CPAP Treatment at Home after Acute Decompensated Heart Failure in Patients with Obstructive Sleep Apnea
Abstract
1. Introduction
2. Aim
3. Materials and Methods
3.1. Study Design
3.2. Study Participants
3.3. Statistical Analysis
4. Results
4.1. Patient Demographics and Clinical Characteristics
4.2. OSA Patients
4.2.1. BMI Follow-Up in the CPAP and Non-CPAP Groups
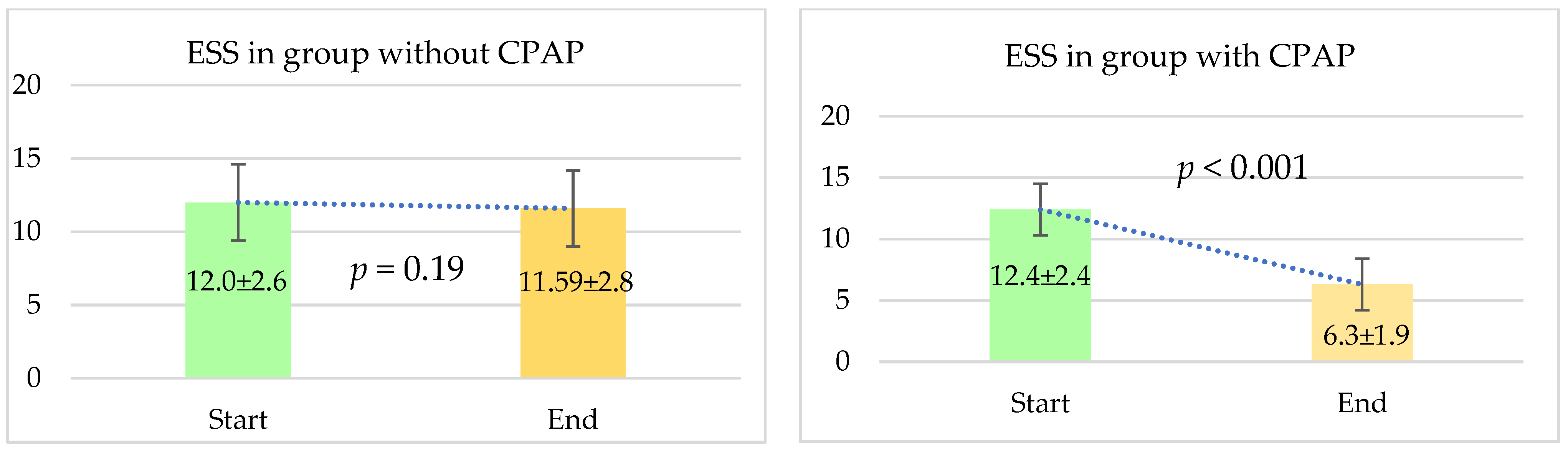
4.2.2. Comparison Regarding Daytime Sleepiness
4.2.3. Comparison in Terms of Systolic and Diastolic Blood Pressure
4.2.4. Change in LVEF% in Patients with and without CPAP Therapy
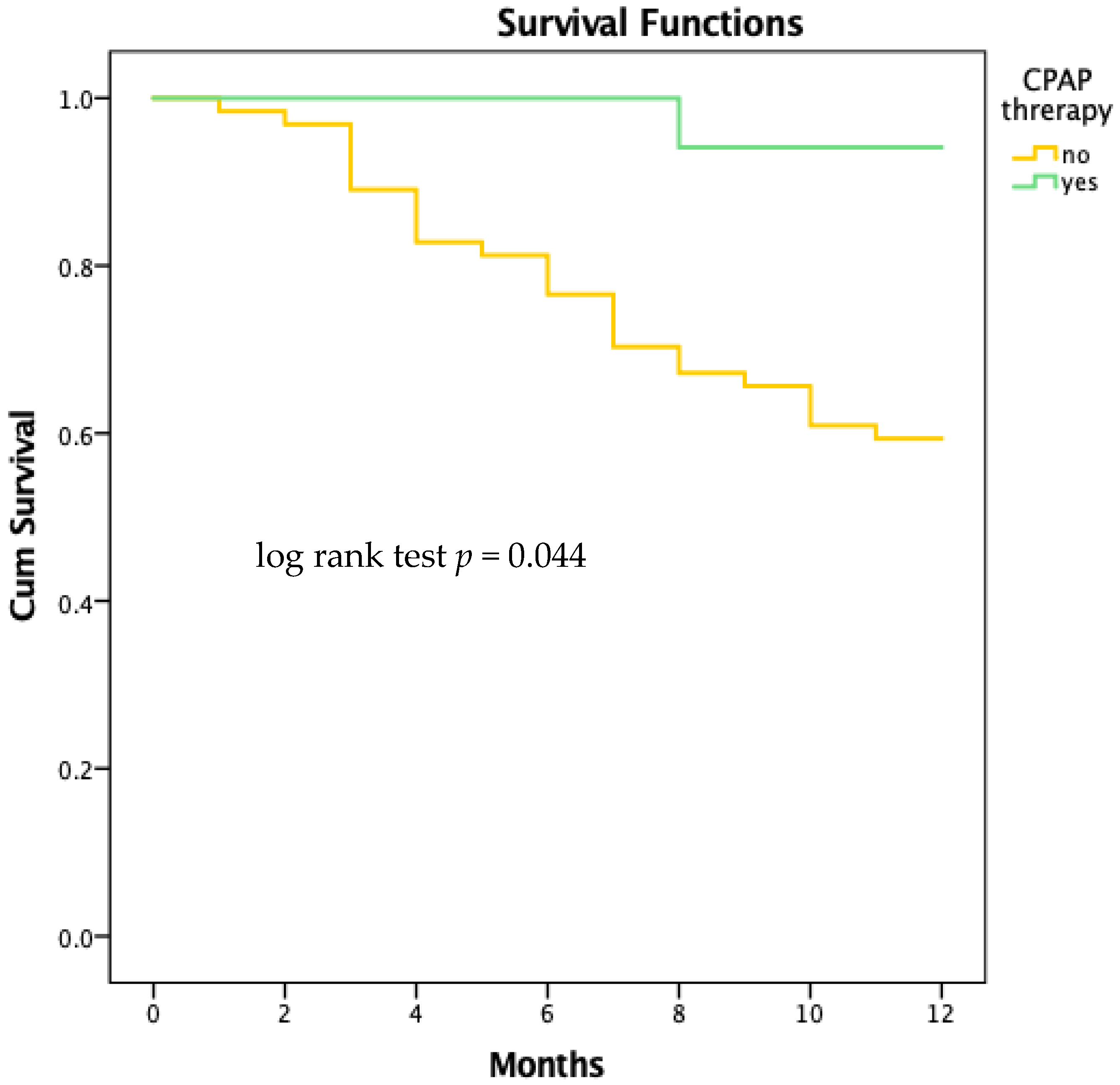
4.2.5. Survival Analysis
5. Discussion
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javaheri, S.; Javaheri, S. Obstructive Sleep Apnea in Heart Failure: Current Knowledge and Future Directions. J. Clin. Med. 2022, 11, 3458. [Google Scholar] [CrossRef]
- Bozkurt, B.; Hershberger, R.E.; Butler, J.; Grady, K.L.; Heidenreich, P.A.; Isler, M.L.; Kirklin, J.K.; Weintraub, W.S. 2021 ACC/AHA Key Data Elements and Definitions for Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure) Circ. Cardiovasc. Qual. Outcomes 2021, 14, e000102. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Naughton, M.T.; Tamisier, R.; Cowie, M.R.; Bradley, T.D. Sleep apnoea and heart failure. Eur. Respir. J. 2022, 59, 2101640. [Google Scholar] [CrossRef]
- Kishan, S.; Rao, M.S.; Ramachandran, P.; Devasia, T.; Samanth, J. Prevalence and Patterns of Sleep-Disordered Breathing in Indian Heart Failure Population. Pulm. Med. 2021, 2021, 9978906. [Google Scholar] [CrossRef]
- Khayat, R.; Jarjoura, D.; Porter, K.; Sow, A.; Wannemacher, J.; Dohar, R.; Pleister, A.; Abraham, W.T. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur. Heart J. 2015, 36, 1463–1469. [Google Scholar] [CrossRef]
- Lu, M.; Wang, Z.; Zhan, X.; Wei, Y. Obstructive sleep apnea increases the risk of cardiovascular damage: A systematic review and meta-analysis of imaging studies. Syst. Rev. 2021, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Gupta, G.; Narang, R.; Ray, A.; Pandey, R.M.; Malhotra, A.; Sinha, S. The impact of obstructive sleep apnoea severity on cardiac structure and injury. Sleep. Med. 2021, 77, 58–65. [Google Scholar] [CrossRef]
- Menon, T.; Kalra, D.K. Sleep Apnea and Heart Failure-Current State-of-The-Art. Int. J. Mol. Sci. 2024, 25, 5251. [Google Scholar] [CrossRef] [PubMed]
- DiCaro, M.V.; Lei, K.; Yee, B.; Tak, T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. J. Clin. Med. 2024, 13, 3223. [Google Scholar] [CrossRef]
- Uchoa, C.H.G.; Pedrosa, R.P.; Javaheri, S.; Geovanini, G.R.; Carvalho, M.M.B.; Torquatro, A.C.S.; Leite, A.P.D.L.; Gonzaga, C.C.; Bertolami, A.; Amodeo, C.; et al. OSA and Prognosis After Acute Cardiogenic Pulmonary Edema: The OSA-CARE Study. Chest 2017, 152, 1230–1238. [Google Scholar] [CrossRef]
- Kasai, T.; Narui, K.; Dohi, T.; Yanagisawa, N.; Ishiwata, S.; Ohno, M.; Yamaguchi, T.; Momomura, S. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008, 133, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Kourouklis, S.P.; Vagiakis, E.; Paraskevaidis, I.A.; Farmakis, D.; Kostikas, K.; Parissis, J.T.; Katsivas, A.; Kremastinos, D.T.; Anastasiou-Nana, M.; Filippatos, G. Effective sleep apnoea treatment improves cardiac function in patients with chronic heart failure. Int. J. Cardiol. 2013, 168, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Pack, A.I.; Magalang, U.J.; Singh, B.; Kuna, S.T.; Keenan, B.T.; Maislin, G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: Understanding and overcoming bias. Sleep 2021, 44, zsaa229. [Google Scholar] [CrossRef]
- Khayat, R.N.; Javaheri, S.; Porter, K.; Sow, A.; Holt, R.; Randerath, W.; Abraham, W.T.; Jarjoura, D. In-Hospital Management of Sleep Apnea During Heart Failure Hospitalization: A Randomized Controlled Trial. J. Card. Fail. 2020, 26, 705–712. [Google Scholar] [CrossRef]
- Chang, H.-C.; Wu, H.-T.; Huang, P.-C.; Ma, H.-P.; Lo, Y.-L.; Huang, Y.-H. Portable Sleep Apnea Syndrome Screening and Event Detection Using Long Short-Term Memory Recurrent Neural Network. Sensors 2020, 20, 6067. [Google Scholar] [CrossRef]
- Khayat, R.N.; Jarjoura, D.; Patt, B.; Yamokoski, T.; Abraham, W.T. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: Report of prevalence and patient characteristics. J. Card. Fail. 2009, 15, 739–746. [Google Scholar] [CrossRef]
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Oldenburg, O.; Graml, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Suling, A.; Woehrle, H.; SchlaHF Investigators. Phenotyping of Sleep-Disordered Breathing in Patients with Chronic Heart Failure with Reduced Ejection Fraction-the SchlaHF Registry. J. Am. Heart Assoc. 2017, 6, e005899. [Google Scholar] [CrossRef]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Polecka, A.; Olszewska, N.; Danielski, Ł.; Olszewska, E. Association between Obstructive Sleep Apnea and Heart Failure in Adults-A Systematic Review. J. Clin. Med. 2023, 12, 6139. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Cole, K.V.; Malik, A.S.; Pépin, J.L.; Sert Kuniyoshi, F.H.; Cistulli, P.A.; Benjafield, A.V.; Somers, V.K.; medXcloud group. Positive Airway Pressure Adherence and Health Care Resource Utilization in Patients with Obstructive Sleep Apnea and Heart Failure with Reduced Ejection Fraction. J. Am. Heart Assoc. 2023, 12, e028732. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Floras, J.S.; Usui, K.; Plante, J.; Tkacova, R.; Kubo, T.; Ando, S.; Bradley, T.D. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003, 348, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M.; Mehra, R.; Patel, S.R.; Quan, S.F.; Babineau, D.C.; Tracy, R.P.; Rueschman, M.; Blumenthal, R.S.; Lewis, E.F.; et al. CPAP versus oxygen in obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2276–2285. [Google Scholar] [CrossRef]
- Damy, T.; Margarit, L.; Noroc, A.; Bodez, D.; Guendouz, S.; Boyer, L.; Drouot, X.; Lamine, A.; Paulino, A.; Rappeneau, S.; et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur. J. Heart Fail. 2012, 14, 1009–1019. [Google Scholar] [CrossRef]
- Javaheri, S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation 2000, 101, 392–397. [Google Scholar] [CrossRef]
- Johnson, C.B.; Beanlands, R.S.; Yoshinaga, K.; Haddad, H.; Leech, J.; de Kemp, R.; Burwash, I.G. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can. J. Cardiol. 2008, 24, 697–704. [Google Scholar] [CrossRef]
- Naito, R.; Kasai, T.; Dohi, T.; Takaya, H.; Narui, K.; Momomura, S.I. Factors Associated with the Improvement of Left Ventricular Systolic Function by Continuous Positive Airway Pressure Therapy in Patients with Heart Failure With Reduced Ejection Fraction and Obstructive Sleep Apnea. Front. Neurol. 2022, 13, 781054. [Google Scholar] [CrossRef]
- Kim, D.; Shim, C.Y.; Cho, Y.J.; Park, S.; Lee, C.J.; Park, J.H.; Cho, H.J.; Ha, J.W.; Hong, G.R. Continuous Positive Airway Pressure Therapy Restores Cardiac Mechanical Function in Patients with Severe Obstructive Sleep Apnea: A Randomized, Sham-Controlled Study. J. Am. Soc. Echocardiogr. 2019, 32, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Duran, C.A.; Rahman, H.; Lekkala, M.; Saleem, M.A.; Kaluski, E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur. Heart J. 2018, 39, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Whellan, D.J.; Fiuzat, M.; Punjabi, N.M.; Tasissa, G.; Anstrom, K.J.; Benjafield, A.V.; Woehrle, H.; Blase, A.B.; Lindenfeld, J.; et al. Cardiovascular outcomes with minute ventilation- targeted adaptive servoventilation therapy in heart failure. J. Am. Coll. Cardiol. 2017, 69, 15771587. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Woehrle, H.; Wegscheider, K.; Angermann, C.; d’Ortho, M.P.; Erdmann, E.; Levy, P.; Simonds, A.K.; Somers, V.K.; Zannad, F.; et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N. Engl. J. Med. 2015, 373, 1095–1105. [Google Scholar] [CrossRef]
- Wang, Y.; Branco, R.F.; Salanitro, M.; Penzel, T.; Schöbel, C. Effects of sacubitril-valsartan on central and obstructive apneas in heart failure patients with reduced ejection fraction. Sleep Breath. 2023, 27, 283–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelaia, C.; Armentaro, G.; Volpentesta, M.; Mancuso, L.; Miceli, S.; Caroleo, B.; Perticone, M.; Maio, R.; Arturi, F.; Imbalzano, E.; et al. Effects of Sacubitril-Valsartan on Clinical, Echocardiographic, and Polygraphic Parameters in Patients Affected by Heart Failure with Reduced Ejection Fraction and Sleep Apnea. Front. Cardiovasc. Med. 2022, 9, 861663. [Google Scholar] [CrossRef]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Clinical Characteristic | OSA n = 59 | CSA n = 22 | p–Value |
|---|---|---|---|
| Age, years | 8.840 | 9.62 | 0.382 |
| Gender, male % | 57.6 | 54.5 | 0.499 |
| Arterial hypertension, % | 74.6 | 68.1 | 0.565 |
| Ischemic heart disease, % | 47.5 | 86.3 | 0.002 |
| Left ventricular hypertrophy, % | 79.7 | 50 | 0.008 |
| Diabetes mellitus, % | 74.5 | 40.9 | 0.005 |
| Atrial fibrillation, % | 49.1 | 81.8 | 0.008 |
| SBP, mm Hg | 89.07 | 811.1 | <0.001 |
| DBP, mm Hg | 87.08 | 87.9 | <0.001 |
| Mean heart rate, beats/min | 88.2 | 88.9 | <0.001 |
| BMI | 87.07 | 83.5 | 0.002 |
| NT-proBNP, pg/mL | 8897 | 81,453 | <0.001 |
| LVEF% | 8.04 | 9.4 | <0.001 |
| E/e’m | 3.8 | 2.9 | 0.016 |
| AHI | 22.3 | 9.8 | 0.129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaydzhiev, P.; Borizanova, A.; Georgieva, N.; Voynova, G.; Yakov, S.; Kocev, T.; Tomova-Lyutakova, G.; Krastev, B.; Spasova, N.; Ilieva, R.; et al. CPAP Treatment at Home after Acute Decompensated Heart Failure in Patients with Obstructive Sleep Apnea. J. Clin. Med. 2024, 13, 5676. https://doi.org/10.3390/jcm13195676
Kalaydzhiev P, Borizanova A, Georgieva N, Voynova G, Yakov S, Kocev T, Tomova-Lyutakova G, Krastev B, Spasova N, Ilieva R, et al. CPAP Treatment at Home after Acute Decompensated Heart Failure in Patients with Obstructive Sleep Apnea. Journal of Clinical Medicine. 2024; 13(19):5676. https://doi.org/10.3390/jcm13195676
Chicago/Turabian StyleKalaydzhiev, Petar, Angelina Borizanova, Neli Georgieva, Gergana Voynova, Slavi Yakov, Tsvetan Kocev, Galya Tomova-Lyutakova, Bozhidar Krastev, Natalia Spasova, Radostina Ilieva, and et al. 2024. "CPAP Treatment at Home after Acute Decompensated Heart Failure in Patients with Obstructive Sleep Apnea" Journal of Clinical Medicine 13, no. 19: 5676. https://doi.org/10.3390/jcm13195676
APA StyleKalaydzhiev, P., Borizanova, A., Georgieva, N., Voynova, G., Yakov, S., Kocev, T., Tomova-Lyutakova, G., Krastev, B., Spasova, N., Ilieva, R., Kinova, E., & Goudev, A. (2024). CPAP Treatment at Home after Acute Decompensated Heart Failure in Patients with Obstructive Sleep Apnea. Journal of Clinical Medicine, 13(19), 5676. https://doi.org/10.3390/jcm13195676






