Spinal Anesthesia for Awake Spine Surgery: A Paradigm Shift for Enhanced Recovery after Surgery
Abstract
:1. Introduction
2. Historical Background
2.1. The Introduction of Intrathecal Administration
2.2. Potential for Regional Blockade in Spinal Procedures
2.3. Modern Medicine and Awake Spine Surgery
3. Anesthesia for Awake Spine Surgery
3.1. Epidural Administration (EA)
3.2. Local Anesthesia (LA)
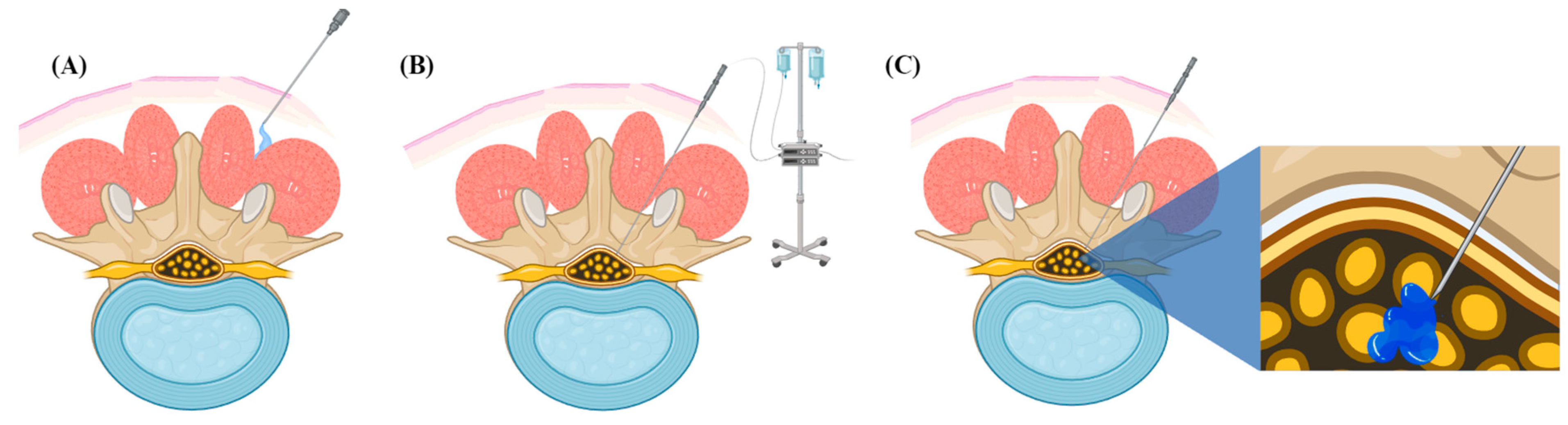
3.3. Spinal Anesthesia with Intrathecal Administration
3.4. Mechanisms
4. Overview of the Application of Awake Spine Surgery
4.1. Patient Selection and Anesthesia Considerations
4.2. Preoperative Stage
4.3. Intraoperative Management
4.4. Postoperative Management
5. Pitfalls Associated with Awake Spine Surgery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garg, B.; Ahuja, K.; Mehta, N.; Sharan, A.D. Awake Spinal Fusion. JBJS Rev. 2021, 9, e20.00163. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.W.; Tabarestani, T.Q.; Chaudhry, N.S.; Salven, D.S.; Shaffrey, C.I.; Bullock, W.M.; Guinn, N.R.; Gadsden, J.; Berger, M.; Abd-El-Barr, M.M. Awake Spinal Fusion Is Associated with Reduced Length of Stay, Opioid Use, and Time to Ambulation Compared to General Anesthesia: A Matched Cohort Study. World Neurosurg. 2023, 176, e91–e100. [Google Scholar] [CrossRef] [PubMed]
- Urick, D.; Sciavolino, B.; Wang, T.Y.; Gupta, D.K.; Sharan, A.; Abd-El-Barr, M.M. Perioperative Outcomes of General versus Spinal Anesthesia in the Lumbar Spine Surgery Population: A Systematic Review and Meta-Analysis of Data from 2005 through 2021. J. Clin. Orthop. Trauma 2022, 30, 101923. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Mehta, N.; Bansal, T.; Shekhar, S.; Khanna, P.; Baidya, D.K. Design and Implementation of an Enhanced Recovery after Surgery Protocol in Elective Lumbar Spine Fusion by Posterior Approach: A Retrospective, Comparative Study. Spine 2021, 46, E679. [Google Scholar] [CrossRef]
- Kolcun, J.P.G.; Brusko, G.D.; Basil, G.W.; Epstein, R.; Wang, M.Y. Endoscopic Transforaminal Lumbar Interbody Fusion without General Anesthesia: Operative and Clinical Outcomes in 100 Consecutive Patients with a Minimum 1-Year Follow-Up. Neurosurg. Focus 2019, 46, E14. [Google Scholar] [CrossRef]
- Letchuman, V.; Agarwal, N.; Mummaneni, V.P.; Wang, M.Y.; Shabani, S.; Patel, A.; Rivera, J.; Haddad, A.; Le, V.; Chang, J.M.; et al. Pearls and Pitfalls of Awake Spine Surgery: A Simplified Patient-Selection Algorithm. World Neurosurg. 2022, 161, 154–155. [Google Scholar] [CrossRef]
- De Biase, G.; Carter, R.E.; Otamendi-Lopez, A.; Garcia, D.; Chen, S.; Bojaxhi, E.; Quinones-Hinojosa, A.; Abode-Iyamah, K. Assessment of Surgeons’ Attitude towards Awake Spine Surgery under Spinal Anesthesia. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2023, 107, 48–53. [Google Scholar] [CrossRef]
- Wang, A.Y.; Liu, P.; Balonov, K.; Riesenburger, R.; Kryzanski, J. Use of Spinal Anesthesia in Lower Thoracic Spine Surgery: A Case Series. Oper. Neurosurg. 2022, 23, 298. [Google Scholar] [CrossRef]
- Chan, A.K.; Gnaedinger, A.; Ayoub, C.; Gupta, D.K.; Abd-El-Barr, M.M. The “In-Parallel” Technique for Awake, Bilateral Simultaneous Minimally Invasive Transforaminal Lumbar Interbody Fusion and Multilevel Lumbar Decompression. Oper. Neurosurg. Hagerstown Md 2023, 24, e160–e169. [Google Scholar] [CrossRef]
- De Biase, G.; Chen, S.; Akinduro, O.; Quinones-Hinojosa, A.; Abode-Iyamah, K. Awake Robotic Minimally Invasive L4-5 Transforaminal Lumbar Interbody Fusion. World Neurosurg. 2021, 148, 93. [Google Scholar] [CrossRef]
- De Biase, G.; Bechtle, P.; Leone, B.; Quinones-Hinojosa, A.; Abode-Iyamah, K. Awake Minimally Invasive Transforaminal Lumbar Interbody Fusion with a Pedicle-Based Retraction System. Clin. Neurol. Neurosurg. 2021, 200, 106313. [Google Scholar] [CrossRef]
- Huang, C.-C.; Fitts, J.; Huie, D.; Bhowmick, D.A.; Abd-El-Barr, M.M. Evolution of Cervical Endoscopic Spine Surgery: Current Progress and Future Directions-A Narrative Review. J. Clin. Med. 2024, 13, 2122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, D.; Zheng, G.; Luo, Y. Systematic Review and Meta-Analysis of the Effect of Nerve Block under Ultrasound in Ilioinguinal/Iliohypogastric in Children. Transl. Pediatr. 2022, 11, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, A.; Pavone, G.; Conticchio, M.; Piacente, C.; Varvara, M.; Ferraro, V.; Stasi, M.; Casella, A.; Filippo, R.; Tedeschi, M.; et al. Awake Robotic Liver Surgery: A Case Report. World J. Gastrointest. Surg. 2023, 15, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Costanzo, G.; Pellicciaro, M.; Materazzo, M.; Buonomo, C.; Federico, T.; Giacobbi, E.; Servadei, F.; Anemona, L.; Noce, A.; et al. Awake Breast Surgery: A Systematic Review. Vivo Athens Greece 2023, 37, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Ewart, L. “I’ve Just Got to Take That Risk and Have Faith…”: The Challenge of Gaining and Maintaining Trust in Patients Undergoing Knee Surgery with a Regional Anaesthetic. J. Perioper. Pract. 2024, 17504589241238847. [Google Scholar] [CrossRef]
- Wou, F.; Narayanan, M. Superior Trunk Block Catheter and 2% Chloroprocaine as a Phrenic Sparing Approach for Awake Arthroscopic Acromioclavicular Joint Surgery: A Case Report. Cureus 2024, 16, e55761. [Google Scholar] [CrossRef]
- Turhan, Ö.; Sivrikoz, N.; Duman, S.; Kara, M.; Sungur, Z. Postoperative Pulmonary Complications in Awake Video-Assisted Thoracoscopic Surgery: Our 10-Year Experience. Turk Gogus Kalp Damar Cerrahisi Derg. 2024, 32, 75–83. [Google Scholar] [CrossRef]
- Fiani, B.; Reardon, T.; Selvage, J.; Dahan, A.; El-Farra, M.H.; Endres, P.; Taka, T.; Suliman, Y.; Rose, A. Awake Spine Surgery: An Eye-Opening Movement. Surg. Neurol. Int. 2021, 12, 222. [Google Scholar] [CrossRef]
- Kehlet, H. Multimodal Approach to Control Postoperative Pathophysiology and Rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Bansal, T.; Sharan, A.D.; Garg, B. Enhanced Recovery after Surgery (ERAS) Protocol in Spine Surgery. J. Clin. Orthop. Trauma 2022, 31, 101944. [Google Scholar] [CrossRef] [PubMed]
- Soffin, E.M.; Vaishnav, A.S.; Wetmore, D.S.; Barber, L.; Hill, P.; Gang, C.H.; Beckman, J.D.; Albert, T.J.; Qureshi, S.A. Design and Implementation of an Enhanced Recovery after Surgery (ERAS) Program for Minimally Invasive Lumbar Decompression Spine Surgery: Initial Experience. Spine 2019, 44, E561. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Chang, P.-Y.; Grossman, J. Development of an Enhanced Recovery after Surgery (ERAS) Approach for Lumbar Spinal Fusion. J. Neurosurg. Spine 2017, 26, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Staartjes, V.E.; de Wispelaere, M.P.; Schröder, M.L. Improving Recovery after Elective Degenerative Spine Surgery: 5-Year Experience with an Enhanced Recovery after Surgery (ERAS) Protocol. Neurosurg. Focus 2019, 46, E7. [Google Scholar] [CrossRef]
- Venkata, H.K.; van Dellen, J.R. A Perspective on the Use of an Enhanced Recovery Program in Open, Non-Instrumented Day Surgery for Degenerative Lumbar and Cervical Spinal Conditions. J. Neurosurg. Sci. 2018, 62, 245–254. [Google Scholar] [CrossRef]
- Muhly, W.T.; Sankar, W.N.; Ryan, K.; Norton, A.; Maxwell, L.G.; DiMaggio, T.; Farrell, S.; Hughes, R.; Gornitzky, A.; Keren, R.; et al. Rapid Recovery Pathway after Spinal Fusion for Idiopathic Scoliosis. Pediatrics 2016, 137, e20151568. [Google Scholar] [CrossRef]
- Grasu, R.M.; Cata, J.P.; Dang, A.Q.; Tatsui, C.E.; Rhines, L.D.; Hagan, K.B.; Bhavsar, S.; Raty, S.R.; Arunkumar, R.; Potylchansky, Y.; et al. Implementation of an Enhanced Recovery after Spine Surgery Program at a Large Cancer Center: A Preliminary Analysis. J. Neurosurg. Spine 2018, 29, 588–598. [Google Scholar] [CrossRef]
- Ali, Z.S.; Ma, T.S.; Ozturk, A.K.; Malhotra, N.R.; Schuster, J.M.; Marcotte, P.J.; Grady, M.S.; Welch, W.C. Pre-Optimization of Spinal Surgery Patients: Development of a Neurosurgical Enhanced Recovery after Surgery (ERAS) Protocol. Clin. Neurol. Neurosurg. 2018, 164, 142–153. [Google Scholar] [CrossRef]
- Wulf, H.F.W. The Centennial of Spinal Anesthesia. Anesthesiology 1998, 89, 500–506. [Google Scholar] [CrossRef]
- Bier, A. Versuche über Cocainisirung des Rückenmarkes. Dtsch. Z. Für Chir. 1899, 51, 361–369. [Google Scholar] [CrossRef]
- Corning, J.L. Spinal Anaesthesia and Local Medication of the Cord. New York Med. J. 1885, 42, 483–485. [Google Scholar]
- Mandabach, M.G. The Early History of Spinal Anesthesia. Int. Congr. Ser. 2002, 1242, 163–168. [Google Scholar] [CrossRef]
- Minagar, A.; Lowis, G.W. Dr Heinrich Irenaeus Quincke (1842–1922): Clinical Neurologist of Kiel. J. Med. Biogr. 2001, 9, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Wynter, W.E. Four cases of tubercular meningitis in which paracentesis of the theca vertebralis was performed for the relief of fluid pressure. Lancet 1891, 137, 981–982. [Google Scholar] [CrossRef]
- Yeung, A.T.; Yeung, C.A. Advances in Endoscopic Disc and Spine Surgery: Foraminal Approach. Surg. Technol. Int. 2003, 11, 255–263. [Google Scholar] [PubMed]
- Yeung, A.T.; Yeung, C.A. In-Vivo Endoscopic Visualization of Patho-Anatomy in Painful Degenerative Conditions of the Lumbar Spine. Surg. Technol. Int. 2006, 15, 243–256. [Google Scholar]
- Yeung, A.T. Minimally Invasive Disc Surgery with the Yeung Endoscopic Spine System (YESS). Surg. Technol. Int. 1999, 8, 267–277. [Google Scholar]
- Wang, M.Y.; Grossman, J. Endoscopic Minimally Invasive Transforaminal Interbody Fusion without General Anesthesia: Initial Clinical Experience with 1-Year Follow-Up. Neurosurg. Focus 2016, 40, E13. [Google Scholar] [CrossRef]
- Garg, B.; Ahuja, K.; Sharan, A.D. Regional Anesthesia for Spine Surgery. J. Am. Acad. Orthop. Surg. 2022, 30, 809–819. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Wood, C.; Roulaud, M.; Page, P.; Lorgeoux, B.; Baron, S.; Nivole, K.; Many, M.; et al. Combining Awake Anesthesia with Minimal Invasive Surgery Optimizes Intraoperative Surgical Spinal Cord Stimulation Lead Placement. J. Clin. Med. 2022, 11, 5575. [Google Scholar] [CrossRef]
- Ajayan, N.; Hrishi, A.P.; Rath, G.P. Anesthesia for Same Day Neurosurgery with Updates on Awake Craniotomy and Awake Spine Surgery. Curr. Opin. Anesthesiol. 2023, 36, 500. [Google Scholar] [CrossRef]
- Turcotte, J.J.; Gelfand, J.M.; Jones, C.M.; Jackson, R.S. Development of a Low-Resource Operating Room and a Wide-Awake Orthopedic Surgery Program during the COVID-19 Pandemic. Surg. Innov. 2021, 28, 183–188. [Google Scholar] [CrossRef]
- Rizzo, P.; Hann, H.; Coombs, B.; Ali, A.A.H.; Stretton, A.; Sikander, M. The Hitchhiker’s Guide to Spine Awake Surgery. The Oxford SAS Protocol and Early Outcomes. World Neurosurg. 2023, 176, e289–e296. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Park, J.H.; Hyun, S.-J.; Hodel, D.; Hausmann, O.N. Regional Anesthesia for Lumbar Spine Surgery: Can It Be a Standard in the Future? Neurospine 2021, 18, 733–740. [Google Scholar] [CrossRef]
- Patel, T.D.; McNicholas, M.N.; Paschell, P.A.; Arnold, P.M.; Lee, C.-T. Thoracolumbar Interfascial Plane (TLIP) Block Verses Other Paraspinal Fascial Plane Blocks and Local Infiltration for Enhanced Pain Control after Spine Surgery: A Systematic Review. BMC Anesthesiol. 2024, 24, 122. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, R.J.C.; Koopman, J.S.H.A.; Postema, J.M.C.; Verberkmoes, N.J.; Chin, K.J.; Bouwman, R.A.; Versyck, B.J.B. Continuous Erector Spinae Plane Block versus Thoracic Epidural Analgesia in Video-Assisted Thoracic Surgery: A Study Protocol for a Prospective Randomized Open Label Non-Inferiority Trial. Trials 2021, 22, 321. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.; Gore, S. Endoscopic Foraminal Decompression for Failed Back Surgery Syndrome under Local Anesthesia. Int. J. Spine Surg. 2014, 8, 22. [Google Scholar] [CrossRef]
- Feng, W.-L.; Yang, J.-S.; Wei, D.; Gong, H.-L.; Xi, Y.; Lv, H.-Q.; Wang, X.-G.; Xia, B.; Wei, J.-M. Gradient Local Anesthesia for Percutaneous Endoscopic Interlaminar Discectomy at the L5/S1 Level: A Feasibility Study. J. Orthop. Surg. 2020, 15, 413. [Google Scholar] [CrossRef]
- Azad, T.D.; Alomari, S.; Khalifeh, J.M.; Ahmed, A.K.; Musharbash, F.N.; Mo, K.; Lubelski, D.; Witham, T.F.; Bydon, A.; Theodore, N. Adoption of Awake Spine Surgery-Trends from a National Registry over 14 Years. Spine J. 2022, 22, 1601–1609. [Google Scholar] [CrossRef]
- Prabhakar, N.K.; Chadwick, A.L.; Nwaneshiudu, C.; Aggarwal, A.; Salmasi, V.; Lii, T.R.; Hah, J.M. Management of Postoperative Pain in Patients Following Spine Surgery: A Narrative Review. Int. J. Gen. Med. 2022, 15, 4535–4549. [Google Scholar] [CrossRef]
- Mooney, J.; Erickson, N.; Salehani, A.; Laskay, N.; Mahavadi, A.; Ilyas, A.; Mainali, B.; Agarwal, N.; Godzik, J. Microendoscopic Lumbar Discectomy with General versus Local Anesthesia: A Systematic Review and Meta-Analysis. N. Am. Spine Soc. J. NASSJ 2022, 10, 100129. [Google Scholar] [CrossRef]
- Mooney, J.; Laskay, N.; Erickson, N.; Salehani, A.; Mahavadi, A.; Ilyas, A.; Mainali, B.; Nowak, B.; Godzik, J. General vs. Local Anesthesia for Percutaneous Endoscopic Lumbar Discectomy (PELD): A Systematic Review and Meta-Analysis. Glob. Spine J. 2023, 13, 1671–1688. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.; Erickson, N.; Laskay, N.; Salehani, A.; Mahavadi, A.; Ilyas, A.; Mainali, B.; Godzik, J. Epidural Versus Local Anesthesia for Percutaneous Endoscopic Lumbar Discectomy: A Systematic Review and Meta-Analysis. Clin. Spine Surg. 2023, 36, 458–469. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhao, J.; Chen, C.; Sun, X.; Zhang, B.; Yang, Q. Comparing the Effectiveness and Safety between Local Anesthesia versus Epidural Anesthesia for Percutaneous Transforaminal Endoscopic Discectomy: A Systematic Review and Meta-Analysis. World Neurosurg. 2022, 166, e528–e535. [Google Scholar] [CrossRef]
- Zheng, B.; Guo, C.; Xu, S.; Li, H.; Wu, Y.; Liu, H. Anesthesia Methods for Full-Endoscopic Lumbar Discectomy: A Review. Front. Med. 2023, 10, 1193311. [Google Scholar] [CrossRef] [PubMed]
- Towne, E.B. Laminectomy and removal of spinal cord tumors under local anesthesia. Calif. West. Med. 1926, 24, 194–197. [Google Scholar]
- Nygaard, O.P.; Romner, B.; Thoner, J.; Due-Tønnessen, B. Local Anesthesia in Posterior Cervical Surgery. Anesthesiology 1997, 86, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.V.; Kaptan, A.Y.; Orhan, Ö.; Altay, M.A.; Altay, N.; Demir, S.; Demir, M. Comparison of the Effectiveness of the WALANT Method in Soft Tissue and Bone Tissue Surgeries in Lower Extremities. Jt. Dis. Relat. Surg. 2023, 34, 439–444. [Google Scholar] [CrossRef]
- Ayhan, E.; Akaslan, F. Patients’ Perspective on Carpal Tunnel Release with WALANT or Intravenous Regional Anesthesia. Plast. Reconstr. Surg. 2020, 145, 1197–1203. [Google Scholar] [CrossRef]
- Ramos, P.R.; Sakata, R.K.; Ribeiro, H.C.; Bonfanti, A.; Ferraro, L.H. da C. A Prospective, Comparative Study of the Analgesic Effect between the WALANT Technique and Local Anesthesia Associated with Sedation for Hand Surgery. Acta Cir. Bras. 2023, 38, e384323. [Google Scholar] [CrossRef]
- Kot, P.; Rodriguez, P.; Granell, M.; Cano, B.; Rovira, L.; Morales, J.; Broseta, A.; Andrés, J.D. The Erector Spinae Plane Block: A Narrative Review. Korean J. Anesthesiol. 2019, 72, 209–220. [Google Scholar] [CrossRef]
- Rajjoub, R.; Ghaith, A.K.; El-Hajj, V.G.; Rios-Zermano, J.; De Biase, G.; Atallah, E.; Tfaily, A.; Saad, H.; Akinduro, O.O.; Elmi-Terander, A.; et al. Comparative Outcomes of Awake Spine Surgery under Spinal versus General Anesthesia: A Comprehensive Systematic Review and Meta-Analysis. Eur. Spine J. 2024, 33, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Waguia, R.; Touko, E.K.; Sykes, D.A.W.; Kelly-Hedrick, M.; Hijji, F.Y.; Sharan, A.D.; Foster, N.; Abd-El-Barr, M.M. How to Start an Awake Spine Program: Protocol and Illustrative Cases. IBRO Neurosci. Rep. 2022, 13, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.-H.; Choy, W.; Miller, C.A.; Robinson, L.C.; Mummaneni, P.V. A Novel Technique for Awake, Minimally Invasive Transforaminal Lumbar Interbody Fusion: Technical Note. Neurosurg. Focus 2019, 46, E16. [Google Scholar] [CrossRef]
- Openanesthesia Spinal Anesthesia in Adults: Pharmacology, Technique, and Side Effects. Available online: https://www.openanesthesia.org/keywords/spinal-anesthesia-in-adults-pharmacology-technique-and-side-effects/ (accessed on 20 April 2024).
- Jadczak, C.N.; Vanjani, N.N.; Pawlowski, H.; Cha, E.D.K.; Lynch, C.P.; Prabhu, M.C.; Hartman, T.J.; Nie, J.W.; MacGregor, K.R.; Zheng, E.; et al. The Current Status of Awake Endoscopic Surgery: A Systematic Review and Meta-Analysis. World Neurosurg. 2023, 180, e198–e209. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.W.; Tabarestani, T.Q.; Salven, D.S.; Chaudhry, N.S.; Wang, T.Y.; Gottfried, O.N.; Shaffrey, C.I.; Guinn, N.R.; Gadsden, J.; Ayoub, C.M.; et al. Awake Spinal Anesthesia Facilitates Spine Surgery in Poor Surgical Candidates: A Case Series. Neurochirurgie 2023, 69, 101444. [Google Scholar] [CrossRef]
- Hausman, M.S.J.; Jewell, E.S.; Engoren, M. Regional Versus General Anesthesia in Surgical Patients with Chronic Obstructive Pulmonary Disease: Does Avoiding General Anesthesia Reduce the Risk of Postoperative Complications? Anesth. Analg. 2015, 120, 1405. [Google Scholar] [CrossRef]
- De Biase, G.; Gruenbaum, S.E.; West, J.L.; Chen, S.; Bojaxhi, E.; Kryzanski, J.; Quiñones-Hinojosa, A.; Abode-Iyamah, K. Spinal versus General Anesthesia for Minimally Invasive Transforaminal Lumbar Interbody Fusion: Implications on Operating Room Time, Pain, and Ambulation. Neurosurg. Focus 2021, 51, E3. [Google Scholar] [CrossRef]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A Review of ASA Physical Status–Historical Perspectives and Modern Developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef]
- Berthoud, M.C.; Reilly, C.S. Adverse Effects of General Anaesthetics. Drug Saf. 1992, 7, 434–459. [Google Scholar] [CrossRef]
- Scharwächter, W.H.; Keet, S.W.M.; Stoecklein, K.; Loer, S.A.; Boer, C. Health Risk Factors in the Anesthesia Population. J. Clin. Anesth. 2016, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, Y. Mechanisms of Anesthetic Actions and the Brain. J. Anesth. 2007, 21, 187–199. [Google Scholar] [CrossRef]
- Perez-Roman, R.J.; Govindarajan, V.; Bryant, J.-P.; Wang, M.Y. Spinal Anesthesia in Awake Surgical Procedures of the Lumbar Spine: A Systematic Review and Meta-Analysis of 3709 Patients. Neurosurg. Focus 2021, 51, E7. [Google Scholar] [CrossRef]
- Fong, T.G.; Inouye, S.K. The Inter-Relationship between Delirium and Dementia: The Importance of Delirium Prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.D.; Feng, R.; Carson, J.L.; Gaskins, L.J.; Dillane, D.; Sessler, D.I.; Frederick, S.; Jay, M.-Z.; Edward, R.M.; Samir, M.; et al. Spinal Anesthesia or General Anesthesia for Hip Surgery in Older Adults. N. Engl. J. Med. 2021, 385, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.; Sundaram, K.; Anis, H.; Kamath, A.F.; Mont, M.A.; Higuera, C.A.; Piuzzi, N.S. Spinal Anesthesia Is Associated with Decreased Complications after Total Knee and Hip Arthroplasty. JAAOS-J. Am. Acad. Orthop. Surg. 2020, 28, e213. [Google Scholar] [CrossRef]
- Ma, N.; Yi, P.; Xiong, Z.; Ma, H.; Tan, M.; Tang, X. Efficacy and Safety of Perioperative Use of Non-Steroidal Anti-Inflammatory Drugs for Preemptive Analgesia in Lumbar Spine Surgery: A Systematic Review and Meta-Analysis. Perioper. Med. Lond. Engl. 2023, 12, 61. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, X.; Guan, L.C.; Zhang, C.; Liu, J.; Ford, N.C.; He, S.; Chen, X.; Cao, X.; Zang, L.; et al. Chronic Pain after Spine Surgery: Insights into Pathogenesis, New Treatment, and Preventive Therapy. J. Orthop. Transl. 2023, 42, 147–159. [Google Scholar] [CrossRef]
- Rivkin, A.; Rivkin, M.A. Perioperative Nonopioid Agents for Pain Control in Spinal Surgery. Am. J. Health-Syst. Pharm. 2014, 71, 1845–1857. [Google Scholar] [CrossRef]
- Wehab, Z.; Tabarestani, T.Q.; Abd-El-Barr, M.M.; Husain, A.M. Intraoperative Electromyography in Awake Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Case Study on Nerve Activation Under the Effects of Local Anesthesia. J. Clin. Neurophysiol. 2022, 39, e26–e29. [Google Scholar] [CrossRef]
- Letchuman, V.; Agarwal, N.; Mummaneni, V.P.; Wang, M.Y.; Shabani, S.; Patel, A.; Rivera, J.; Haddad, A.F.; Le, V.; Chang, J.M.; et al. Awake Spinal Surgery: Simplifying the Learning Curve with a Patient Selection Algorithm. Neurosurg. Focus 2021, 51, E2. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year of Publication | Summary |
|---|---|---|
| Yeung et al. [47] | 2014 |
|
| Wang et al. [38] | 2016 |
|
| Kolcun et al. [5] | 2019 |
|
| Feng et al. [48] | 2020 |
|
| De Biase et al. [11] | 2021 |
|
| De Biase et al. [10] | 2021 |
|
| Azad et al. [49] | 2022 |
|
| Chan et al. [9] | 2023 |
|
| Patel et al. [45] | 2024 |
|
| Protocol | Medications | Goals | |
|---|---|---|---|
| Preoperative Stage | Administration of preoperative analgesics | Methocarbamol, Gabapentin, Acetaminophen | Initial administration to minimize postoperative pain |
| Intraoperative Stage | Begins with a single-injection administration of spinal anesthesia to the intradural space. Patients may be given a second injection intraoperatively if they complain of discomfort. | Bupivacaine (most widely used) or lidocaine, mild sedation maintained via midazolam | Keep the patient in a semi-conscious, mildly sedated state that prevents limb rigidity but avoids the need for intubation |
| Postoperative Stage | Postoperative care consists of analgesics, physical therapy, and monitoring | Methocarbamol, Gabapentin, Acetaminophen | Begin physical therapy with the goal of having the patient ambulate by postop day 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, J.P.; Bonin, B.; Quinones, C.; Kumbhare, D.; Guthikonda, B.; Hoang, S. Spinal Anesthesia for Awake Spine Surgery: A Paradigm Shift for Enhanced Recovery after Surgery. J. Clin. Med. 2024, 13, 5326. https://doi.org/10.3390/jcm13175326
Wilson JP, Bonin B, Quinones C, Kumbhare D, Guthikonda B, Hoang S. Spinal Anesthesia for Awake Spine Surgery: A Paradigm Shift for Enhanced Recovery after Surgery. Journal of Clinical Medicine. 2024; 13(17):5326. https://doi.org/10.3390/jcm13175326
Chicago/Turabian StyleWilson, John Preston, Bryce Bonin, Christian Quinones, Deepak Kumbhare, Bharat Guthikonda, and Stanley Hoang. 2024. "Spinal Anesthesia for Awake Spine Surgery: A Paradigm Shift for Enhanced Recovery after Surgery" Journal of Clinical Medicine 13, no. 17: 5326. https://doi.org/10.3390/jcm13175326
APA StyleWilson, J. P., Bonin, B., Quinones, C., Kumbhare, D., Guthikonda, B., & Hoang, S. (2024). Spinal Anesthesia for Awake Spine Surgery: A Paradigm Shift for Enhanced Recovery after Surgery. Journal of Clinical Medicine, 13(17), 5326. https://doi.org/10.3390/jcm13175326






