Advances in Modern Microsurgery
Abstract
:1. Introduction
2. Robotic Microsurgery
3. Advances in Peripheral Nerve Surgery
3.1. Advances in Diagnosis
3.2. Advances of Nerve Bridging and Transfer
3.3. Advances in Sensitivity and Pain Management
4. Free Microsurgical Tissue Transfer
5. Replantation and Allotransplantation
6. Lymphatic Surgery
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CE | Conformité Européenne (European Conformity) |
| CTA | Composite tissue allografts |
| MUSA | MicroSure surgical assistant |
| DIEP flap | Deep inferior epigastric perforator flap |
| MR | Magnetic resonance |
| LVA | Lymphovenous anastomosis |
| VCA | Vascularized composite tissue allografts |
| VLNT | Vascularized lymph node transfer |
References
- Tamai, S. History of microsurgery. Plast. Reconstr. Surg. 2009, 124 (Suppl. S6), e282–e294. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Chien, Y.C.; Pao, Y.S. Salvage of the Forearm following Complete Traumatic Amputation: Report of a Case. Chin. Med. J. 1963, 82, 633–638. [Google Scholar] [PubMed]
- Taylor, G.I.; Palmer, J.H. The vascular territories (angiosomes) of the body: Experimental study and clinical applications. Br. J. Plast. Surg. 1987, 40, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Devauchelle, B.; Badet, L.; Lengelé, B.; Morelon, E.; Testelin, S.; Michallet, M.; D’Hauthuille, C.; Dubernard, J.-M. First human face allograft: Early report. Lancet 2006, 368, 203–209. [Google Scholar] [CrossRef]
- Yamamoto, T. Onco-reconstructive supermicrosurgery. Eur. J. Surg. Oncol. 2019, 45, 1146–1151. [Google Scholar] [CrossRef]
- Shademan, A.; Decker, R.S.; Opfermann, J.D.; Leonard, S.; Krieger, A.; Kim, P.C.W. Supervised autonomous robotic soft tissue surgery. Sci. Transl. Med. 2016, 8, 337ra64. [Google Scholar] [CrossRef]
- Barbon, C.; Grünherz, L.; Uyulmaz, S.; Giovanoli, P.; Lindenblatt, N. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open 2022, 34, 126–133. [Google Scholar] [CrossRef]
- van Mulken, T.J.; Schols, R.M.; Scharmga, A.M.; Winkens, B.; Cau, R.; Schoenmakers, F.B.F.; Qiu, S.S.; van der Hulst, R.R.W.J.; MicroSurgical Robot Research Group. First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: A randomized pilot trial. Nat. Commun. 2020, 11, 757. [Google Scholar] [CrossRef]
- Ferrari-Light, D.; Geraci, T.C.; Sasankan, P.; Cerfolio, R.J. The Utility of Near-Infrared Fluorescence and Indocyanine Green During Robotic Pulmonary Resection. Front. Surg. 2019, 6, 47. [Google Scholar] [CrossRef]
- Henn, D.; Trotsyuk, A.A.; Barrera, J.A.; Sivaraj, D.B.; Chen, K.; Mittal, S.B.; Mermin-Bunnell, A.M.B.; Chattopadhyay, A.; Larson, M.R.B.; Kinney, B.M.; et al. Robotics in Plastic Surgery: It’s Here. Plast. Reconstr. Surg. 2023, 152, 239–249. [Google Scholar] [CrossRef]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, eaaz7946. [Google Scholar] [CrossRef] [PubMed]
- Fagogenis, G.; Mencattelli, M.; Machaidze, Z.; Rosa, B.; Price, K.; Wu, F.; Weixler, V.; Saeed, M.; Mayer, J.E.; Dupont, P.E. Autonomous Robotic Intracardiac Catheter Navigation Using Haptic Vision. Sci. Robot. 2019, 4, eaaw1977. [Google Scholar] [CrossRef]
- Vargo, M.; Ding, P.; Sacco, M.; Duggal, R.; Genther, D.J.; Ciolek, P.J.; Byrne, P.J. The psychological and psychosocial effects of facial paralysis: A review. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Goubier, J.-N.; Battiston, B.; Casanas, J.; Quick, T. Adult traumatic brachial plexus injuries: Advances and current updates. J. Hand Surg. (Eur. Vol.) 2024, 49, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.M.; Hadlock, T.A.; Klebuc, M.J.; Simpson, R.L.; Zenn, M.R.; Marcus, J.R. Contemporary solutions for the treatment of facial nerve paralysis. Plast. Reconstr. Surg. 2015, 135, 1025e–1046e. [Google Scholar] [CrossRef]
- Moore, A.M.; Novak, C.B. Advances in nerve transfer surgery. J. Hand Ther. 2014, 27, 96–104, quiz 105. [Google Scholar] [CrossRef]
- Raman, S.; Daniele, E.; Daniele, K.A.; Choudhary, A.M.; Purnell, C.A.; Ranzer, M. A Scoping Review of Innervated Breast Reconstruction. Ann. Plast. Surg. 2024, 92, 591–596. [Google Scholar] [CrossRef]
- Mauch, J.T.; Kao, D.S.; Friedly, J.L. Targeted muscle reinnervation and regenerative peripheral nerve interfaces for pain prophylaxis and treatment: A systematic review. PM&R 2023, 15, 1457–1465. [Google Scholar]
- Holzgrefe, R.E.; Wagner, E.R.; Singer, A.D.; Daly, C.A. Imaging of the Peripheral Nerve: Concepts and Future Direction of Magnetic Resonance Neurography and Ultrasound. J. Hand Surg. Am. 2019, 44, 1066–1079. [Google Scholar] [CrossRef]
- Finkelstein, E.R.; Hui-Chou, H.; Fullerton, N.; Jose, J. Experience with ultrasound neurography for postoperative evaluation of targeted muscle reinnervation. Skelet. Radiol. 2024, 53, 811–816. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Y.; Shi, X.; Wang, K.; Qu, Q.; Liang, Q.; Ma, X.; He, K.; Chi, C.; Tang, J.; et al. NIR-II light in clinical oncology: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2024, 21, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Braga Silva, J.; Becker, A.S.; Leal, B.L.M.; Busnello, C.V. Advances of Direct Peripheral Nerve Repair Techniques: Do We Already Have Enough Scientific Evidence? Indian J. Orthop. 2023, 57, 189–202. [Google Scholar] [CrossRef]
- Leckenby, J.I.; Chacon, M.A.; Milek, D.; Lichtman, J.W.; Grobbelaar, A.O. Axonal Regeneration through Autologous Grafts: Does the Axonal Load Influence Regeneration? J. Surg. Res. 2022, 280, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.C.; Stonner, M.M.; Dy, C.J. Key Considerations for Nerve Transfer Rehabilitation After Surgical Reconstruction for Brachial Plexus and Peripheral Nerve Injuries. J. Hand Surg. Am. 2024, 49, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.E.S.; Jeyaratnam, S.; Lim, A.Y.T. Updates in peripheral nerve surgery of the upper extremity: Diagnosis and treatment options. Ann. Transl. Med. 2023, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Kaufman Goldberg, T.; Trzcinski, L.O.; McGonagle, E.R.; Hadlock, T. A Does supercharging with cross-face nerve graft enhance smile in non-flaccid facial paralysis patients undergoing selective neurectomy? Microsurgery 2024, 44, e31118. [Google Scholar] [CrossRef]
- Curran, M.W.T.; Olson, J.L.; Morhart, M.J.; Wu, S.S.Z.; Midha, R.; Berger, M.J.; Chan, K.M. Reverse End-to-Side Nerve Transfer for Severe Ulnar Nerve Injury: A Western Canadian Multicentre Prospective Nonrandomized Cohort Study. Neurosurgery 2022, 91, 856–862. [Google Scholar] [CrossRef]
- Ernst, J.; Hahne, J.M.; Markovic, M.; Schilling, A.F.; Lorbeer, L.; Grade, M.; Felmerer, G. Combining Surgical Innovations in Amputation Surgery-Robotic Harvest of the Rectus Abdominis Muscle, Transplantation and Targeted Muscle Reinnervation Improves Myocontrol Capability and Pain in a Transradial Amputee. Medicina 2023, 59, 2134. [Google Scholar] [CrossRef]
- Goodyear, E.G.B.; O’brien, A.L.; West, J.M.M.; Huayllani, M.T.; Huffman, A.C.B.; Souza, J.M.; Schulz, S.A.; Moore, A.M. Targeted Muscle Reinnervation at the Time of Amputation Decreases Recurrent Symptomatic Neuroma Formation. Plast. Reconstr. Surg. 2024, 153, 154–163. [Google Scholar] [CrossRef]
- Eseme, E.A.; Remy, K.; Mené, B.L.; Walz, S.N.; Madduri, S.; Oranges, C.M.; Kalbermatten, D.F. Sensory and pain outcomes of neurotized skin-grafted free gracilis muscle flaps for lower extremity reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2024, 92, 216–224. [Google Scholar] [CrossRef]
- Jacobson, J.H., 2nd; Suarez, E.L. Microvascular surgery. Dis. Chest 1962, 41, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Kriss, T.C.; Kriss, V.M. History of the operating microscope: From magnifying glass to microneurosurgery. Neurosurgery 1998, 42, 899–907; discussion 907–908. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.H.; Buncke, H.J., Jr. Autotransplant of omentum to a large scalp defect, with microsurgical revascularization. Plast. Reconstr. Surg. 1972, 49, 268–274. [Google Scholar] [CrossRef]
- Taylor, G.I.; Miller, G.D.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Koshima, I.; Soeda, S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br. J. Plast. Surg. 1989, 42, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Hartrampf, C.R.; Scheflan, M.; Black, P.W. Breast reconstruction with a transverse abdominal island flap. Plast. Reconstr. Surg. 1982, 69, 216–225. [Google Scholar] [CrossRef]
- Allen, R.J.; Heitmann, C. Perforator flaps—The history of evolution. Handchir. Mikrochir. Plast. Chir. 2002, 34, 216–218. [Google Scholar] [CrossRef]
- Wei, F.C.; Jain, V.; Suominen, S.; Chen, H.C. Confusion among perforator flaps: What is a true perforator flap? Plast. Reconstr. Surg. 2001, 107, 874–876. [Google Scholar] [CrossRef]
- Garcia, J.P.; Avila, F.R.; Torres, R.A.; Maita, K.C.; Borna, S.; Rinker, B.D.; Forte, A.J.; Ho, O.A. Evaluating the exoscope as an alternative to the operating microscope in plastic surgery. J. Plast. Reconstr. Aesthetic Surg. 2023, 85, 376–386. [Google Scholar] [CrossRef]
- Koshima, I.; Yamamoto, T.; Narushima, M.; Mihara, M.; Iida, T. Perforator flaps and supermicrosurgery. Clin. Plast. Surg. 2010, 37, 683–689, vii-iii. [Google Scholar] [CrossRef]
- Malt, R.A.; McKhann, C. Replantation of severed ARMS. JAMA 1964, 189, 716–722. [Google Scholar] [CrossRef]
- Chen, Z.W.; Yu, H.L. Current procedures in China on replantation of severed limbs and digits. Clin. Orthop. Relat. Res. 1987, 215, 15–23. [Google Scholar] [CrossRef]
- Kleinert, H.E.; Kasdan, M.L. Anastamosis of digital vessels. J. Ky. Med. Assoc. 1965, 63, 106–108. [Google Scholar] [PubMed]
- Komatsu, S.; Tamai, S. Successful replantation of a completely cut-off thumb. Plast. Reconstr. Surg. 1968, 42, 374–377. [Google Scholar] [CrossRef]
- Gu, Y.D.; Zhang, G.M.; Cheng, D.S.; Yan, J.G.; Chen, X.M. Free toe transfer for thumb and finger reconstruction in 300 cases. Plast. Reconstr. Surg. 1993, 91, 693–700; discussion 701–702. [Google Scholar] [PubMed]
- Ono, S.; Chung, K.C. Efficiency in Digital and Hand Replantation. Clin. Plast. Surg. 2019, 46, 359–370. [Google Scholar] [CrossRef]
- Peacock, E.E., Jr. Homologous composite tissue grafts of the digital flexor mechanism in human beings. Plast. Reconstr. Surg. Transplant. Bull. 1960, 25, 418–421. [Google Scholar] [CrossRef]
- Doi, K. Homotransplantation of limbs in rats. A preliminary report on an experimental study with nonspecific immunosuppressive drugs. Plast. Reconstr. Surg. 1979, 64, 613–621. [Google Scholar]
- Lance, E.M.; Inglis, A.E.; Figarola, F.; Veith, F.J. Transplantation of the canine hind limb. Surgical technique and methods of immunosuppression for allotransplantation. A preliminary report. J. Bone Jt. Surg. Am. 1971, 53, 1137–1149. [Google Scholar]
- Canadian Multicentre Transplant Study Group. A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N. Engl. J. Med. 1983, 309, 809–815. [Google Scholar]
- Francois, C.G.; Breidenbach, W.C.; Maldonado, C.; Kakoulidis, T.P.; Hodges, A.; Dubernard, J.-M.; Owen, E.; Pei, G.; Ren, X.; Barker, J.H. Hand transplantation: Comparisons and observations of the first four clinical cases. Microsurgery 2000, 20, 360–371. [Google Scholar] [CrossRef]
- Dubernard, J.-M.; Lengelé, B.; Morelon, E.; Testelin, S.; Badet, L.; Moure, C.; Beziat, J.-L.; Dakpé, S.; Kanitakis, J.; D’Hauthuille, C.; et al. Outcomes 18 months after the first human partial face transplantation. N. Engl. J. Med. 2007, 357, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Barret, J.P.; Gavaldà, J.; Bueno, J.; Nuvials, X.; Pont, T.; Masnou, N.; Colomina, M.J.; Serracanta, J.; Arno, A.; Huguet, P.; et al. Full face transplant: The first case report. Ann. Surg. 2011, 254, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.T.; Brandacher, G.; Lee, W.P.A. Hand and upper extremity transplantation: An update of outcomes in the worldwide experience. Plast. Reconstr. Surg. 2015, 135, 351e–360e. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, N.; Kikuchi, K.; Todokoro, T.; Iida, T.; Koshima, I. Dynamic Indocyanine Green (ICG) lymphography for breast cancer-related arm lymphedema. Ann. Plast. Surg. 2014, 73, 706–709. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: A novel severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Araki, J.; Kikuchi, K.; Narushima, M.; Yamamoto, T.; Iida, T.; Yoshimatsu, H.; Murai, N.; Mitsui, K.; et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS ONE 2012, 7, e38182. [Google Scholar] [CrossRef]
- van Heumen, S.; Riksen, J.J.; Bramer, W.M.; van Soest, G.; Vasilic, D. Imaging of the Lymphatic Vessels for Surgical Planning: A Systematic Review. Ann. Surg. Oncol. 2023, 30, 462–479. [Google Scholar] [CrossRef]
- Yang, J.C.; Wu, S.; Chiang, M.; Lin, W.; Hsieh, C. Intraoperative identification and definition of “functional” lymphatic collecting vessels for supermicrosurgical lymphatico-venous anastomosis in treating lymphedema patients. J. Surg. Oncol. 2018, 117, 994–1000. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Sørensen, J.A. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: A systematic review and meta-analysis. Microsurgery 2018, 38, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Huang, J.J.; Wu, C.W.; Yang, C.Y.; Lin, C.Y.; Henry, S.L.; Kolios, L. The mechanism of vascularized lymph node transfer for lymphedema: Natural lymphaticovenous drainage. Plast. Reconstr. Surg. 2014, 133, 192e–198e. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Suami, H. Overview of lymph node transfer for lymphedema treatment. Plast. Reconstr. Surg. 2014, 134, 548–556. [Google Scholar] [CrossRef]
- Ito, R.; Zelken, J.; Yang, C.-Y.; Lin, C.-Y.; Cheng, M.-H. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol. Oncol. 2016, 141, 182–188. [Google Scholar] [CrossRef]
- Dayan, J.H.; Dayan, E.; Smith, M.L. Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast. Reconstr. Surg. 2015, 135, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Pasko, J.L.; Garreau, J.; Carl, A.; Ansteth, M.; Glissmeyer, M.; Johnson, N. Axillary reverse lymphatic mapping reduces patient perceived incidence of lymphedema after axillary dissection in breast cancer. Am. J. Surg. 2015, 209, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Skoracki, R.; Eiferman, D. Vascularized jejunal mesenteric lymph node transfer for treatment of extremity lymphedema. Microsurgery 2017, 37, 177–178. [Google Scholar] [CrossRef]
- Coriddi, M.; Wee, C.; Meyerson, J.; Eiferman, D.; Skoracki, R. Vascularized Jejunal Mesenteric Lymph Node Transfer: A Novel Surgical Treatment for Extremity Lymphedema. J. Am. Coll. Surg. 2017, 225, 650–657. [Google Scholar] [CrossRef]

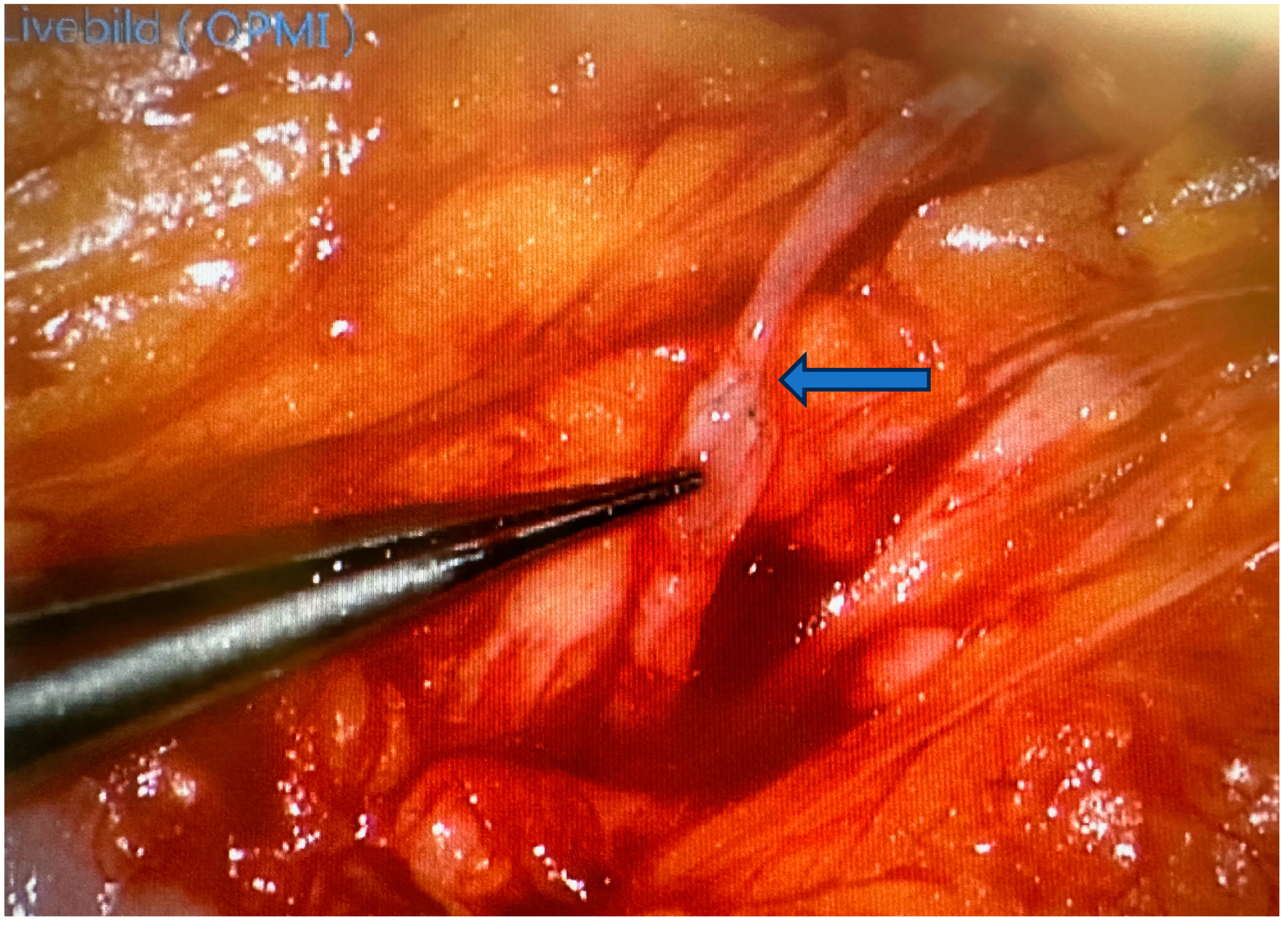
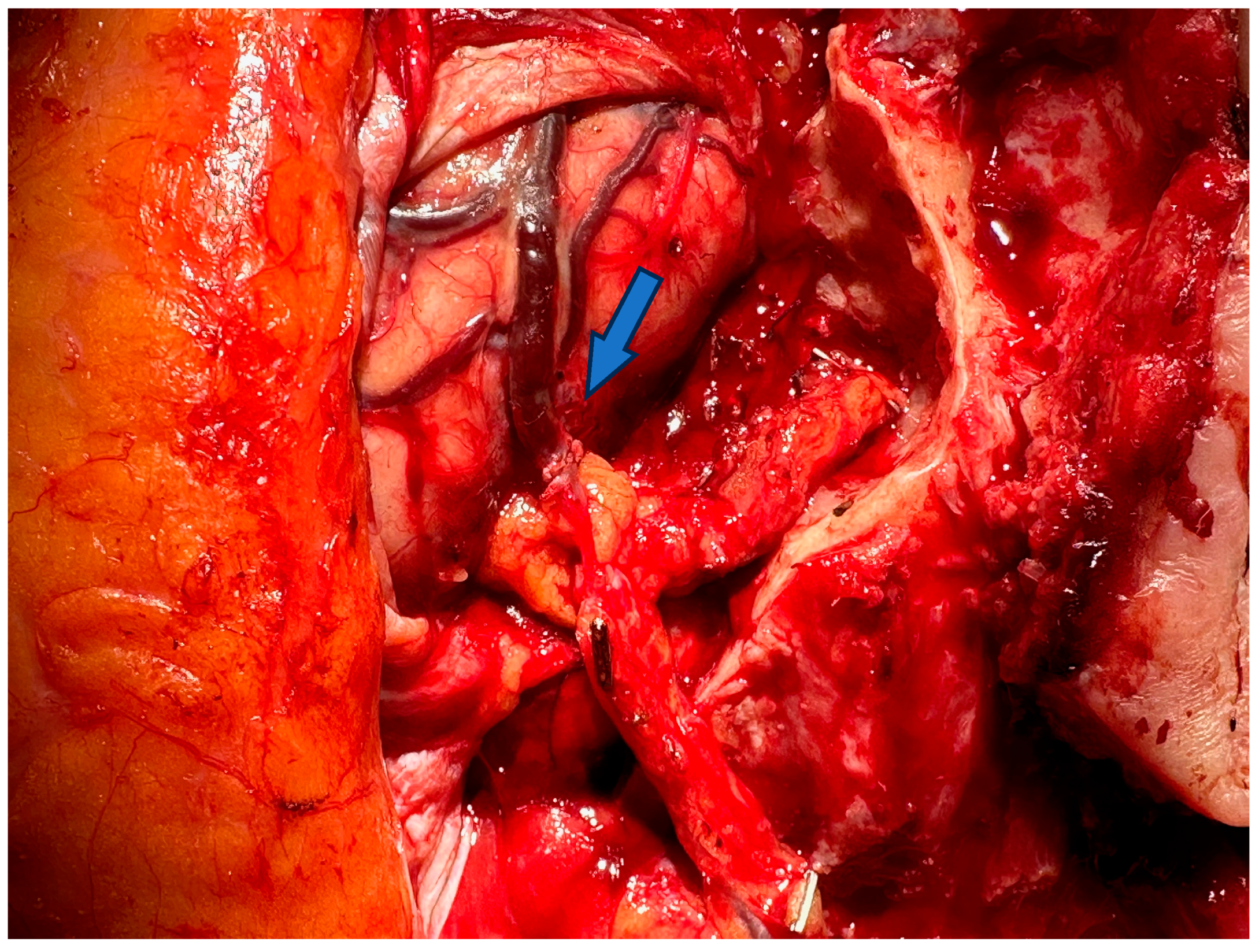
| Category | Microsurgery | Supermicrosurgery |
|---|---|---|
| Vascular Anastomosis | Commonly used for vessels larger than 0.8 mm | Used for very small vessels (less than 0.8 mm) |
| Flap Reconstruction | Used for transferring tissue flaps | Used for thin, perforator-based flaps |
| Lymphedema Treatment | - | Lymphaticovenous anastomosis |
| Nerve Repair and Reconstruction | Nerve grafting and coaptation | Repair of very small nerve branches |
| Breast Reconstruction | DIEP, TRAM, and other large flap transfers | - |
| Hand Surgery | Replantation and composite tissue transfer | Replantation of distal fingers, fine vascular anastomosis |
| Facial Reanimation | Cross-facial nerve grafting | Supermicrosurgical nerve grafting, muscle transfers |
| Peripheral Nerve Surgery | Repair of larger peripheral nerves | Supermicrosurgical repair of small nerve branches |
| Year | Advancement/Development | Vessel Caliber | Description |
|---|---|---|---|
| 1921 | Introduction of the first surgical microscope | ~1.0 mm | Enabled more precise surgical procedures under magnification |
| 1960s | Development of microsurgical instruments | 0.5–1.0 mm | Specialized tools for handling tiny vessels and nerves |
| 1964 | First successful digital replantation | ~1.0 mm | Marked the beginning of reconstructive microsurgery |
| 1970s | Advent of microsurgical training programs | ~0.8–1.0 mm | Formalized training improved the precision and success rates of microsurgery |
| 1980s | Introduction of free tissue transfer (flap surgery) | 0.3–1.0 mm | Allowed for complex reconstructions using tissue from distant body sites |
| 1990s | Development of perforator flaps | 0.5–1.0 mm | Advanced techniques for tissue transfer with minimal donor site morbidity |
| 2000s | Emergence of supermicrosurgery | <0.8 mm | Enabled suturing of extremely small vessels and lymphatic vessels |
| 2010s | Advancements in lymphaticovenular anastomosis (LVA) | <0.5 mm | Refined supermicrosurgical techniques for treating lymphedema |
| 2020s | Introduction of robotic-assisted microsurgery | ~0.5 mm and smaller | Enhanced precision and dexterity in microsurgical procedures using robotics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thamm, O.C.; Eschborn, J.; Schäfer, R.C.; Schmidt, J. Advances in Modern Microsurgery. J. Clin. Med. 2024, 13, 5284. https://doi.org/10.3390/jcm13175284
Thamm OC, Eschborn J, Schäfer RC, Schmidt J. Advances in Modern Microsurgery. Journal of Clinical Medicine. 2024; 13(17):5284. https://doi.org/10.3390/jcm13175284
Chicago/Turabian StyleThamm, Oliver C., Johannes Eschborn, Ruth C. Schäfer, and Jeremias Schmidt. 2024. "Advances in Modern Microsurgery" Journal of Clinical Medicine 13, no. 17: 5284. https://doi.org/10.3390/jcm13175284






