Intrauterine Shaping of Fetal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
3. Discussion
3.1. Differentiation of Views—Sterile (Acquired at Birth)/Non-Sterile Environment
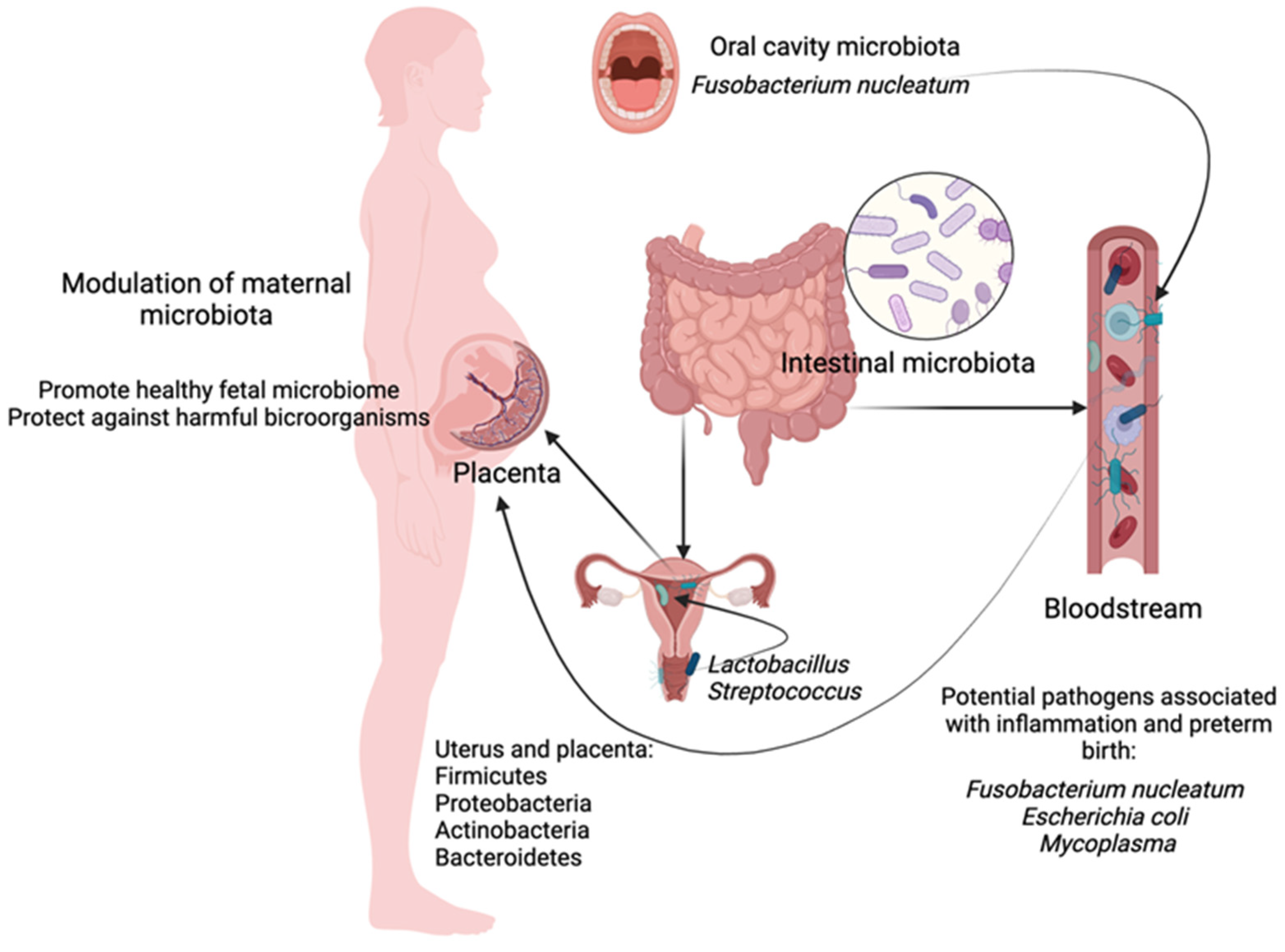
3.2. The Way of the Colonization of the Uterus: By Continuity, by Blood
3.3. The Microbiota of the Oral Cavity and the Placenta of a Pregnant Woman
3.4. Maternal Placental and Uterine Microbiome
3.5. Meconium Microbiota Depending on Gestational Age and Specific Diseases
3.6. The Intestinal Microbiome in Newborns
3.7. Influence of the Intrauterine Microbiome on the Child’s Immune System
3.8. Pregnancy Complications—Pre-Eclampsia and Its Spectrum
4. Conclusions
Funding
Conflicts of Interest
References
- Gałęcka, M.; Basińska, A.M.; Bartnicka, A. Znaczenie mikrobioty jelitowej w kształtowaniu zdrowia człowieka—Implikacje w praktyce lekarza rodzinnego. Forum Med. Rodz. 2018, 12, 50–59. [Google Scholar]
- Healy, D.B.; Ryan, C.A.; Ross, R.P.; Stanton, C. Clinical implications of preterm infant gut microbiome development. Nat. Microbiol. 2022, 7, 22–33. [Google Scholar] [CrossRef]
- Indrio, F.; Neu, J.; Pettoello-Mantovani, M. Development of the Gastrointestinal Tract in Newborns as a Challenge for an Appropriate Nutrition: A Narrative Review. Nutrients 2022, 14, 1405. [Google Scholar] [CrossRef] [PubMed]
- Weström, B.; Sureda, E.A.; Pierzynowska, K. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, L.; Han, Y.; Wu, L.; Wang, B. The Development of Early Life Microbiota in Human Health and Disease. Engineering 2022, 12, 101–114. [Google Scholar] [CrossRef]
- Campisciano, G.; Quadrifoglio, M.; Comar, M.; De Seta, F. Evidence of bacterial DNA presence in chorionic villi and amniotic fluid in the first and second trimester of pregnancy. Future Microbiol. 2021, 16, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sharlandjieva, V.; Beristain, A.G.; Terry, J. Assessment of the human placental microbiome in early pregnancy. Front. Med. 2023, 10, 1096262. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, A.; Gałęcka, M.; Mazela, J. Wpływ czynników prenatalnych i postnatalnych na mikrobiotę jelitową noworodków. Stand. Med. Pediatr. 2016, 13, 112–116. [Google Scholar]
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef]
- Lee, J.K.-F.; Hern Tan, L.T.; Ramadas, A.; Ab Mutalib, N.-S.; Lee, L.-H. Exploring the Role of Gut Bacteria in Health and Disease in Preterm Neonates. Int. J. Environ. Res. Public. Health 2020, 17, 6963. [Google Scholar] [CrossRef] [PubMed]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Gopel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. Biomed. Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef] [PubMed]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.C.; Salari, R.C.; Saxena, D.; Davidson, L.; O’Toole, G.A.; Moore, J.H.; Sogin, M.L.; Foster, J.A.; Edwards, W.H.; Palumbo, P.; et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch. Dis. Child. Fetal Neonat. 2012, 97, 456–462. [Google Scholar] [CrossRef]
- Carl, M.A.; Ndao, I.M.; Springman, A.C.; Manning, S.D.; Johnson, J.R.; Johnston, B.D.; Burnham, C.A.; Weinstock, E.S.; Weinstock, G.M.; Wylie, T.N.; et al. Sepsis from the gut: The enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin. Infect. Dis. 2014, 58, 1211–1218. [Google Scholar] [CrossRef]
- Shaw, A.G.; Sim, K.; Randell, P.; Cox, M.J.; McClure, Z.E.; Li, M.S.; Donaldson, H.; Langford, P.R.; Cookson, W.O.; Moffatt, M.F.; et al. Late-Onset Bloodstream Infection and Perturbed Maturation of the Gastrointestinal Microbiota in Premature Infants. PLoS ONE 2015, 10, e0132923. [Google Scholar] [CrossRef]
- Mai, V.; Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Li, N.; Shuster, J.; Sharma, R.; Hudak, M.L.; Neu, J. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 2013, 8, e52876. [Google Scholar] [CrossRef]
- Groer, M.; Luciano, A.A.; Dishaw, L.; Ashmeade, T.; Miller, E.; Gilbert, J. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef]
- Younge, N.E.; Newgard, C.B.; Cotten, C.M.; Goldberg, R.N.; Muehlbauer, M.J.; Bain, J.R.; Stevens, R.D.; O’Connell, T.M.; Rawls, J.F.; Seed, P.C.; et al. Disrupted Maturation of the Microbiota and Metabolome among Extremely Preterm Infants with Postnatal Growth Failure. Sci. Rep. 2019, 9, 8167. [Google Scholar] [CrossRef]
- Henderickx, J.G.E.; Zwittink, R.D.; van Lingen, R.A.; Knol, J.; Belzer, C. The Preterm Gut Microbiota: An Inconspicuous Challenge in Nutritional Neonatal Care. Front. Cell. Infect. Microbiol. 2019, 9, 85. [Google Scholar] [CrossRef]
- Lee, J.K.; Yu, V.Y. Calorie intake in sick versus respiratory stable very low birthweight babies. Pediatr. Int. 1996, 38, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.; Wynn, J.L.; Weitkamp, J.-H. Infectious causes of necrotizing enterocolitis. Clin. Perinatol. 2015, 42, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Kosloske, A.M.; Ulrich, J.A. A bacteriologic basis for the clinical presentations of necrotizing enterocolitis. J. Pediatr. Surg. 1980, 15, 558–564. [Google Scholar] [CrossRef]
- Kosloske, A.; Ulrich, J.; Hoffman, H. Fulminant necrotising enterocolitis associated with clostridia. Lancet 1978, 312, 1014–1016. [Google Scholar] [CrossRef]
- Lindberg, T.P.; Caimano, M.J.; Hagadorn, J.I.; Bennett, E.M.; Maas, K.; Brownell, E.A.; Matson, A.P. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J. Matern.-Fetal Neonatal Med. 2020, 33, 349–358. [Google Scholar] [CrossRef]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2022, 65, 438–447. [Google Scholar] [CrossRef]
- Houghteling, P.D.; Walker, W.A. Why is initial bacterial colonization of the intestine important to the infant’s and child’s health? J. Pediatr. Gastroenterol. Nutr. 2015, 60, 294–307. [Google Scholar] [CrossRef]
- Bardos, J.; Fiorentino, D.; Longman, R.E.; Paidas, M. Immunological Role of the Maternal Uterine Microbiome in Pregnancy: Pregnancies Pathologies and Alterated Microbiota. Front. Immunol. 2019, 10, 2823. [Google Scholar] [CrossRef]
- NIH Hmp Working Group; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH human microbiome project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16s ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, C.; Wei, W.; Wang, Z.; Dai, J.; Hao, L.; Song, L.; Zhang, X.; Zeng, L.; Du, H.; et al. The metagenome of the female upper reproductive tract. Gigascience 2018, 7, Giy107. [Google Scholar] [CrossRef] [PubMed]
- Donnet-Hughes, A.; Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr. Soc. 2010, 69, 407–415. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Hanley, J. Child health neonatal infections: Group B streptococcus search date March 2007 prophylactic treatment of at-risk neonates: GBS child health neonatal infections: Group B streptococcus. Clin. Evid. 2008, 1, 1–6. [Google Scholar]
- Kliman, H.J. Comment on the placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 254le4. [Google Scholar] [CrossRef]
- Garmi, G.; Okopnik, M.; Keness, Y.; Zafran, N.; Berkowitz, E.; Salim, R. Correlation between clinical, placental histology and microbiological findings in spontaneous preterm births. Fetal Diagn. Ther. 2016, 40, 141–149. [Google Scholar] [CrossRef]
- Sweeney, E.L.; Kallapur, S.G.; Gisslen, T.; Lambers, D.S.; Chougnet, C.A.; Stephenson, S.-A.; Jobe, A.H.; Knox, C.L. Placental infection with ureaplasma species is associated with histologic chorioamnionitis and adverse outcomes in moderately preterm and late-preterm infants. J. Infect. Dis. 2016, 213, 1340–1347. [Google Scholar] [CrossRef]
- Quinn, P.A.; Butany, J.; Taylor, J.; Hannah, W. Chorioamnionitis: Its association with pregnancy outcome and microbial infection. Am. J. Obstet. Gynecol. 1987, 156, 379–387. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Sood, R.; Zehnder, J.L.; Druzin, M.L.; Brown, P.O. Gene expression patterns in human placenta. Proc. Natl. Acad. Sci. USA 2006, 103, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Egbase, P.E.; al-Sharhan, M.; al-Othman, S.; al-Mutawa, M.; Udo, E.E.; Grudzinskas, J.G. Incidence of microbial growth from the tip of the embaryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization. Hum. Reprod. 1996, 11, 1687–1689. [Google Scholar] [CrossRef]
- Heinonen, P.K.; Teisala, K.; Punnonen, R.; Miettinen, A.; Lehtinen, M.; Paavonen, J. Anatomic sites of upper genital tract infection. Obstet. Gynecol. 1985, 66, 384–390. [Google Scholar]
- Eschenbach, D.A.; Rosene, K.; Tompkins, L.S.; Watkins, H.; Gravett, M.G. Endometrial cultures obtained by a triple-lumen method from afebrile and febrile postpartum women. J. Infect. Dis. 1986, 153, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Hemsell, D.L.; Obregon, V.L.; Heard, M.C.; Nobles, B.J. Endometrial bacteria in asymptomatic, nonpregnant women. J. Reprod. Med. 1989, 34, 872–874. [Google Scholar] [PubMed]
- Moller, B.R.; Kristiansen, F.V.; Thorsen, P.; Frost, L.; Mogensen, S.C. Sterility of the uterine cavity. Acta Obstet. Gynecol. Scand. 1995, 74, 216–219. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–189. [Google Scholar] [CrossRef]
- Leon, R.; Silva, N.; Ovalle, A.; Chaparro, A.; Ahumada, A.; Gajardo, M.; Martinez, M.; Gamonal, J. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J. Periodontol. 2007, 78, 1249–1255. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Lahra, M.M.; Beeby, P.J.; Jeffery, H.E. Maternal versus fetal inflammation and respiratory distress syndrome: A 10-year hospital cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F13–F16. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Kim, C.J.; et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol. 2014, 71, 330–358. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef]
- Cobb, C.M.; Kelly, P.J.; Williams, K.B.; Babbar, S.; Angolkar, M.; Derman, R.J. The oral microbiome and adverse pregnancy outcomes. Int. J. Womens Health 2017, 9, 551–559. [Google Scholar] [CrossRef]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef]
- Han, Y.W.; Ikegami, A.; Bissada, N.F.; Herbst, M.; Redline, R.W.; Ashmead, G.G. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J. Clin. Microbiol. 2006, 44, 1475–1483. [Google Scholar] [CrossRef]
- Han, Y.W.; Fardini, Y.; Chen, C.; Iacampo, K.G.; Peraino, V.A.; Shamonki, J.M.; Redline, R.W. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet. Gynecol. 2010, 115, 442–445. [Google Scholar] [CrossRef]
- Han, Y.W.; Shen, T.; Chung, P.; Buhimschi, I.A.; Buhimschi, C.S. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J. Clin. Microbiol. 2009, 47, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.; Alber, D.; Jones, H.; Harris, K.; Fitzgerald, F.; Peebles, D.; Klein, N. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta 2014, 35, 1099–1101. [Google Scholar] [CrossRef]
- Parnell, L.A.; Briggs, C.M.; Cao, B.; Delannoy-Bruno, O.; Schrieffer, A.E.; Mysorekar, I.U. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci. Rep. 2017, 7, 11200. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Schaudinn, C.; Kusanovic, J.P.; Gorur, A.; Gotsch, F.; Webster, P.; Nhan-Chang, C.L.; Erez, O.; Kim, C.J.; Espinoza, J.; et al. Detection of a microbial biofilm in intraamniotic infection. Am. J. Obstet. Gynecol. 2008, 198, 135.e1–135.e5. [Google Scholar] [CrossRef]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef]
- Moore, L.V.; Johnson, J.L.; Moore, W.E. Descriptions of Prevotella tannerae sp. nov. and Prevotella enoeca sp. nov. from the human gingival crevice and emendation of the description of Prevotella zoogleoformans. Int. J. Syst. Bacteriol. 1994, 44, 599–602. [Google Scholar] [CrossRef]
- Gosalbes, M.J.; Llop, S.; Vallès, Y.; Moya, A.; Ballester, F.; Francino, M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 2013, 43, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernandez, N.; Solis, G.; Hernandez-Barranco, A.; Margolles, A.; de Los Reyes-Gavilan, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Adlerberth, I.; Wold, A.E. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009, 98, 229–238. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17. [Google Scholar]
- Unger, S.; Stintzi, A.; Shah, P.; Mack, D.; O’Connor, D.L. Gut microbiota of the very-low-birth-weight infant. Pediatr. Res. 2015, 77, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, M.V.; Hall, L.; Soderlund, S.; Hakansson, E.G.; Hakansson, S.; Domellof, M. Intestinal flora in very low-birth weight infants. Acta Paediatr. 2009, 98, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Carlisle, E.M.; Bik, E.M.; Morowitz, M.J.; Relman, D.A. Microbiome assembly across multiple body sites in low-birthweight infants. mBio 2013, 4, e00782-13. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Wade, W. Unculturable bacteria–the uncharacterized organisms that cause oral infections. J. R. Soc. Med. 2002, 95, 81–83. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T.; Rajchel, J.; Bedard, J.; Newby, R.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16s ribosomal gene. Hum. Microb. J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Fang, R.L.; Chen, L.X.; Shu, W.S.; Yao, S.Z.; Wang, S.W.; Chen, Y.Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16s rRNA gene. PeerJ 2016, 4, E1602. [Google Scholar] [CrossRef]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef]
- Miles, S.M.; Hardy, B.L.; Merrell, D.S. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil. Steril. 2017, 107, 813–820.e1. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 69–75. [Google Scholar] [CrossRef]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identyfikacja i ocena mikrobiomu w żeńskich i męskich drogach rozrodczych. Aktual. Hum. Reprod. 2019, 25, 298–325. [Google Scholar] [CrossRef]
- Brotman, R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Keelan, J.A.; Payne, M.S. Comparison of Meconium DNA Extraction Methods for Use in Microbiome Studies. Front. Microbiol. 2018, 9, 270. [Google Scholar] [CrossRef]
- Hu, J.; Nomura, Y.; Bashir, A.; Fernandez-Hernandez, H.; Itzkowitz, S.; Pei, Z.; Stone, J.; Loudon, H.; Peter, I. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS ONE 2013, 8, e78257. [Google Scholar] [CrossRef]
- Ardissone, A.N.; De La Cruz, D.M.; Davis-Richardson, A.G.; Rechcigl, K.T.; Li, N.; Drew, J.C.; Murgas-Torrazza, R.; Sharma, R.; Hudak, M.L.; Triplett, E.W.; et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE 2014, 9, e90784. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Sánchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D.; Hale, E.C.; et al. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics 2011, 127, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar] [CrossRef]
- Kang, H.M.; Kim, S.; Hwang-Bo, S.; Yoo, I.H.; Seo, Y.-M.; Oh, M.Y.; Im, S.-A.; Youn, Y.-A. Compositional Differences of Meconium Microbiomes of Preterm and Term Infants, and Infants That Developed Necrotizing Enterocolitis or Feeding Intolerance. Pathogens 2023, 12, 55. [Google Scholar] [CrossRef]
- Jalanka-Tuovinen, J.; Salonen, A.; Nikkilä, J.; Immonen, O.; Kekkonen, R.; Lahti, L.; Palva, A.; de Vos, W.M. Intestinal microbiota in healthy adults: Temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE 2011, 6, e23035. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Klopp, J.; Ferretti, P.; Meyer, C.U.; Hilbert, K.; Haiß, A.; Marißen, J.; Henneke, P.; Hudalla, H.; Pirr, S.; Viemann, D.; et al. Meconium Microbiome of Very Preterm Infants across Germany. mSphere 2022, 7, e00808-21. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J.; Suau, A.; Campeotto, F.; Magne, F.; Aires, J.; Ferraris, L.; Kalach, N.; Leroux, B.; Dupont, C. Conditions of Bifidobacterial Colonization in Preterm Infants: A Prospective Analysis. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Liu, C.; Mitchell, C.M.; Fiedler, T.L.; Thomas, K.K.; Agnew, K.J.; Marrazzo, J.M.; Fredricks, D.N. Temporal variability of human vaginal bacteria and relationship with bacterial. PLoS ONE 2010, 5, e10197. [Google Scholar] [CrossRef]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, womens health and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Di Nicuolo, F.; Pontecorvi, A.; Gratta, M.; Scambia, G.; Di Simone, N. Endometrial microbes and microbiome: Recent insights on the inflammatory and immune players of the human endometrium. Am. J. Reprod. Immunol. 2018, 80, e13065. [Google Scholar] [CrossRef]
- McQueen, D.B.; Perfetto, C.O.; Hazard, F.K.; Lathi, R.B. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil. Steril. 2015, 104, 927–931. [Google Scholar] [CrossRef]
- McQueen, D.B.; Bernardi, L.A.; Stephenson, M.D. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil. Steril. 2014, 101, 1026–1030. [Google Scholar] [CrossRef]
- Matteo, M.; Cicinelli, E.; Greco, P.; Massenzio, F.; Baldini, D.; Falagario, T.; Rosenberg, P.; Castellana, L.; Specchia, G.; Liso, A. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am. J. Reprod. Immunol. 2009, 61, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Farland, L.V.S.; Prescott, J.; Sasamoto, N.; Tobias, D.K.S.; Gaskins, A.J.S.; Stuart, J.J.S.; Carusi, D.A.; Chavarro, J.E.M.; Horne, A.W.M.C.; Rich-Edwards, J.W.S.; et al. Endometriosis and risk of adverse pregnancy outcomes. Obstet. Gynecol. 2019, 134, 527–536. [Google Scholar] [CrossRef]
- Stencel-Gabriel, K.; Gabriel, I.; Wiczkowski, A.; Paul, M.; Olejek, A. Prenatal priming of cord blood T lymphocytes by microbiota in the maternal vagina. Am. J. Reprod. Immunol. 2009, 61, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-infant microbial transmission from different body sites shapes the developing Infant gut microbiome. Cell Host Microbe 2018, 24, 133–145. [Google Scholar] [CrossRef]
- Wu, Y.W.; Colford, J.J.M. Chorioamnionitis as a Risk Factor for Cerebral Palsy: A Meta-analysis. JAMA 2000, 284, 1417–1424. [Google Scholar] [CrossRef]
- Shatrov, J.G.; Birch, S.C.M.; Lam, L.T.; Quinlivan, J.A.; McIntyre, S.; Mendz, G.L. Chorioamnionitis and Cerebral Palsy: A Meta-Analysis. Obstet. Gynecol. 2010, 116, 387–392. [Google Scholar] [CrossRef]
- Ogunyemi, D.; Murillo, M.; Jackson, U.; Hunter, N.; Alperson, B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J. Matern. Fetal Neonatal Med. 2003, 13, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Andrews, W.W.; Goldenberg, R.L.; Faye-Petersen, O.; Cliver, S.; Goepfert, A.R.; Hauth, J.C. The Alabama Preterm Birth study: Polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am. J. Obstet. Gynecol. 2006, 195, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Soraisham, A.S.; Singhal, N.; McMillan, D.D.; Sauve, R.S.; Lee, S.K.; Canadian Neonatal, N. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am. J. Obstet. Gynecol. 2009, 200, e371–e376. [Google Scholar] [CrossRef]
- Dammann, O.; Brinkhaus, M.J.; Bartels, D.B.; Dordelmann, M.; Dressler, F.; Kerk, J.; Dork, T.; Dammann, C.E. Immaturity, perinatal inflammation, and retinopathy of prematurity: A multi-hit hypothesis. Early Hum. Dev. 2009, 85, 325–329. [Google Scholar] [CrossRef]
- Been, J.V.; Lievense, S.; Zimmermann, L.J.; Kramer, B.W.; Wolfs, T.G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and meta-analysis. J. Pediatr. 2013, 162, 236–242. [Google Scholar] [CrossRef]
- Klinger, G.; Levy, I.; Sirota, L.; Boyko, V.; Reichman, B.; Lerner-Geva, L.; Israel Neonatal, N. Epidemiology and risk factors for early onset sepsis among very-low-birthweight infants. Am. J. Obstet. Gynecol. 2009, 201, 38.e1–38.e6. [Google Scholar] [CrossRef] [PubMed]
- Strunk, T.; Doherty, D.; Jacques, A.; Simmer, K.; Richmond, P.; Kohan, R.; Charles, A.; Burgner, D. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 2012, 129, e134–e141. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Chernikova, D.A.; Madan, J.C.; Housman, M.L.; Zain-Ul-Abideen, M.; Lundgren, S.N.; Morrison, H.G.; Sogin, M.L.; Williams, S.M.; Moore, J.H.; Karagas, M.R.; et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr. Res. 2018, 84, 71–79. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Butel, M.J.; Roland, N.; Hibert, A.; Popot, F.; Favre, A.; Tessedre, A.C.; Bensaada, M.; Rimbault, A.; Szylit, O. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J. Med. Microbiol. 1998, 47, 391–399. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Telesford, K.M.; Yan, W.; Ochoa-Reparaz, J.; Pant, A.; Kircher, C.; Christy, M.A.; Begum-Haque, S.; Kasper, D.L.; Kasper, L.H. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39+Foxp3+ T cells and Treg function. Gut Microbes 2015, 6, 234–242. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Sánchez, B.; Milani, C.; Duranti, S.; Solís, G.; Fernández, N.; de Los Reyes-Gavilan, C.G.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 2015, 166, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Minassian, C.; Thomas, S.L.; Williams, D.J.; Campbell, O.; Smeeth, L. Acute maternal infection and risk of preeclampsia: A population based case control study. PLoS ONE 2013, 8, e73047. [Google Scholar] [CrossRef]
- Kalinderi, K.; Delkos, D.; Kalinderis, M.; Athanasiadis, A.; Kalogiannidis, I. Urinary tract infection during pregnancy: Current concepts on a common multifaceted problem. J. Obstet. Gynaecol. 2018, 38, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Hollander, W.J.D.; Schalekamp-Timmermans, S.; Holster, I.L.; Jaddoe, V.W.; Hofman, A.; Moll, H.A.; Perez-Perez, G.I.; Blaser, M.J.; Steegers, E.A.P.; Kuipers, E.J. Helicobacter pylori colonization and pregnancies complicated by preeclampsia, spontaneous prematurity, and small for gestational age birth. Helicobacter 2017, 22, e12364. [Google Scholar] [CrossRef]
- Lv, L.-J.; Li, S.-H.; Li, S.-C.; Zhong, Z.-C.; Duan, H.-L.; Tian, C.; Li, H.; He, W.; Chen, M.-C.; He, T.-W.; et al. Early onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front. Cell Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef]
- Dunn, A.B.; Hanson, L.; VandeVusse, L.; Leslie, S. Through the microbial looking glass: Premature labor, preclampsia, and gestational diabetes: A scoping review. J. Perinat. Neonatal Nurs. 2019, 33, 35–51. [Google Scholar] [CrossRef]
- Amarasekara, R.; Jayasekara, R.W.; Senanayake, H.; Dissanayake, V.H. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J. Obstet. Gynaecol. Res. 2015, 41, 662–669. [Google Scholar] [CrossRef]

| Disease | NEC | EONS | LONS | |
|---|---|---|---|---|
| Organism | ||||
| Escherichia coli | + | + | + | |
| Streptococcus agalactiae | + | |||
| Staphylococcus spp. | + | + | ||
| Klebsiella pneumoniae | + | + | ||
| Cronobacter sakazakii | + | |||
| Candida spp. | + | |||
| Researcher | Ardissone et al. [87] | Kang et al. [90] | Klopp et al. [93] | |
|---|---|---|---|---|
| Time of Delivery | Phylum | Occurrence | ||
| <33 weeks | Firmicutes | 8.67% Enterococcus 0.82% Lactobacillus | most of the neonatal meconium microbiota; the dominant ones: Prevotella Bacteroides | detected |
| Actinobacteria | Bifidobacterium 5.47% | - | detected | |
| Proteobacteria | 6.35% Enterobacter 0.98% Photorhabdus | detected | detected | |
| Tannerella | low bacterial count | - | - | |
| Bacteroidetes | - | most of the neonatal meconium microbiota | detected | |
| >33 weeks | Firmicutes | 0.41% Enterococcus 0.07% Lactobacillus | most of the neonatal meconium microbiota | - |
| Actinobacteria | 0.35% Bifidobacterium | - | - | |
| Proteobacteria | 0.06% Enterobacter 0.01% Photorhabdus | lower compared to newborns delivered at lower gestational ages | - | |
| Tannerella | very low bacterial count | - | - | |
| Bacteroidetes | - | higher compared to newborns delivered at lower gestational ages; the dominant ones: Prevotella Bacteroides | - | |
| Researcher | Sood et al. [42] | Bartnicka et al. [9] | Ardissone et al. [87] | Collado et al. [94] | Jianzhong Hu et al. [86] | |
|---|---|---|---|---|---|---|
| Taxa | ||||||
| Actinobacteria | ++ (including Bifidobacterium) | ++ | ++ Propionibacterium | ++ | ||
| Proteobacteria | ++ | ++ Stenotrophomonas, Escherichia | ++ Enterobacteriaceae, Escherichia, Shigella | ++ | ||
| Bacteroides | ++ | ++ | ||||
| Firmicutes | + (including Lactobacillus) | ++ Lactobacillus, Streptococcus | ++ Streptococcus, Lactobacillus | ++ | ||
| Researcher | Arboleya et al. [68] | Bartnicka et al. [9] | Aagaard et al. Intestinal Microbiome of a Newborn with the Body Weight < 1200 g [41] | Ardissone et al. [87] | Juliette Madan et al. [14] | |
|---|---|---|---|---|---|---|
| Taxa | ||||||
| Actinobacteria | + | + | ||||
| Tenericutes | ++ | + | ||||
| Firmicutes | ++ Enterococcus Lactobacillus Staphylococcus | ++ Clostridium | ++ | ++ | ++ Lactobacillus Staphylococcus | |
| Proteobacteria | ++ Enterobacter | + Enterobacteriaceae | ++ | ++ Enterobacteriales | ||
| Bacteroidetes | + | |||||
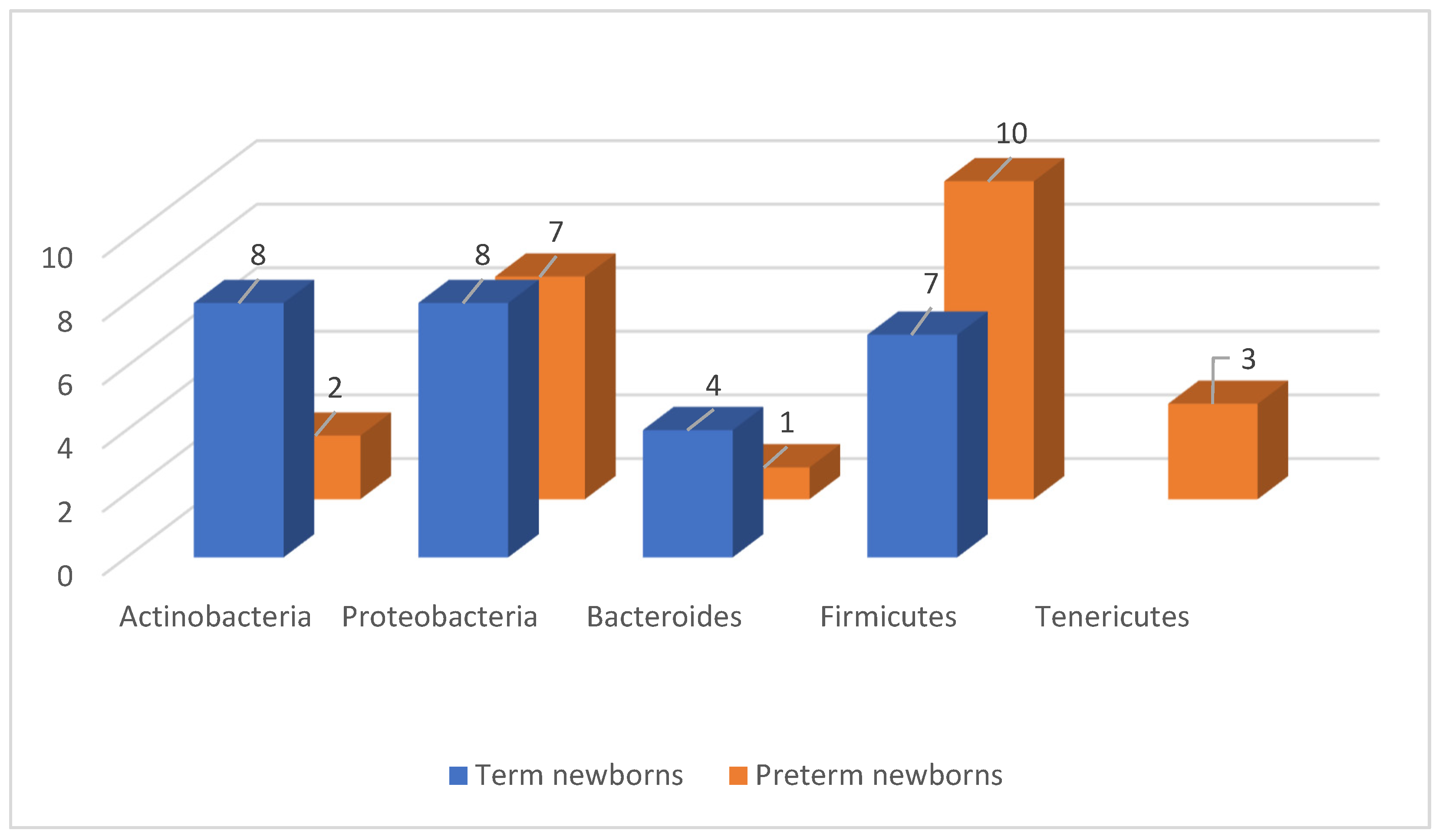
| Full-Term versus Premature Newborn | The Intestinal Microbiome in Term Newborns | The Intestinal Microbiome in Term and Preterm Newborns | The Intestinal Microbiome in Preterm Newborns | |
|---|---|---|---|---|
| Phylum | ||||
| Firmicutes | Streptococcus | Lactobacillus | Staphylococcus Enterococcus Clostridium | |
| Proteobacteria | Escherichia Shigella Stenotrophomonas | Enterobacteriaceae | Enterobacter | |
| Actinobacteria | Bifidobacterium Propionibacterium | The authors did not perform species differentiation | insignificant amount | |
| Bacteroidetes | insignificant amount | The authors did not perform species differentiation | trace amount | |
| Tenericutes | lack | The authors did not perform species differentiation | insignificant amount | |
| Site | Intestine | Vagina | Amniotic Fluid and Placenta | |
|---|---|---|---|---|
| Organism | ||||
| Firmicutes | ++ including Streptococcus | + including Lactobacillus | + Lactobacillus, Streptococcus | |
| Tenericutes | ++ | |||
| Proteobacteria | ++ including E. Coli (+++), non-pathogenic Neisseria species | ++ Escherichia/Shigella, Enterobacter | ||
| Bacteroidetes | ++ including Prevotella tannerae | ++ | ||
| Fusobacteria | ++ | |||
| Actinobacteria | ++ including Bifidobacterium | ++ Propionibacterium | ||
| Site | Intestine | Vagina | Amniotic Fluid and Placenta | |
|---|---|---|---|---|
| Organism | ||||
| Firmicutes | +++ Enterococcus, Staphylococcus, Clostridium | +++ including Lactobacillus, Staphylococcus | ++ | |
| Proteobacteria | +++ Enterobacter | ++ | ||
| Bacteroidetes | + | + | + | |
| Actinobacteria | + Bifidobacterium | + Atopobium | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dera, N.; Żeber-Lubecka, N.; Ciebiera, M.; Kosińska-Kaczyńska, K.; Szymusik, I.; Massalska, D.; Dera, K.; Bubień, K. Intrauterine Shaping of Fetal Microbiota. J. Clin. Med. 2024, 13, 5331. https://doi.org/10.3390/jcm13175331
Dera N, Żeber-Lubecka N, Ciebiera M, Kosińska-Kaczyńska K, Szymusik I, Massalska D, Dera K, Bubień K. Intrauterine Shaping of Fetal Microbiota. Journal of Clinical Medicine. 2024; 13(17):5331. https://doi.org/10.3390/jcm13175331
Chicago/Turabian StyleDera, Norbert, Natalia Żeber-Lubecka, Michał Ciebiera, Katarzyna Kosińska-Kaczyńska, Iwona Szymusik, Diana Massalska, Kacper Dera, and Katarzyna Bubień. 2024. "Intrauterine Shaping of Fetal Microbiota" Journal of Clinical Medicine 13, no. 17: 5331. https://doi.org/10.3390/jcm13175331





