Critically Ill Patients with Newly Diagnosed Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: Case Series and Literature Review
Abstract
1. Introduction
2. Case Presentations
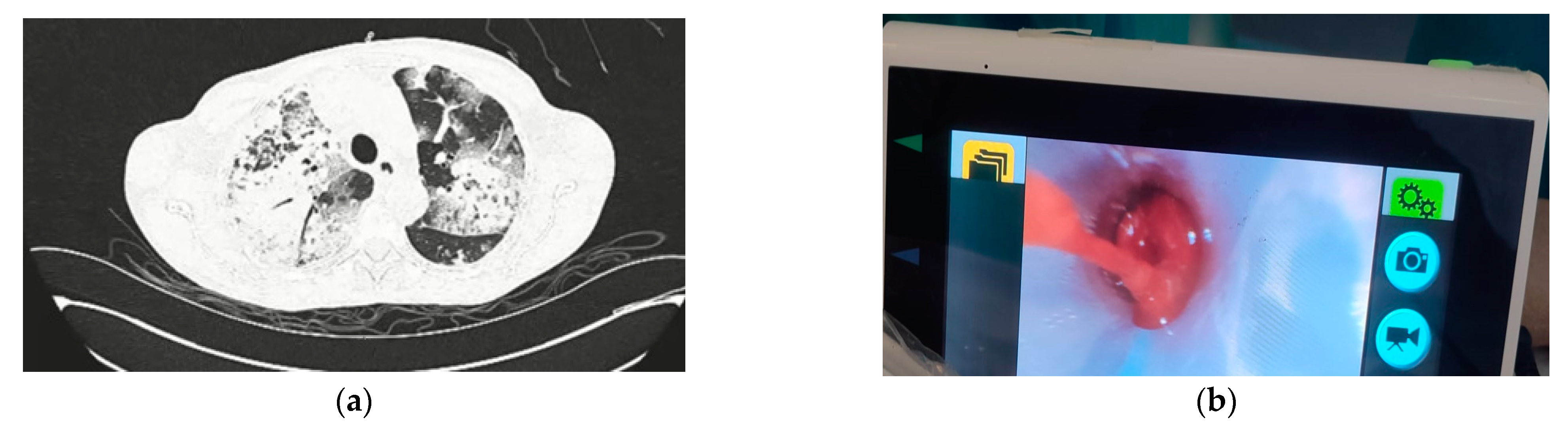
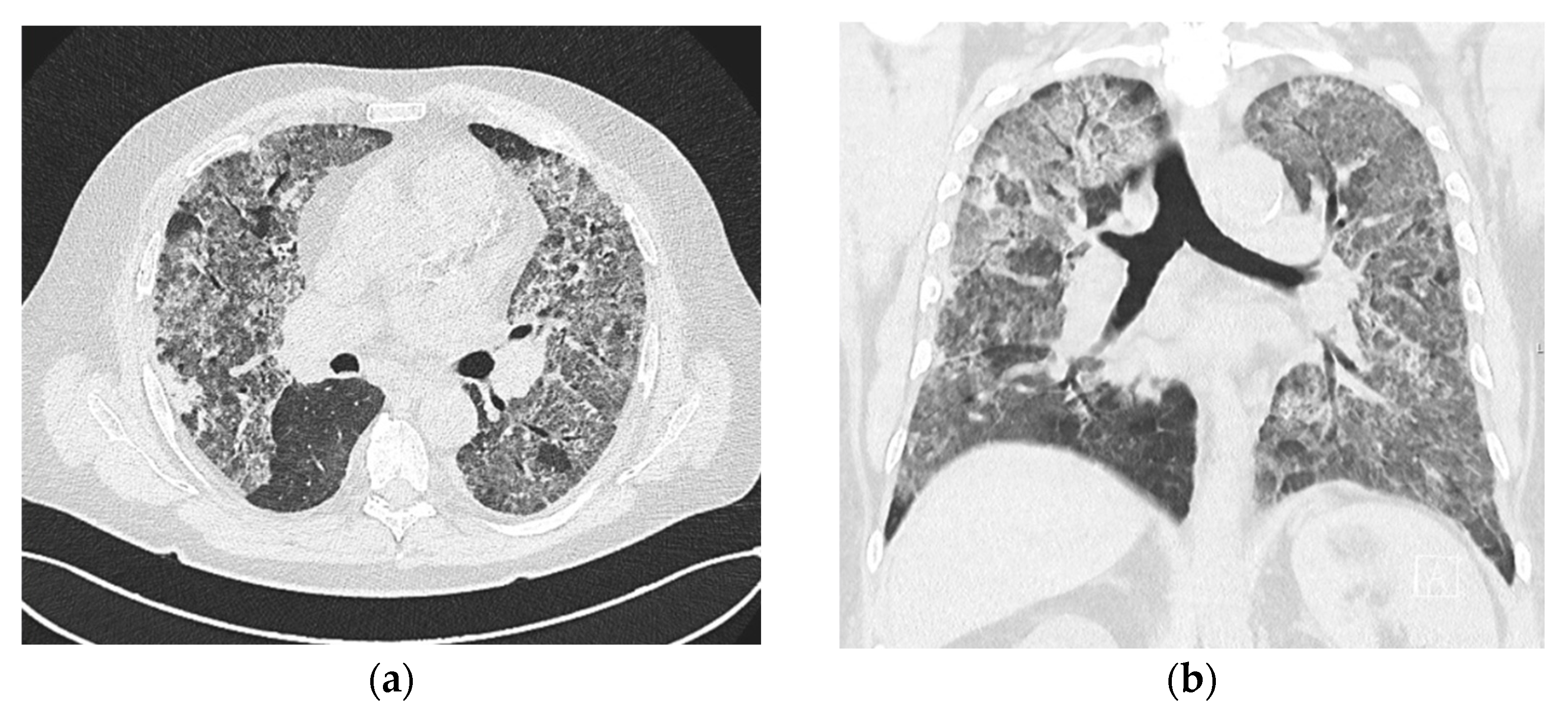
2.1. Case No. 1
2.2. Case No. 2
2.3. Case No. 3
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franca, S.A.; Toufen, C.; Hovnanian, A.L.D.; Albuquerque, A.L.P.; Borges, E.R.; Pizzo, V.R.P.; Carvalho, C.R.R. The epidemiology of acute respiratory failure in hospitalized patients: A Brazilian prospective cohort study. J. Crit. Care 2011, 26, 330.e1–330.e8. [Google Scholar] [CrossRef] [PubMed]
- Villgran, V.D.; Lyons, C.; Nasrullah, A.; Clarisse Abalos, C.; Bihler, E.; Alhajhusain, A. Acute Respiratory Failure. Crit. Care Nurs. Q. 2022, 45, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Gómez-Carballo, C.; Soto-Peleteiro, A.; Olmo-Velasco, M.; Bueno, L.; Ruiz-Arruza, I. Ruiz-Irastorza, G. ANCA-associated pulmonary-renal syndrome treated with cyclophosphamide, rituximab, repeated methyl-prednisolone pulses and a reduced oral glucocorticoid regime: An observational study. Clin. Exp. Rheumatol. 2023, 41, 928–935. [Google Scholar]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primer. 2020, 6, 71. [Google Scholar] [CrossRef]
- Duarte, A.C.; Ribeiro, R.; Macedo, A.M.; Santos, M.J. ANCA-associated vasculitis: Overview and practical issues of diagnosis and therapy from a European perspective. Porto Biomed. J. 2023, 8, e237. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Xu, Y.; Jiao, J.; Xie, L.; Mo, G. A novel therapeutic strategy using extracorporeal membrane oxygenation in patients with anti-neutrophil cytoplasmic antibodies-associated vasculitis: A case report and literature review. Ann. Transl. Med. 2021, 9, 1267. [Google Scholar] [CrossRef]
- Wu, S.J.; Hsu, Y.C.; Wang, K.L.; Fu, P.K. Prone Positioning May Improve the Treatment of Diffuse Alveolar Hemorrhage and Severe Acute Respiratory Distress Syndrome (ARDS) Secondary to ANCA Associated Vasculitis: A Case Report. Life 2022, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, H.; Malagon, I.; Robson, K.; Parmar, J.; Hamilton, P.; Falter, F. Extracorporeal membrane oxygenation for Life-threatening ANCA-positive pulmonary capillaritis. A review of UK experience. Heart Lung Vessel 2015, 7, 159–167. [Google Scholar]
- Alamo, B.S.; Moi, L.; Bajema, I.; Berden, A.; Flossmann, O.; Hruskova, Z.; Jayne, D.; Wester-Trejo, M.; Wallquist, C.; Westman, K. Long-term outcome of kidney function in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2024, 39, 1483–1493. [Google Scholar]
- Demiselle, J.; Auchabie, J.; Beloncle, F.; Gatault, P.; Grangé, S.; Du Cheyron, D.; Dellamonica, J.; Boyer, S.; Beauport, D.T.; Piquilloud, L.; et al. Patients with ANCA-associated vasculitis admitted to the intensive care unit with acute vasculitis manifestations: A retrospective and comparative multicentric study. Ann. Intensive Care 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.; O’Callaghan, M.; Ataya, A.; Gupta, N.; Keane, M.P.; Murphy, D.J.; McCarthy, C. Pulmonary renal syndrome: A clinical review. Breathe Sheff. Engl. 2022, 18, 220208. [Google Scholar] [CrossRef] [PubMed]
- Bantis, K.; Stangou, M.J.; Kalpakidis, S.; Nikolaidou, C.; Lioulios, G.; Mitsoglou, Z.; Iatridi, F.; Fylaktou, A.; Papagianni, A. Different Types of ANCA Determine Different Clinical Phenotypes and Outcome in ANCA-Associated Vasculitis (AAV). Front. Med. 2021, 8, 783757. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D. The diagnosis of vasculitis. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 445–453. [Google Scholar] [CrossRef]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef]
- Hogan, S.L.; Nachman, P.H.; Wilkman, A.S.; Jennette, J.C.; Falk, R.J. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J. Am. Soc. Nephrol. JASN. 1996, 7, 23–32. [Google Scholar] [CrossRef]
- Alexandre, A.T.; Vale, A.; Gomes, T. Diffuse alveolar hemorrhage: How relevant is etiology? Sarcoidosis Vasc. Diffuse Lung Dis. Off. J. WASOG 2019, 36, 47–52. [Google Scholar]
- Quadrelli, S.; Dubinsky, D.; Solis, M.; Yucra, D.; Hernández, M.; Karlen, H.; Brigante, A. Immune diffuse alveolar hemorrhage: Clinical presentation and outcome. Respir. Med. 2017, 129, 59–62. [Google Scholar] [CrossRef]
- Haas, M.; Eustace, J.A. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: A study of 126 cases. Kidney Int. 2004, 65, 2145–2152. [Google Scholar] [CrossRef]
- Neumann, I.; Regele, H.; Kain, R.; Birck, R.; Meisl, F.T. Glomerular immune deposits are associated with increased proteinuria in patients with ANCA-associated crescentic nephritis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2003, 18, 524–531. [Google Scholar] [CrossRef]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St. Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2021, 73, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, R.; Zonozi, R.; Rosenthal, J.; Laliberte, K.; Niles, J.L.; Cortazar, F.B. Treatment of Aggressive Antineutrophil Cytoplasmic Antibody-Associated Vasculitis with Eculizumab. Kidney Int. Rep. 2020, 5, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Jawa, P.; Derebail, V.K.; Falk, R.J. Treatment Updates in Antineutrophil Cytoplasmic Antibodies (ANCA) Vasculitis. Kidney360 2021, 2, 763–770. [Google Scholar] [CrossRef]
- Tang, P.F.; Xu, L.C.; Hong, W.T.; Shi, H.Y. Successful treatment of granulomatosis with polyangiitis using tocilizumab combined with glucocorticoids: A case report. World J. Clin. Cases. 2023, 11, 1144–1151. [Google Scholar] [CrossRef]
| Patient No. 1 | Patient No. 2 | Patient No. 3 | |||||
|---|---|---|---|---|---|---|---|
| Reference Range | ED | ICU | ED | ICU | ED | ICU | |
| RBCs (×1012/L) | 3.86–5.08 | 2.52 | 2.70 | 2.26 | 2.93 | 3.66 | 3.8 |
| Hemoglobin, g/L | 119–157 | 76 | 78 | 66 | 84 | 116 | 116 |
| WBCs (×109/L) | 3.4–9.7 | 7.4 | 8 | 4.44 | 6.1 | 6.2 | 6.2 |
| Leukocyte Differential Count (×109/L) | |||||||
| ANC | 2.06–6.49 | 6.1 | 7.43 | 3.69 | 5.53 | 4.07 | 5.82 |
| Lymphocytes | 1.19–3.35 | 0.94 | 0.4 | 0.27 | 0.28 | 1.39 | 0.23 |
| Monocytes | 0.12–0.84 | 0.23 | 0.17 | 0.36 | 0.23 | 0.54 | 0.12 |
| Basophils | 0.0–0.6 | 0.01 | 0.0 | 0.03 | 0.03 | 0.03 | 0.01 |
| Eosinophils | 0.0–0.43 | 0.04 | 0.0 | 0.09 | 0.03 | 0.17 | 0.01 |
| Platelets (×109/L) | 158–424 | 286 | 222 | 169 | 166 | 199 | 142 |
| PT | >0.7 | 0.87 | 0.98 | 0.96 | 1.01 | 1.29 | 1 |
| Fibrinogen, g/L | 1.8–4.1 | 6.31 | 4.5 | 6.81 | >7.0 | 5.69 | >7.0 |
| LDH, U/L | <241 | N/A | 257 | 458 | 544 | N/A | 155 |
| Urea, mmol/L | 2.8–8.3 | 52.9 | 31.5 | 31.2 | 36.9 | 4.7 | 7.7 |
| Creatinine, µmol/L | 60–104 | 1406 | 848 | 660 | 690 | 71 | 66 |
| Urinalysis | |||||||
| E | neg. | +++ | Anuria | +++ | +++ | +++ | +++ |
| LE | neg. | ++ | Anuria | +/− | neg. | + | neg. |
| Prot. | neg. | ++ | Anuria | + | + | + | +/− |
| CRP, mg/L | <5 | 124.1 | 96.7 | 237.7 | 269.3 | 7.1 | 269.3 |
| Procalcitonin, ug/L | <0.25 | 3 | 2.7 | N/A | 5.71 | 0.16 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukavina, K.; Zlopasa, O.; Vukovic Brinar, I.; Dzubur, F.; Anic, B.; Vujaklija Brajkovic, A. Critically Ill Patients with Newly Diagnosed Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: Case Series and Literature Review. J. Clin. Med. 2024, 13, 5688. https://doi.org/10.3390/jcm13195688
Rukavina K, Zlopasa O, Vukovic Brinar I, Dzubur F, Anic B, Vujaklija Brajkovic A. Critically Ill Patients with Newly Diagnosed Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: Case Series and Literature Review. Journal of Clinical Medicine. 2024; 13(19):5688. https://doi.org/10.3390/jcm13195688
Chicago/Turabian StyleRukavina, Kresimir, Ozrenka Zlopasa, Ivana Vukovic Brinar, Feda Dzubur, Branimir Anic, and Ana Vujaklija Brajkovic. 2024. "Critically Ill Patients with Newly Diagnosed Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: Case Series and Literature Review" Journal of Clinical Medicine 13, no. 19: 5688. https://doi.org/10.3390/jcm13195688
APA StyleRukavina, K., Zlopasa, O., Vukovic Brinar, I., Dzubur, F., Anic, B., & Vujaklija Brajkovic, A. (2024). Critically Ill Patients with Newly Diagnosed Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: Case Series and Literature Review. Journal of Clinical Medicine, 13(19), 5688. https://doi.org/10.3390/jcm13195688






