Left Ventricular Diastolic Dysfunction across Levels of Kidney Function: A Cross-Sectional Study Based on Routine Clinical Practice Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Determinants and Covariates
2.3. Outcome of Interest
2.4. Statistical Analysis
2.5. Sensitivity Analyses
3. Results
3.1. Baseline Characteristics
3.2. Echocardiography Parameters
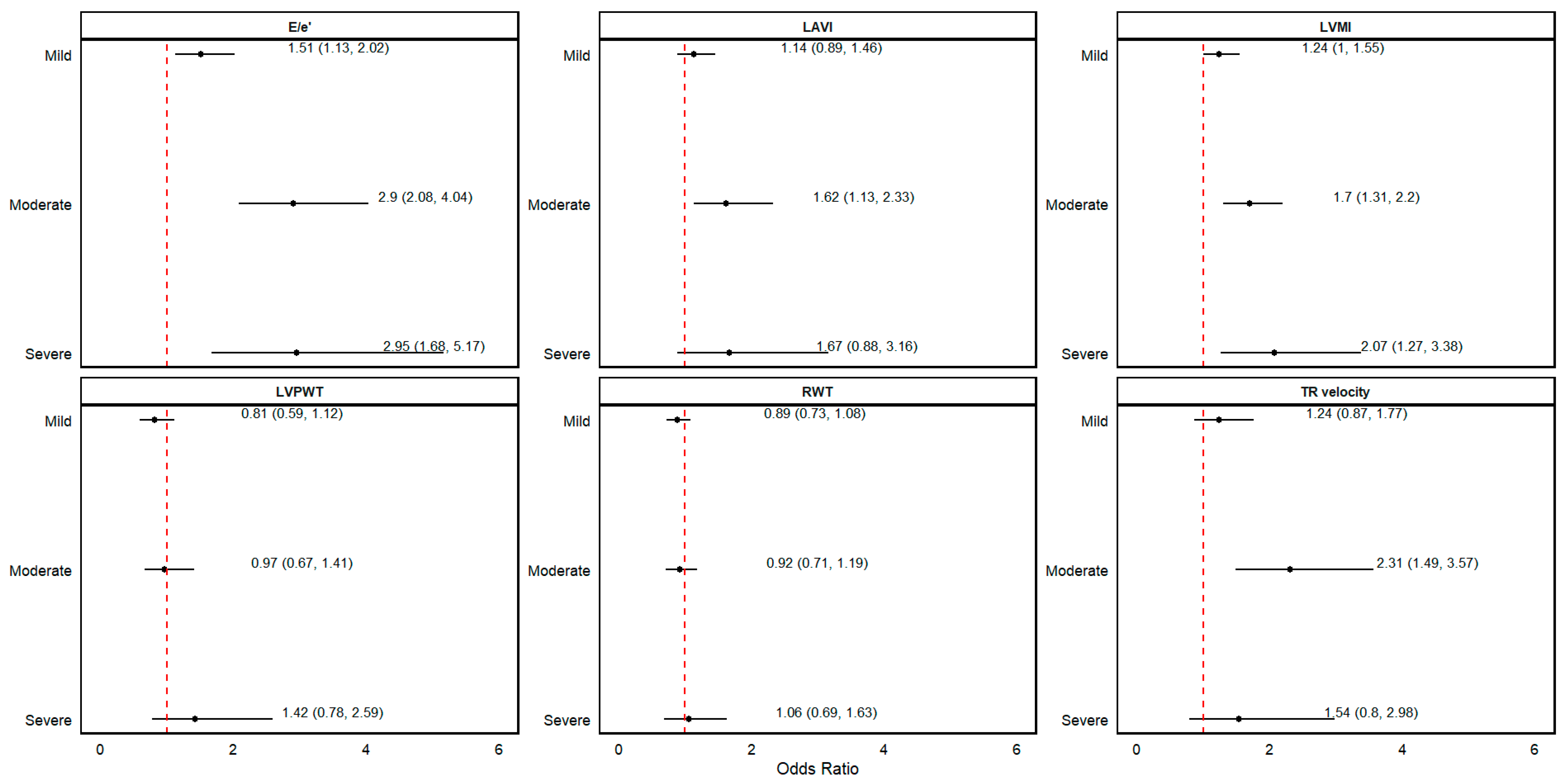
3.3. Association between Kidney Function and LVDD
3.4. Sensitivity Analysis
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Lamprea-Montealegre, J.A.; Shlipak, M.G.; Estrella, M.M. Chronic kidney disease detection, staging and treatment in cardiovascular disease prevention. Heart 2021, 107, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.M.; Dudley, S.C., Jr. Diastolic dysfunction. Circ. J. 2015, 79, 470–477. [Google Scholar] [CrossRef]
- Nadruz, W.; Shah, A.M.; Solomon, S.D. Diastolic Dysfunction and Hypertension. Med. Clin. N. Am. 2017, 101, 7–17. [Google Scholar] [CrossRef]
- Edwards, N.C.; Price, A.M.; Steeds, R.P.; Ferro, C.J.; Townend, J.N.; Group, B.C.-R. Management of heart failure in patients with kidney disease—Updates from the 2021 ESC guidelines. Nephrol. Dial. Transplant. 2023, 38, 1798–1806. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Sturdà, F.; Russo, R.; Basile, P.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Bertandino, F.; Monitillo, F.; et al. Assessment and management of heart failure in patients with chronic kidney disease. Heart Fail. Rev. 2023, 29, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, B.; Lee, J.Y.; Kim, J.S.; Han, B.G.; Choi, S.O.; Yang, J.W. Tissue Doppler-derived E/e’ ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J. Intern. Med. 2013, 28, 35–44. [Google Scholar] [CrossRef]
- Galderisi, M. Diastolic dysfunction and diastolic heart failure: Diagnostic, prognostic and therapeutic aspects. Cardiovasc. Ultrasound 2005, 3, 9. [Google Scholar] [CrossRef]
- Ogawa, T.; Nitta, K. Clinical Impact of Left Ventricular Diastolic Dysfunction in Chronic Kidney Disease. Contrib. Nephrol. 2018, 195, 81–91. [Google Scholar] [CrossRef]
- Romejko, K.; Rymarz, A.; Szamotulska, K.; Bartoszewicz, Z.; Rozmyslowicz, T.; Niemczyk, S. Left Ventricular Diastolic Dysfunction in Chronic Kidney Disease Patients Not Treated with Dialysis. Nutrients 2022, 14, 4664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yang, J.W.; Yoo, J.S.; Choi, S.O.; Han, B.G. Association between E/e’ ratio and fluid overload in patients with predialysis chronic kidney disease. PLoS ONE 2017, 12, e0184764. [Google Scholar] [CrossRef] [PubMed]
- Nerpin, E.; Ingelsson, E.; Risérus, U.; Sundström, J.; Andren, B.; Jobs, E.; Larsson, A.; Lars, L.; Ärnlöv, J. The association between glomerular filtration rate and left ventricular function in two independent community-based cohorts of elderly. Nephrol. Dial. Transplant. 2014, 29, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Kitzman, D.W.; Davies, M.J.; Rasmussen, S.; Barros, E.; Butler, J.; Einfeldt, M.N.; Hovingh, G.K.; Møller, D.V.; Petrie, M.C.; et al. Semaglutide in HFpEF across obesity class and by body weight reduction: A prespecified analysis of the STEP-HFpEF trial. Nat. Med. 2023, 29, 2358–2365. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Stachteas, P.; Patoulias, D.; Bougioukas, K.I.; Fragakis, N. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: An overview of 36 systematic reviews. Heart Fail. Rev. 2023, 28, 1033–1051. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef]
- Overmars, L.M.; Niemantsverdriet, M.S.A.; Groenhof, T.K.J.; De Groot, M.C.H.; Hulsbergen-Veelken, C.A.R.; Van Solinge, W.W.; Musson, R.E.A.; Ten Berg, M.J.; Hoefer, I.E.; Haitjema, S. A Wolf in Sheep’s Clothing: Reuse of Routinely Obtained Laboratory Data in Research. J. Med. Internet Res. 2022, 24, e40516. [Google Scholar] [CrossRef]
- ten Berg, M.J.; Huisman, A.; van den Bemt, P.M.; Schobben, A.F.; Egberts, A.C.; van Solinge, W.W. Linking laboratory and medication data: New opportunities for pharmacoepidemiological research. Clin. Chem. Lab. Med. 2007, 45, 13–19. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Kang, E.; Lee, S.W.; Ryu, H.; Kang, M.; Kim, S.; Park, S.K.; Jung, J.Y.; Lee, K.B.; Han, S.H.; Ahn, C.; et al. Left Ventricular Diastolic Dysfunction and Progression of Chronic Kidney Disease: Analysis of KNOW-CKD Data. J. Am. Heart Assoc. 2022, 11, e025554. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, M.G.; Sahlén, A.; Ding, Z.P.; Ugander, M. Diastolic function and its association with diabetes, hypertension and age in an outpatient population with normal stress echocardiography findings. Cardiovasc. Ultrasound 2020, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 10 August 2023).
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Cho, G.Y. Diastolic dysfunction and chronic kidney disease. Korean J. Intern. Med. 2013, 28, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Jain, A.; Scott, C.; Chen, H.H. The renal-cardiac connection in subjects with preserved ejection fraction: A population based study. ESC Heart Fail. 2017, 4, 266–273. [Google Scholar] [CrossRef]
- Fitzpatrick, J.K.; Parikh, R.V.; Hamilton, S.A.; Ambrosy, A.P.; Tan, T.C.; Bansal, N.; Go, A.S. The association between changes in echocardiography and risk of heart failure hospitalizations and death in adults with chronic kidney disease. Sci. Rep. 2023, 13, 8863. [Google Scholar] [CrossRef]
- Hayashi, S.Y.; Rohani, M.; Lindholm, B.; Brodin, L.A.; Lind, B.; Barany, P.; Alvestrand, A.; Seeberger, A. Left ventricular function in patients with chronic kidney disease evaluated by colour tissue Doppler velocity imaging. Nephrol. Dial. Transplant. 2006, 21, 125–132. [Google Scholar] [CrossRef]
- Vernooij, R.W.M.; van Ommen, A.L.N.; Valstar, G.B.; Cramer, M.J.; Teske, A.J.; Menken, R.; Hofstra, L.; Rutten, F.H.; Bots, M.L.; den Ruijter, H.M.; et al. Association of mild kidney dysfunction with diastolic dysfunction and heart failure with preserved ejection fraction. ESC Heart Fail. 2024, 11, 315–326. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Do, C.V.; Dang, D.T.V.; Do, L.D.; Doan, L.H.; Dang, H.T.V. Progressive alterations of left atrial and ventricular volume and strain across chronic kidney disease stages: A speckle tracking echocardiography study. Front. Cardiovasc. Med. 2023, 10, 1197427. [Google Scholar] [CrossRef] [PubMed]
- Alaini, A.; Malhotra, D.; Rondon-Berrios, H.; Argyropoulos, C.P.; Khitan, Z.J.; Raj, D.S.C.; Rohrscheib, M.; Shapiro, J.I.; Tzamaloukas, A.H. Establishing the presence or absence of chronic kidney disease: Uses and limitations of formulas estimating the glomerular filtration rate. World J. Methodol. 2017, 7, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Han, B.G.; Seol, J.H.; Choi, S.; Shin, D.; Kim, J.S.; Kim, Y.H. Comparing Left Ventricular Diastolic Function between Peritoneal Dialysis and Non-Dialysis Patients with Stage 5 Chronic Kidney Disease: A Propensity Score-Matched Analysis. J. Clin. Med. 2023, 12, 5092. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Oh, K.-H.; Choi, K.H.; Oh, Y.K.; Ma, S.K.; Kim, S.W. Association of Left Ventricular Diastolic Dysfunction With Cardiovascular Outcomes in Patients With Pre-dialysis Chronic Kidney Disease: Findings From KNOW-CKD Study. Front. Cardiovasc. Med. 2022, 9, 844312. [Google Scholar] [CrossRef]
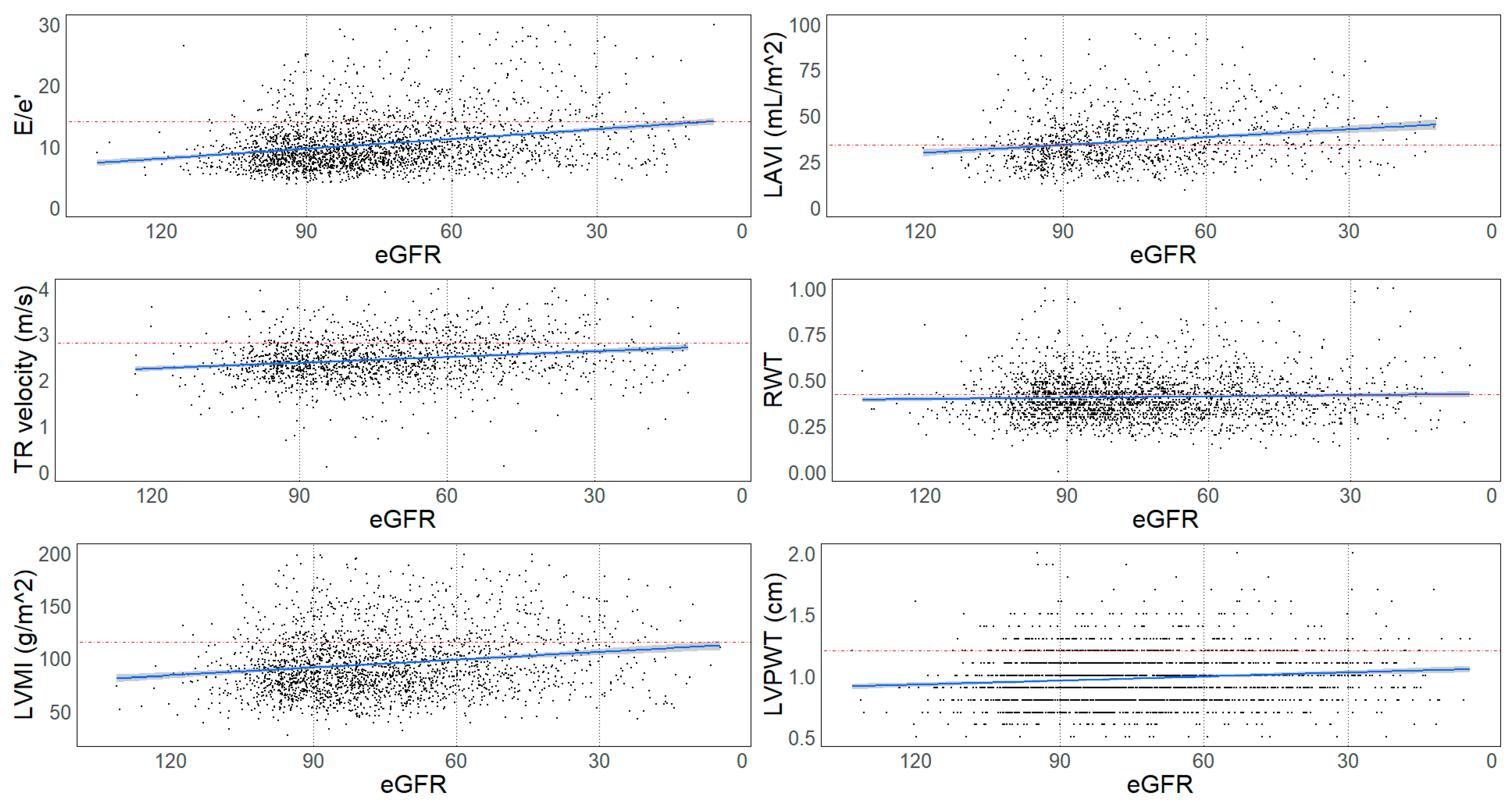
| Normal Kidney Function | Mildly Decreased Kidney Function | p * | Moderately Decreased Kidney Function | p ** | Severely Decreased Kidney Function | p *** | p **** | |
|---|---|---|---|---|---|---|---|---|
| N (%) | 1050 (26) | 2007 (50) | 818 (20%) | 147 (4%) | ||||
| Age (mean (SD) | 61.83 (11.50) | 66.95 (11.84) | <0.001 | 70.71 (11.43) | <0.001 | 71.01 (12.64) | <0.001 | <0.001 |
| Women (%) | 437 (41.6) | 850 (42.4) | 0.726 | 310 (37.9) | 0.114 | 69 (46.9) | 0.257 | 0.08 |
| Medical history | ||||||||
| Hypertension (%) | 663 (63.1) | 1271 (63.3) | 0.951 | 568 (69.4) | 0.005 | 125 (85.0) | <0.001 | <0.001 |
| Diabetes (%) | 91 (8.7) | 122 (6.1) | 0.009 | 88 (10.8) | 0.149 | 26 (17.7) | 0.001 | <0.001 |
| CVD (%) | 722 (68.8) | 1525 (76.0) | <0.001 | 693 (84.7) | <0.001 | 117 (79.6) | 0.010 | <0.001 |
| Heart failure (%) | 130 (12.4) | 316 (15.7) | 0.014 | 208 (25.4) | <0.001 | 17 (11.6) | 0.882 | <0.001 |
| COPD/asthma (%) | 285 (27.1) | 502 (25.0) | 0.217 | 276 (33.7) | 0.002 | 58 (39.5) | 0.003 | <0.001 |
| Laboratories | ||||||||
| eGFR (ml/min/1.73 m2), median (IQR) | 96.10 (92.92, 100.23) | 77.30 (69.80, 84.13) | <0.001 | 49.01 (41.20, 55.18) | <0.001 | 22.36 (17.77, 27.27) | <0.001 | <0.001 |
| Potassium (mmol/L), mean (SD) | 4.11 (0.32) | 4.18 (0.34) | <0.001 | 4.32 (0.40) | <0.001 | 4.52 (0.43) | <0.001 | <0.001 |
| CRP (mg/L), median (IQR) | 10.40 (2.30, 51.20) | 8.30 (2.20, 48.50) | 0.556 | 18.05 (3.80, 70.75) | <0.001 | 32.85 (6.78, 106.45) | <0.001 | <0.001 |
| Hba1c (mmol/mol), median (IQR) | 39.00 (35.15, 47.25) | 40.00 (36.00, 46.00) | 0.197 | 42.00 (38.00, 50.38) | <0.001 | 48.50 (38.62, 61.75) | 0.001 | <0.001 |
| Albumin (g/L), mean (SD) | 36.84 (6.18) | 37.23 (6.12) | 0.346 | 36.01 (6.97) | 0.102 | 33.65 (6.30) | <0.001 | <0.001 |
| Cholesterol (mmol/L), mean (SD) | 4.99 (1.39) | 4.83 (1.29) | 0.013 | 4.55 (1.34) | <0.001 | 4.55 (1.34) | 0.016 | <0.001 |
| HDL (mmol/L), median [IQR] | 1.20 [1.00, 1.50] | 1.20 [1.00, 1.55] | 0.770 | 1.10 [0.90, 1.30] | <0.001 | 1.20 [0.95, 1.40] | 0.057 | <0.001 |
| LDL (mmol/L), median [IQR] | 2.80 [2.10, 3.60] | 2.70 [2.00, 3.50] | 0.209 | 2.40 [1.78, 3.10] | <0.001 | 2.20 [1.70, 3.00] | 0.002 | <0.001 |
| Triglycerides (mmol/L), median [IQR] | 1.60 [1.10, 2.30] | 1.50 [1.10, 2.20] | 0.556 | 1.70 [1.20, 2.60] | 0.010 | 1.70 [1.20, 2.50] | 0.001 | 0.005 |
| Normal Kidney Function | Mildly Decreased Kidney Function | p * | Moderately Decreased Kidney Function | p ** | Severely Decreased Kidney Function | p *** | p **** | |
|---|---|---|---|---|---|---|---|---|
| N | 1050 | 2007 | 818 | 147 | ||||
| E/e′ > 14 (%) | 50 (4.8) | 179 (8.9) | <0.001 | 134 (16.4) | <0.001 | 22 (15.0) | <0.001 | <0.001 |
| LAVI > 34 mL/m2 | 120 (11.4) | 278 (13.9) | 0.011 | 133 (16.3) | <0.001 | 16 (10.9) | 0.001 | <0.001 |
| TR velocity > 2.8 m/s (%) | 46 (4.4) | 153 (7.6) | 0.001 | 131 (16.0) | <0.001 | 12 (8.2) | 0.128 | <0.001 |
| RWT > 0.42 (%) | 256 (24.4) | 495 (24.7) | 0.985 | 190 (23.2) | 0.003 | 41 (27.9) | 0.205 | 0.004 |
| LMVI >95 g/m2 in women or >115 g/m2 in men (%) | 129 (12.3) | 333 (16.6) | 0.002 | 169 (20.7) | <0.001 | 32 (21.8) | <0.001 | <0.001 |
| LVPWT > 1.2 (%) | 57 (5.4) | 96 (4.8) | 0.725 | 46 (5.6) | 0.005 | 14 (9.5) | 0.054 | 0.001 |
| eGFR | E/e′ | LAVI (mL/m2) | TR velocity (m/s) | RWT | LMVI (g/m2) | LVPWT (cm) |
|---|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Crude | ||||||
| eGFR | −1.3 (−1.5, −1.1) | −2.7 (−3.4, −1.9) | −0.08 (−0.11, −0.06) | −0.01 (−0.01, 0.00) | −5.6 (−6.9, −4.3) | −0.02 (−0.03, −0.01) |
| Model 1 | ||||||
| eGFR | −1.1 (−1.3, −0.87) | −1.9 (−2.7, −1.1) | −0.06 (−0.09, −0.04) | 0.00 (0.00, 0.01) | −4.8 (−6.1, −3.4) | −0.01 (−0.02, 0.00) |
| Model 2 | ||||||
| eGFR | −0.85 (−1.1, −0.62) | −1.3 (−2.1, −0.56) | −0.05 (−0.07, −0.03) | 0.00 (0.00, 0.01) | −3.1 (−4.3, −1.8) | −0.01 (−0.02, 0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras, C.P.; Dal Canto, E.; van Ommen, A.-M.L.; Handoko, M.L.; Haitjema, S.; de Groot, M.C.H.; Bots, M.L.; Verhaar, M.C.; Vernooij, R.W.M. Left Ventricular Diastolic Dysfunction across Levels of Kidney Function: A Cross-Sectional Study Based on Routine Clinical Practice Data. J. Clin. Med. 2024, 13, 5313. https://doi.org/10.3390/jcm13175313
Porras CP, Dal Canto E, van Ommen A-ML, Handoko ML, Haitjema S, de Groot MCH, Bots ML, Verhaar MC, Vernooij RWM. Left Ventricular Diastolic Dysfunction across Levels of Kidney Function: A Cross-Sectional Study Based on Routine Clinical Practice Data. Journal of Clinical Medicine. 2024; 13(17):5313. https://doi.org/10.3390/jcm13175313
Chicago/Turabian StylePorras, Cindy P., Elisa Dal Canto, Anne-Mar L. van Ommen, M. Louis Handoko, Saskia Haitjema, Mark C. H. de Groot, Michiel L. Bots, Marianne C. Verhaar, and Robin W. M. Vernooij. 2024. "Left Ventricular Diastolic Dysfunction across Levels of Kidney Function: A Cross-Sectional Study Based on Routine Clinical Practice Data" Journal of Clinical Medicine 13, no. 17: 5313. https://doi.org/10.3390/jcm13175313
APA StylePorras, C. P., Dal Canto, E., van Ommen, A.-M. L., Handoko, M. L., Haitjema, S., de Groot, M. C. H., Bots, M. L., Verhaar, M. C., & Vernooij, R. W. M. (2024). Left Ventricular Diastolic Dysfunction across Levels of Kidney Function: A Cross-Sectional Study Based on Routine Clinical Practice Data. Journal of Clinical Medicine, 13(17), 5313. https://doi.org/10.3390/jcm13175313







