Heart Failure with Preserved Ejection Fraction Correlates with Fibrotic Atrial Myopathy in Patients Undergoing Atrial Fibrillation Ablation
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Study Participants
2.2. HFA-PEFF Score
2.3. High-Density Bipolar Voltage Map
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Electrophysiological Findings
4. Discussion
4.1. Main Findings
- -
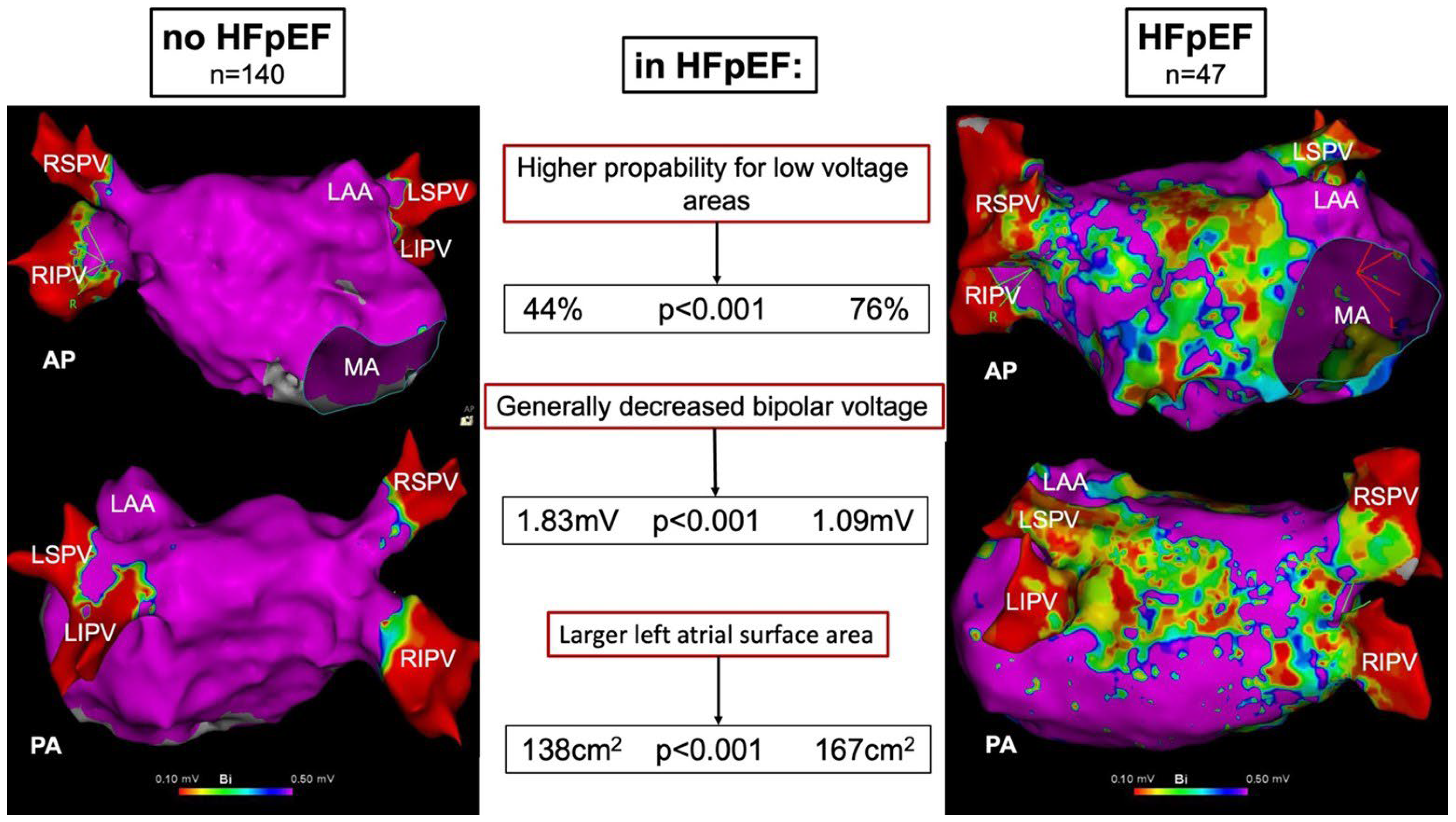
- The presence of LA low-voltage areas is significantly associated with the diagnosis of HFpEF;
- -
- Besides the presence of low-voltage areas, HFpEF patients show a generally decreased LA bipolar voltage;
- -
- HFpEF is associated with a larger LA surface area.
4.2. Prevalence of Low-Voltage Areas
4.3. Atrial Fibrillation Ablation in HFpEF
4.4. The Prognostic Importance of Atrial Myopathy
5. Clinical Implications
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anter, E.; Jessup, M.; Callans, D.J. Atrial Fibrillation and Heart Failure. Circulation 2009, 119, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Lam, C.S.P.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Monte, I.P.; Faro, D.C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. Left Atrial Strain Imaging by Speckle Tracking Echocardiography: The Supportive Diagnostic Value in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2023, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Miranda, W.R.; Yeung, D.F.; Kane, G.C.; Oh, J.K. Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1490–1499. [Google Scholar] [CrossRef]
- Lau, C.; Elshibly, M.M.M.; Kanagala, P.; Khoo, J.P.; Arnold, J.R.; Hothi, S.S. The role of cardiac magnetic resonance imaging in the assessment of heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2022, 9, 922398. [Google Scholar] [CrossRef]
- Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. J. Clin. Med. 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- Cau, R.; Bassareo, P.; Suri, J.S.; Pontone, G.; Saba, L. The emerging role of atrial strain assessed by cardiac MRI in different cardiovascular settings: An up-to-date review. Eur. Radiol. 2022, 32, 4384–4394. [Google Scholar] [CrossRef]
- Schönbauer, R.; Kammerlander, A.A.; Duca, F.; Aschauer, S.; Binder, C.; Zotter-Tufaro, C.; Nitsche, C.; Fiedler, L.; Roithinger, F.X.; Loewe, C.; et al. Impact of Left Atrial Phasic Function in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2020, 13, 2254–2255. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Sardana, M.; Ansari, B.; Satija, V.; Kuriakose, D.; Edelstein, I.; Oldland, G.; Miller, R.; Gaddam, S.; Lee, J.; et al. Left Atrial Phasic Function by Cardiac Magnetic Resonance Feature Tracking Is a Strong Predictor of Incident Cardiovascular Events. Circ. Cardiovasc. Imaging 2018, 11, e007512. [Google Scholar] [CrossRef]
- Schönbauer, R.; Tomala, J.; Kirstein, B.; Huo, Y.; Gaspar, T.; Richter, U.; Piorkowski, J.; Schönbauer, M.-S.; Fiedler, L.; Roithinger, F.X.; et al. Left Atrial Phasic Transport Function Closely Correlates with Fibrotic and Arrhythmogenic Atrial Tissue Degeneration in Atrial Fibrillation Patients: Cardiac Magnetic Resonance Feature Tracking and Voltage Mapping. EP Eur. 2021, 23, 1400–1408. [Google Scholar] [CrossRef]
- Verma, A.; Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Kilicaslan, F.; Minor, S.; Schweikert, R.A.; Saliba, W.; Cummings, J.; Burkhardt, J.D.; et al. Pre-Existent Left Atrial Scarring in Patients Undergoing Pulmonary Vein Antrum Isolation. J. Am. Coll. Cardiol. 2005, 45, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Gaspar, T.; Schönbauer, R.; Wójcik, M.; Fiedler, L.; Roithinger, F.X.; Martinek, M.; Pürerfellner, H.; Kirstein, B.; Richter, U.; et al. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evid. 2022, 1, EVIDoa2200141. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Xintarakou, A.; Tzeis, S.; Psarras, S.; Asvestas, D.; Vardas, P. Atrial Fibrosis as a Dominant Factor for the Development of Atrial Fibrillation: Facts and Gaps. EP Eur. 2020, 22, 342–351. [Google Scholar] [CrossRef]
- Schönbauer, R.; Duca, F.; Kammerlander, A.A.; Aschauer, S.; Binder, C.; Zotter-Tufaro, C.; Koschutnik, M.; Fiedler, L.; Roithinger, F.X.; Loewe, C.; et al. Persistent atrial fibrillation in heart failure with preserved ejection fraction: Prognostic relevance and association with clinical, imaging and invasive haemodynamic parameters. Eur. J. Clin. Investig. 2020, 50, e13184. [Google Scholar] [CrossRef]
- Bonderman, D.; Agis, H.; Kain, R.; Mascherbauer, J. Amyloid in the heart: An under-recognized threat at the interface of cardiology, haematology, and pathology. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 978–980. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Huo, Y.; Gaspar, T.; Pohl, M.; Sitzy, J.; Richter, U.; Neudeck, S.; Mayer, J.; Kronborg, M.B.; Piorkowski, C. Prevalence and Predictors of Low Voltage Zones in the Left Atrium in Patients with Atrial Fibrillation. Europace 2017, 20, 956–962. [Google Scholar] [CrossRef]
- Kosiuk, J.; Dinov, B.; Kornej, J.; Acou, W.J.; Schönbauer, R.; Fiedler, L.; Buchta, P.; Myrda, K.; Gąsior, M.; Poloński, L.; et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm 2015, 12, 2207–2212. [Google Scholar] [CrossRef]
- Chen, S.; Pürerfellner, H.; Meyer, C.; Acou, W.-J.; Schratter, A.; Ling, Z.; Liu, S.; Yin, Y.; Martinek, M.; Kiuchi, M.G.; et al. Rhythm Control for Patients with Atrial Fibrillation Complicated with Heart Failure in the Contemporary Era of Catheter Ablation: A Stratified Pooled Analysis of Randomized Data. Eur. Heart J. 2019, 41, 2863–2873. [Google Scholar] [CrossRef]
- Sugumar, H.; Nanayakkara, S.; Vizi, D.; Wright, L.; Chieng, D.; Leet, A.; Mariani, J.A.; Voskoboinik, A.; Prabhu, S.; Taylor, A.J.; et al. A Prospective STudy Using InvAsive Haemodynamic Measurements FoLLowing Catheter Ablation for AF and Early HFpEF: STALL AF-HFpEF. Eur. J. Heart Fail. 2021, 23, 785–796. [Google Scholar] [CrossRef]
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B. Ablation versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation 2021, 143, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jaswaney, R.; Jani, C.; Zuzek, Z.; Thakkar, S.; Patel, H.P.; Patel, M.; Patel, N.; Tripathi, B.; Lahewala, S.; et al. Catheter Ablation for Atrial Fibrillation in Patients with Concurrent Heart Failure. Am. J. Cardiol. 2020, 137, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Qi, B.; Wang, Z.; Li, F.; Chen, C.; Li, C.; Yuan, S.; Yao, S.; Zhou, J.; Ge, J. Ablation for Atrial Fibrillation Improves the Outcomes in Patients with Heart Failure with Preserved Ejection Fraction. Europace 2023, 26, euad363. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.-J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left Atrial Remodeling and Function in Advanced Heart Failure with Preserved or Reduced Ejection Fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef]
- Schönbauer, R.; Kammerlander, A.A.; Duca, F.; Aschauer, S.; Koschutnik, M.; Dona, C.; Nitsche, C.; Loewe, C.; Hengstenberg, C.; Mascherbauer, J. Prognostic Impact of Left Atrial Function in Heart Failure with Preserved Ejection Fraction in Sinus Rhythm vs. Persistent Atrial Fibrillation. ESC Heart Fail. 2021, 9, 465–475. [Google Scholar] [CrossRef]
| Variable | HFpEF No (n = 140) | Yes (n = 47) | p-Value |
|---|---|---|---|
| Age, y | 63 ± 11 | 70 ± 9 | <0.001 |
| Female sex, n (%) | 58 (41) | 27 (57) | 0.064 |
| Body mass index, kg/m2 | 27 ± 5 | 28 ± 5 | 0.984 |
| Persistent AF, n (%) | 62 (44) | 24 (51) | 0.230 |
| Arterial hypertension, n (%) | 90 (64) | 30 (64) | 1.000 |
| Renal failure, n (%) | 4 (3) | 5 (11) | 0.043 |
| Diabetes mellitus type II, n (%) | 23 (16) | 8 (17) | 1.000 |
| Cardiovascular disease, n (%) | 41 (29) | 17 (36) | 0.361 |
| Hyperlipidemia, n (%) | 67 (48) | 18 (38) | 0.313 |
| Stroke, n (%) | 16 (11) | 3 (6) | 0.414 |
| CHA2DS2-VASc score | 2.3 ± 1.5 | 3.2 ± 1.5 | <0.001 |
| NT-proBNP, ng/mL (SR) | 354 ± 572 | 638 ± 526 | <0.001 |
| NT-proBNP, ng/mL (AF) | 1193 ± 968 | 1915 ± 1382 | 0.001 |
| LAVI, mL/m2 | 31 ± 14 | 45 ± 15 | <0.001 |
| Interventricular Septum, mm | 12 ± 2.1 | 12.6 ± 2.4 | 0.696 |
| HMG-CoA reductase inhibitor, n (%) | 66 (47) | 20 (43) | 0.501 |
| Betablocker, n (%) | 105 (75) | 37 (79) | 0.550 |
| Diuretics, n (%) | 51 (36) | 26 (55) | 0.024 |
| ACE inhibitor, n (%) | 23 (16) | 11 (23) | 0.275 |
| AT II receptor antagonist, n (%) | 46 (33) | 21 (45) | 0.156 |
| Variable | HFpEF No (n = 140) | Yes (n = 47) | p-Value |
|---|---|---|---|
| Mapping points | 1258 ± 719 | 1685 ± 818 | <0.001 |
| LA surface area, cm2 | 138 ± 40 | 167 ± 42 | <0.001 |
| Bipolar voltage, mV | 1.83 ± 0.91 | 1.09 ± 0.64 | <0.001 |
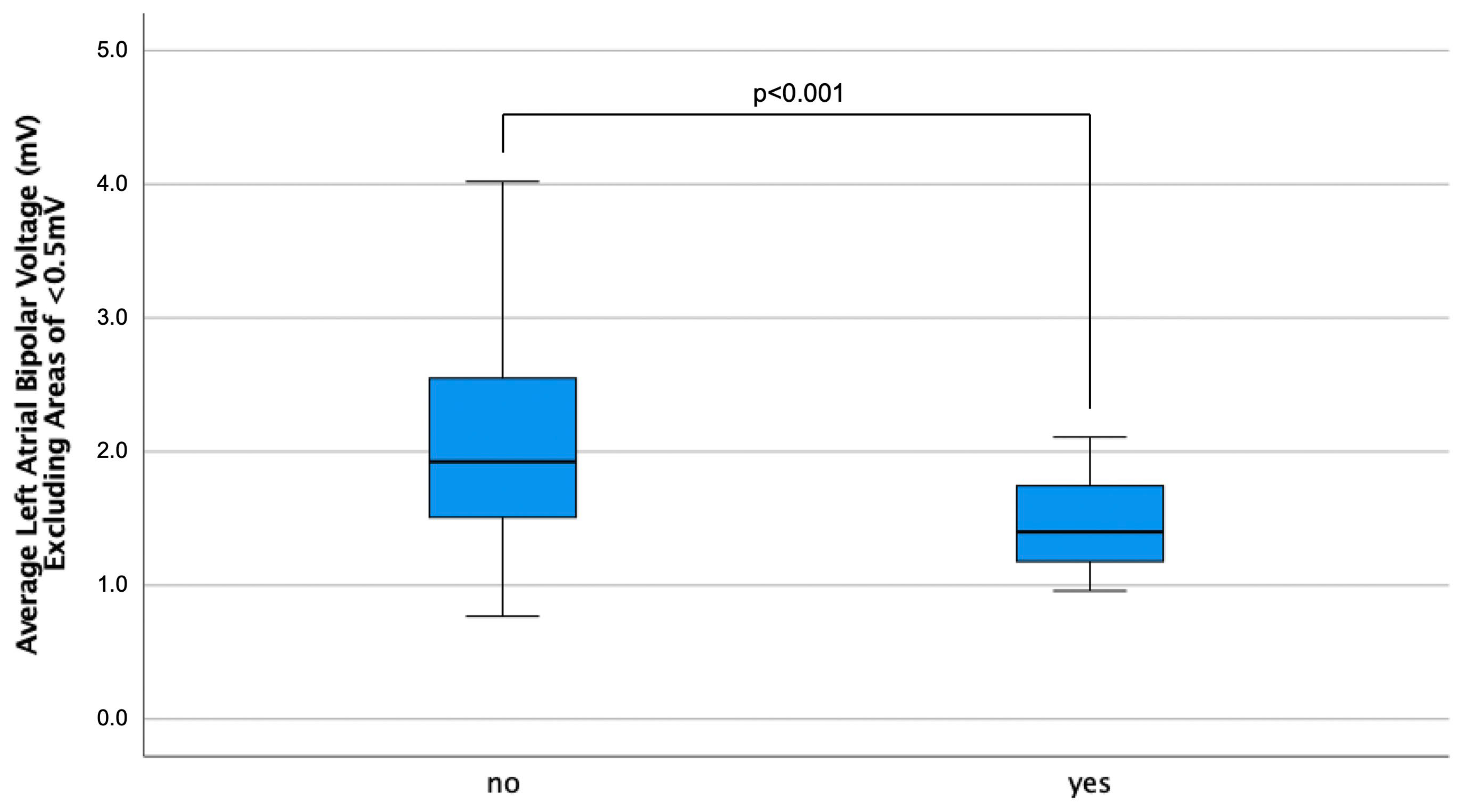
| Low-Voltage Area, n (%) | 61 (44) | 36 (76) | <0.001 |
| Low-Voltage Area, cm2 | 29.5 ± 27.0 | 30.2 ± 24.5 | 0.786 |
| Low-Voltage Area, % of LA surface Area | 19.0 ± 14.3 | 23.5 ± 15.8 | 0.163 |
| Bipolar Voltage >0.5 mV, mV | 2.04 ± 0.70 | 1.51 ± 0.45 | <0.001 |
| HFA-PEFF SCORE | 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|---|
| ρ | p-Value | ||||||||
| N | 14 | 18 | 25 | 41 | 42 | 21 | 26 | ||
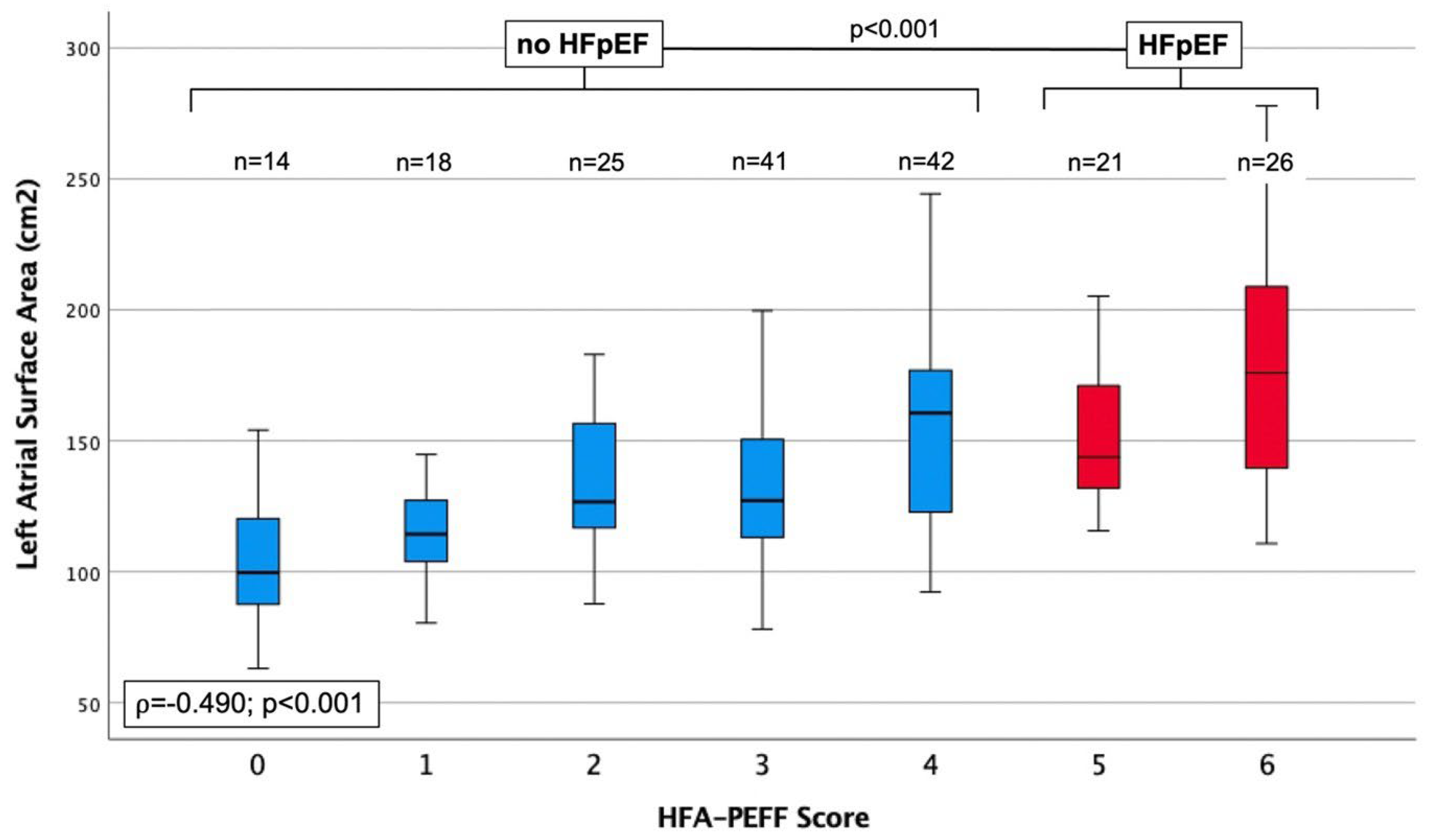
| LA SURFACE AREA, cm2 | 110 ± 35 | 115 ± 19 | 134 ± 26 | 139 ± 39 | 159 ± 44 | 153 ± 32 | 180 ± 46 | 0.490 | <0.001 |
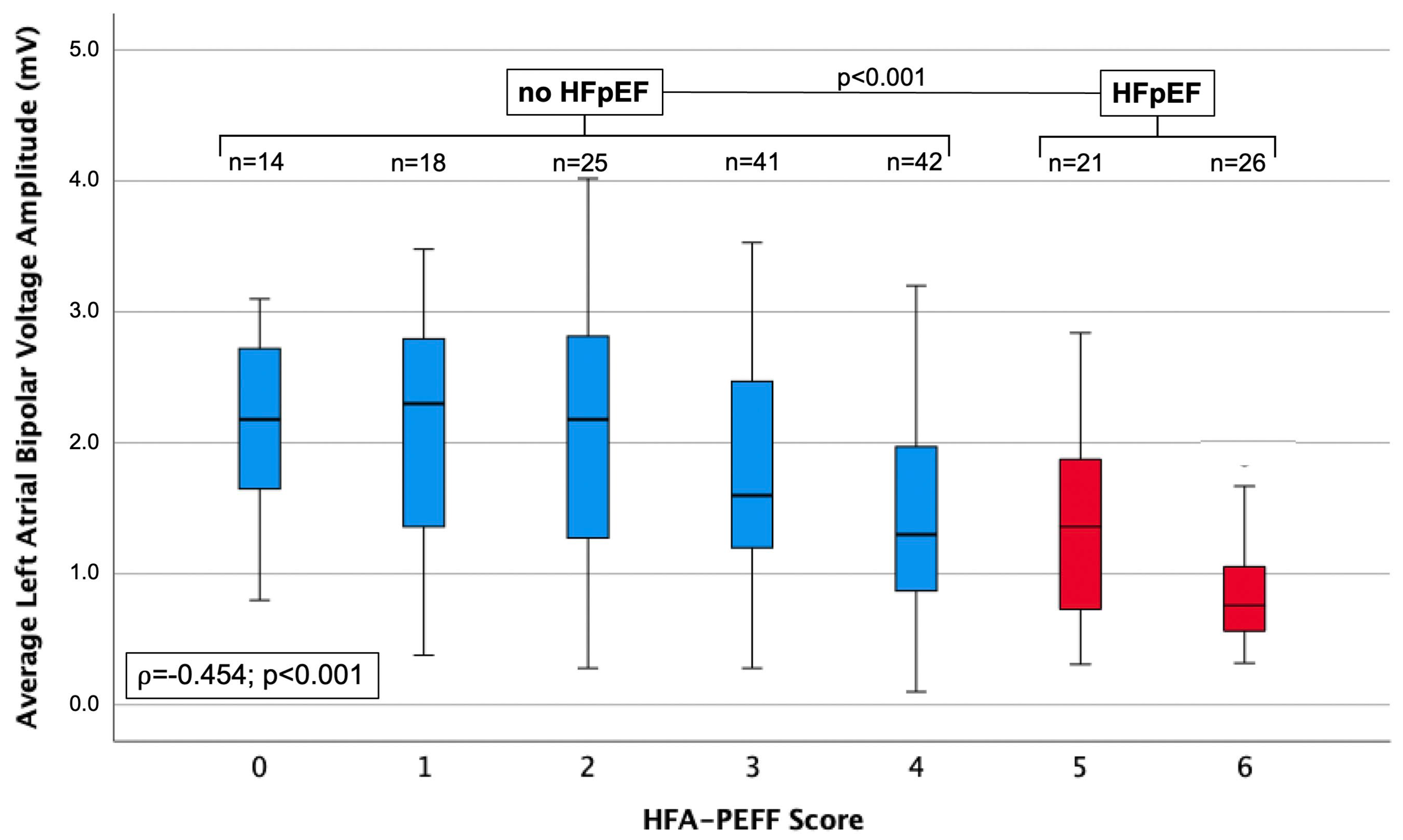
| BIPOLAR VOLTAGE, mV | 2.21 ± 0.95 | 2.13 ± 0.98 | 2.09 ± 1.02 | 1.79 ± 0.80 | 1.43 ± 0.78 | 1.35 ± 0.74 | 0.86 ± 0.42 | −0.454 | <0.001 |
| LVA, N (%) | 3 (21) | 6 (33) | 5 (20) | 19 (46) | 28 (67) | 13 (62) | 23 (88) | 0.418 | <0.001 |
| LVA, cm2 | 21.2 ± 9.9 | 30.7 ± 16.6 | 28.1 ± 12.6 | 27.0 ± 27.5 | 31.9 ± 32.1 | 22.1 ± 26.2 | 35.7 ± 22.2 | 0.070 | 0.527 |
| LVA, % OF LA SURFACE AREA | 15.2 ± 11.2 | 23.2 ± 11.6 | 23.0 ± 11.3 | 17.4 ± 17.1 | 18.7 ± 14.2 | 24.2 ± 19.3 | 23.0 ± 13.5 | 0.101 | 0.358 |
| BIPOLAR VOLTAGE > 0.5 mV, mV | 2.35 ± 0.80 | 2.30 ± 0.75 | 2.21 ± 0.85 | 2.05 ± 0.56 | 1.72 ± 0.56 | 1.67 ± 0.54 | 1.37 ± 0.29 | −0.450 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Sponder, M.; Stojkovic, S.; Riesenhuber, M.; Hammer, A.; Hofbauer, T.M.; Sulzgruber, P.; Burger, S.; Kastl, S.; Duca, F.; et al. Heart Failure with Preserved Ejection Fraction Correlates with Fibrotic Atrial Myopathy in Patients Undergoing Atrial Fibrillation Ablation. J. Clin. Med. 2024, 13, 5685. https://doi.org/10.3390/jcm13195685
Lee J, Sponder M, Stojkovic S, Riesenhuber M, Hammer A, Hofbauer TM, Sulzgruber P, Burger S, Kastl S, Duca F, et al. Heart Failure with Preserved Ejection Fraction Correlates with Fibrotic Atrial Myopathy in Patients Undergoing Atrial Fibrillation Ablation. Journal of Clinical Medicine. 2024; 13(19):5685. https://doi.org/10.3390/jcm13195685
Chicago/Turabian StyleLee, Jonghui, Michael Sponder, Stefan Stojkovic, Martin Riesenhuber, Andreas Hammer, Thomas M. Hofbauer, Patrick Sulzgruber, Stefanie Burger, Stefan Kastl, Franz Duca, and et al. 2024. "Heart Failure with Preserved Ejection Fraction Correlates with Fibrotic Atrial Myopathy in Patients Undergoing Atrial Fibrillation Ablation" Journal of Clinical Medicine 13, no. 19: 5685. https://doi.org/10.3390/jcm13195685
APA StyleLee, J., Sponder, M., Stojkovic, S., Riesenhuber, M., Hammer, A., Hofbauer, T. M., Sulzgruber, P., Burger, S., Kastl, S., Duca, F., & Schönbauer, R. (2024). Heart Failure with Preserved Ejection Fraction Correlates with Fibrotic Atrial Myopathy in Patients Undergoing Atrial Fibrillation Ablation. Journal of Clinical Medicine, 13(19), 5685. https://doi.org/10.3390/jcm13195685






