | Low | Low |
| Dennis et al., 2010 [99] | Moderate | Moderate | Low | Low | Low | Low | Low |
| Treble-Barna et al., 2015 [86] | Low | Low | Low | Low | Low | Low | Low |
| Ware et al., 2017 [100] | Low | Low | Low | Low | Low | Low | Low |
| Authors, Year | Test or Task Used | Subtest Applied | Type of Memory Assessed |
|---|---|---|---|
| Mammarella et al., 2003 [76] | House Visual Span | — | Visuospatial WM |
| VPT | — | Visuospatial WM | |
| CBT | The Forward and Backward version | Visuospatial WM | |
| Burmeister et al., 2005 [63] | WJ-R | Numbers Reversed | WM |
| CVLT-C | — | Verbal memory | |
| Vachha and Adams, 2005 [75] | Lists of 14 words with seven exemplars each of two distinct semantic categories | — | Verbal memory |
| Boyer et al., 2006 [91] | WISC-III | Digit Span and Arithmetic | WM |
| Jansen-Osmann et al., 2008 [92] | KABC | Spatial Memory | Visuospatial WM |
| Virtual maze | — | Visuospatial memory | |
| Lindquist et al., 2008 [93] | CBT | — | Visuospatial WM |
| WISC | Digit Span | WM | |
| STORDEL | — | Verbal memory | |
| ROCF | — | Visuospatial memory | |
| SLDEL | — | Visuospatial memory | |
| RAVLT | — | Verbal memory | |
| English et al., 2010 [94] | N-back test (1-back, 2-back and 3-back) | — | WM |
| Hampton et al., 2013 [95] | CVLT-C | — | Verbal memory |
| Raghubar et al., 2015 [60] | WJ-R | Numbers Reversed | WM |
| WISC-IV-Integrated | Spatial Span backward | Visuospatial WM | |
| Burro et al., 2018 [49] | WISC-IV | Digit Span and Letter-Number Sequencing | WM |
| Attout et al., 2020 [96] | Forward letter span | — | Short-term storage of verbal information |
| Category-span task | — | Short-term storage and manipulation of verbal information | |
| Visuospatial span task | — | Short-term storage of visual information | |
| Bartonek et al., 2021 [97] | CBT | — | Visuospatial WM |
| WalCT | — | Topographic WM |
| Authors, Year | Test or Task Used | Subtest Applied | Type of Memory Assessed |
|---|---|---|---|
| Iddon et al., 2004 [98] | CANTAB | - | Visual and spatial memory, spatial memory span and spatial WM |
| HVLT | - | Verbal memory (immediate recall, learning, recognition and delayed recall) | |
| Dennis et al., 2007 [77] | RBMT-E | Belongings, Appointments, Messages and Immediate and Delayed Story | Prospective memory and verbal episodic memory (recall) |
| The MicroCogTM computerized test | Numbers forward, Numbers reversed, Tic Tac, Story (immediate) 1 and 2, Story (delayed) 1 and 2, Wordlist 1, Wordlist 2, Address and Timers | WM (maintenance and manipulation), immediate and delayed verbal episodic memory (recognition), semantic memory and non-declarative memory | |
| TEA | Lottery and Elevator Counting with Reversal | WM | |
| Dennis et al., 2010 [99] | CAMPTROMPT | - | Prospective memory |
| Treble-Barna et al., 2015 [86] | RBMT-E | Belongings, Appointments, Messages and Immediate and Delayed Story | Prospective memory and verbal episodic memory (recall) |
| Ware et al., 2017 [100] | WMST and WMMT | - | Visual WM |
| Authors, Year | Sample (N) | Demographic Variables | Results | |
|---|---|---|---|---|
| Age (M/SD) (min.–max.) | Sex (n/%) | |||
| Mammarella et al., 2003 [76] | N = 40 Clinical group (SB): N = 20 Control group (TD): N = 20 | SB group: 10.5 (-) TD group: 10.5 (-) (8–13 years) | SB group: F = 11 (55%) M = 9 (45%) TD group: F = 10 (50%) M = 10 (50%) | Statistically significant differences were observed in the performance of the House Visual Span task (p < 0.01) between the clinical SB group (M = 27.30; SD = 2.79) and the control group (M = 29.85; SD = 1.98). However, no differences were observed in the rest of the visuospatial WM tests and tasks used (p > 0.05). |
| Burmeister et al., 2005 [63] | N = 205 Clinical group (SB): N = 164 Non-ADHD: N = 112 ADHD-I: N = 38 ADHD-C: N = 14 Control group (TD): N = 41 | (Age by months) SB group: Non-ADHD: 129.7 (30.8) ADHD-I: 142.0 (27.0) ADHD-C: 119.0 (22.0) TD group: 139.2 (33.8) (7–16 years) | SB group: Non-ADHD: F = 60 (54%) M = 52 (46%) ADHD-I: F = 19 (50%) M = 19 (50%) ADHD-C: F = 5 (36%) M = 9 (64%) TD group: F = 22 (54%) M = 19 (46%) | No differences were observed in the performance of the different cognitive tasks in children with SB when divided into groups based on the ADHD classification. The performance on the “Numbers Reversed” subtest, that assessed WM, was significantly poorer in children with SB compared to the TD participants (p < 0.05). The performance on the California Verbal Learning Test—Children’s Version, that assessed verbal memory, was significantly poorer in children with SB compared to the TD participants (p < 0.0001) |
| Vachha and Adams, 2005 [75] | N = 52 Clinical group (SB): N = 26 Control group (TD): N = 26 | SB group: 12.3 (2.7) TD group: 11.2 (2.6) (7–16 years) | - | The mean memory span was significantly lower across the three trials in participants with SB compared to the TD participants (p < 0.001). In this way, children with SB remembered fewer words than their healthy peers. |
| Boyer et al., 2006 [91] | N = 58 Clinical group (SB): N = 31 Control group (TD): N = 27 | SB group: 10.68 (2.23) TD group: 12.26 (2.09) (8–15 years) | SB group: F = 9 (29%) M = 22 (71%) TD group: F = 12 (44%) M = 15 (56%) | Significant differences were found in the score obtained by the SB group compared to the TD group in one of the WM tasks (“Arithmetic”) (p < 0.001) but not for the other one (“Digit Span”) (M = 9.19; SD = 2.63 in the SB group and M = 10.07; SD = 2.35 in the control group). |
| Jansen-Osmann et al., 2008 [92] | N = 40 Clinical group (SB): N = 20 Control group (TD): N = 20 | SB group: 11.4 (1.7) TD group: 11.8 (1.8) (8–14 years) | SB group: F = 13 (65%) M = 7 (35%) TD group: F = 13 (65%) M = 7 (35%) | The SB clinical group (M = 14.40; SE = 3.50) performed worse on the virtual visuospatial WM task compared to their healthy peers (M = 16.90; SE = 2.49) (p < 0.05). A significant correlation was found between the age of walking and the score obtained in the visuospatial WM task, measured by number of points (p < 0.01) and number of learning trials during the performance on the maze of the task (p < 0.01). In this way, children in the clinical SB group that learned to walk later in life obtained fewer points on the visuospatial WM test, needing more trials to figure out the correct path in the virtual maze. |
| Lindquist et al., 2008 [93] | N = 72 Clinical group 1 (SB): N = 16 Clinical group 2 (H): N = 20 Control group (TD): N = 36 | SB group: 11 years and 7 months (-) H group: 11 years and 7 months (-) TD group: 11 years and 7 months (-) (8–13 years) | SB group: - H group: - TD group: F = 13 (36.1%) M = 23 (63.9%) | Participants with SB obtained significantly lower results on the following tasks measuring short-term memory compared to the healthy participants: Story Recall and The Complex Figure of Rey, (1.5 SD under test norm on both tests). Similar results were found, showing a worse performance in the SB group compared to their healthy peers in the Story Recall and Rey Auditory Verbal Learning tests (1.5 SD under test norm on both tests). The SB clinical group’s performance was significantly inferior compared to their healthy peers on the two visuospatial memory tasks (The Complex Figure of Rey and The Spatial Learning Test) (p < 0.01). However, there were no significant differences in the performance of the “Corsi block test” and “Digit Span” subtest. The SB and H groups did not differ in their performance on the different cognitive tasks. |
| English et al., 2010 [94] | N = 118 Clinical group (SB): N = 79 Control group (TD): N = 39 | SB group: 12.5 (2.7) TD group: 12.3 (2.8) (8–19 years) | SB group: F = 38 (48.10%) M = 41 (51.90%) TD group: F = 21 (53.85%) M = 18 (46.15%) | A lower WM score was observed in the SB clinical group (M = 27.6; SD = 11.9) compared to the TD control group (M = 37.8; SD = 9.9) (p < 0.001). Thus, the SB group showed less developed WM abilities compared to the TD group. |
| Hampton et al., 2013 [95] | N = 180 Clinical group 1 (SB): N = 151 Clinical group 2 (AS): N = 29 Control group (TD): N = 60 | SB group: 11.37 (2.80) AS group: 12.52 (3.30) TD group: 12.08 (2.84) (7–18 years) | SB group: F = 66 (44%) M = 85 (56%) AS group: F = 13 (45%) M = 16 (55%) TD group: F = 31 (52%) M = 29 (48%) | The SB clinical group had the lowest average score on verbal memory compared to the clinical AS group and to the TD group. The clinical SB group and the clinical AS group differed significantly in performance in the verbal memory domain (p < 0.05) |
| Raghubar et al., 2015 [60] | N = 94 Clinical group (SB): N = 44 Control group (TD): N = 50 | SB group: 9.91 (-) TD group: 9.81 (not specified) | SB group: F = - (63%) M = - (37%) TD group: F = - (43%) M = - (57%) | A poorer performance on measures of verbal (p = 0.01) and visuospatial WM (p = 0.001) was observed in the SB clinical group when compared to the TD group. |
| Burro et al., 2018 [49] | N = 26 Clinical group (SB): N = 13 Control group (TD): N = 13 | SB group: 12.85 (2.91) TD group: 12.85 (2.91) (7.6–16.0 years) | SB group: F = 5 (38.5%) M = 8 (61.5%) TD group: F = 5 (38.5%) M = 8 (61.5%) | The general performance in the WM Index was lower in the SB clinical group than in the TD group. A significantly lower performance was observed in the SB group compared to the TD group on the “Letter-Number Sequencing” subtest (p < 0.001). However, no statistically significant differences were found between the SB and the TD groups on the “Digit Span” subtest (M = 7.92; SD = 3.33 and M = 10.54; SD = 3.78, respectively). |
| Attout et al., 2020 [96] | N = 46 Clinical group (SB): N = 23 Control group (TD): N = 23 | (Age by months) SB group: 145.26 (28.46) TD group: 145.65 (28.03) (7–16 years) | SB group: F = 10 (43.48%) M = 13 (56.52%) TD group: F = 12 (52.17%) M = 11 (47.82%) | Lower capacities in verbal (p < 0.001) and visuospatial WM (p < 0.001) were found in the SB group compared to the TD group. However, no differences were found between the SB and the TD groups in the performance on the Forward Letter Span Task (p = 0.14). |
| Bartonek et al., 2021 [97] | N = 161 Clinical group 1 (SB): N = 41 Clinical group 2 (AMC): N = 10 Control group (TD): N = 120 | SB group: 11.9 (3.2) AMC group: 10.6 (3.1) TD group: 9.9 (3.1) - | SB and AMC group: F= 19 (-) M = 22 (-) TD group: F = 63 (52.5%) M = 57 (47.5%) | The score obtained in WalCT that measured topographic WM was lower in the SB group compared to the control group. No differences were found between the scores obtained in the SB group and the AMC and TD groups on the CBT visuospatial memory test. However, a significant difference was found between the non-ambulation group (which was conformed by only children with SB that had to use a wheelchair for all their transfers) and the TD group on the CBT span (p = 0.004). In this way, non-ambulant SB children obtained the lowest scores on visuospatial WM, showing a poorer visuospatial function than those SB children who could walk independently. |
| Authors, Year | Sample (N) | Demographic Variables | Results | |
|---|---|---|---|---|
| Age (M/SD) (min.–max.) | Sex (n/%) | |||
| Iddon et al., 2004 [98] | N = not specified. Ranged from 72 to 159 in memory measures. Clinical group 1 (SB with concomitant hydrocephalus) Clinical group 2 (H) Clinical group 3 (SB alone without concomitant hydrocephalus) Control group (TD) | - | - | Statistically significant differences were observed between the clinical groups and the control group (or normative data) on every score from the CANTAB and the Hopkins Verbal Learning Test. SB participants with a hydrocephalus diagnosis obtained lower scores in all the memory tasks compared to participants with SB alone (without a hydrocephalus diagnosis) and the TD group. The majority of the SB group’s results were average or above average. These results suggest that individuals with SB without hydrocephalus do not show the same memory impairments as those with SB and hydrocephalus. |
| Dennis et al., 2007 [77] | N = 58 Clinical group (SB): N = 29 0–5 shunt revisions: N = 17 >5 revisions: N = 12 Control group (TD): N = 29 | SB group: 26.60 (4.82) 0–5 shunt revisions: 27.53 (4.83) >5 revisions: 25.28 (4.69) TD group: 26.88 (5.86) (18.21–36.50 years) | SB group: F = 13 (44.83%) M = 16 (55.17%) TD group: F = 18 (62.07%) M = 11 (37.93%) | SB group performance differed significantly from that of the TD group on prospective memory tasks (p < 0.05). Within the SB group, patients with more than five shunt revisions showed poorer performance than healthy controls (p < 0.02). Verbal episodic memory tasks included recall and recognition (both immediate and delayed). In recognition tasks, the SB group performed similarly to normative data on immediate recall, but scored lower than the population mean on delayed tasks (p < 0.03), regardless of shunt revisions. In recall tasks, the SB group differed from the TD group on both immediate (p < 0.05) and delayed recall (p < 0.01). However, only those with more than five shunt revisions scored significantly lower than the TD group on the immediate recall task (p < 0.02). The SB group performed significantly worse than the population mean on WM tasks, regardless of shunt revisions. This included a maintenance task (p < 0.01) and a maintenance/manipulation task (p < 0.03). However, on another maintenance task, only those with fewer shunt revisions scored lower than normative means on another maintenance task. No significant differences were found on inhibition and set-shifting tasks. Participants with SB and fewer than six revisions scored higher than population means on the non-declarative memory task (p < 0.03). The SB group showed no significant difference from normative data on the semantic memory task (p > 0.05). |
| Dennis et al., 2010 [99] | N = 49 Clinical group (SB): N = 32 Control group (TD): N = 17 | SB group: 34.48 (10.34) TD group: 30.29 (14.94) (18.33–62.67 years) | SB group: F = 18 (56.25%) M = 14 (43.75%) TD group: F = 12 (70.59%) M = 5 (29.41%) | Differences were observed on the performance on the prospective memory test between the SB clinical group and the control group in the total score (p < 0.0001), time-based subscore (p < 0.0001) and event-based subscore (p < 0.001). Twenty-five percent of the participants in the SB group showed an impaired prospective memory. The older adults in the SB group had a threefold higher rate of poor prospective memory compared to the younger adults in the SB group (37.50% and 12.50%, respectively) (p < 0.05). |
| Treble-Barna et al., 2015 [86] | N = 138 Clinical group (SB): N = 97 Control group (TD): N = 41 | SB group: 29.14 (9.7) TD group: 30.45 (11.9) (18–62 years) | SB group: F = 52 (54%) M = 45 (46%) TD group: F = 29 (71%) M = 12 (29%) | Prospective memory performance was significantly lower in participants with SB (M = 18.57; SE = 0.36) compared to the TD participants (M = 21.92; SE = 0.55). Verbal episodic memory performance was significantly lower in participants with SB (M = 9.41; SE = 0.62) compared to the TD participants (M = 15.50; SE = 0.95). |
| Ware et al., 2017 [100] | N = 120 Clinical group (SB): N = 68 Control group (TD): N = 52 | SB group: 28.61 (9.71) TD group: 31.81 (10.48) (18–56 years) | SB group: F = 33 (49%) M = 35 (51%) TD group: F = 38 (73%) M = 14 (27%) | Regarding the WMST, the performance accuracy was significantly lower in the SB group compared to the TD group (p < 0.001). Older and younger adults from the SB group presented a similar performance accuracy (p = 0.301). In contrast, older adults in the TD group had a significantly poorer performance on accuracy (p < 0.05). These findings elucidate a general impaired profile that seem to be maintained across aging in adults with SB. Reaction time was similar for both SBM and TD groups (p = 0.740). Regarding the WMMT, the performance accuracy was significantly lower in the SB group compared to the TD group (p = 0.002). Reaction time was similar for both SB and TD groups (p > 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amayra, I.; Ruiz de Lazcano, A.; Salgueiro, M.; Anguiano, S.; Ureña, M.; Martínez, O. Memory in Spina Bifida, from Childhood to Adulthood: A Systematic Review. J. Clin. Med. 2024, 13, 5273. https://doi.org/10.3390/jcm13175273
Amayra I, Ruiz de Lazcano A, Salgueiro M, Anguiano S, Ureña M, Martínez O. Memory in Spina Bifida, from Childhood to Adulthood: A Systematic Review. Journal of Clinical Medicine. 2024; 13(17):5273. https://doi.org/10.3390/jcm13175273
Chicago/Turabian StyleAmayra, Imanol, Aitana Ruiz de Lazcano, Monika Salgueiro, Samuel Anguiano, Malena Ureña, and Oscar Martínez. 2024. "Memory in Spina Bifida, from Childhood to Adulthood: A Systematic Review" Journal of Clinical Medicine 13, no. 17: 5273. https://doi.org/10.3390/jcm13175273
APA StyleAmayra, I., Ruiz de Lazcano, A., Salgueiro, M., Anguiano, S., Ureña, M., & Martínez, O. (2024). Memory in Spina Bifida, from Childhood to Adulthood: A Systematic Review. Journal of Clinical Medicine, 13(17), 5273. https://doi.org/10.3390/jcm13175273


.png)



