Factors beyond Body Mass Index Associated with Cardiometabolic Risk among Children with Severe Obesity
Abstract
:1. Introduction
2. Materials and Methods
- -
- underwent a physical examination with anthropometric measurements (body weight was measured to the nearest 0.1 kg on a certified medical scale, body height was measured to the nearest 0.1 cm on Harpenden stadiometer, waist circumference was measured at the level of midpoint between the lowest rib and iliac crest, and hip circumference was measured at the level of the greatest convexity of the buttocks on the back and with cardboard applied tangentially to the greatest convexity of the abdomen on the front by measuring tape to the nearest 0.5 cm)
- -
- underwent pubertal stage classification using the Tanner scale
- -
- had their blood pressure measured (SBP—systolic blood pressure, DBP—diastolic blood pressure); blood pressure was measured using a calibrated automatic blood pressure monitor with a cuff size appropriate to the arm size while each participant was in a sitting position following a 15 min rest period before the examination
- -
- underwent blood uptake for biochemical and hormonal tests in fasting (ALT—alanine aminotransferase, AST—aspartate aminotransferase, glucose, insulin, TGD—triglycerides, HDL—high-density lipoprotein, LDL—low-density lipoprotein, total cholesterol); deviation from the blood collection procedure was allowed if the patient provided documentation of having undergone listed laboratory tests within the last 6 months before the examination
- -
- went through BIA; deviation from BIA was allowed for children unable to cooperate (too young to follow the instructions); BIAs were conducted using TANITA MC-580 M S MDD, TANITA MC-780MA-N, and TANITA MC-780 P MA devices to measure fat mass (FM, %) and fat-free mass (FFM, %).
- -
- BMI = weight (kg) ÷ height (m2)
- -
- WHR = waist circumference (cm) ÷ hip circumference (cm)
- -
- WHtR = waist circumference (cm) ÷ height (cm)
- -
- HOMA IR = (glucose (mg/dL) × insulin (µIU/mL)) ÷ 405
- -
- BMI Z-Score was calculated using the Pediatric Z-Score Calculator online [9] for children aged 2–19 years
- -
- MetS Z-score was calculated using the MetS Z-score Calculator online [10]
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, J.; Buoncristiano, M.; Nardone, P.; Rito, A.I.; Spinelli, A.; Hejgaard, T.; Kierkegaard, L.; Nurk, E.; Kunešová, M.; Musić Milanović, S.; et al. A Snapshot of European Children’s Eating Habits: Results from the Fourth Round of the WHO European Childhood Obesity Surveillance Initiative (COSI). Nutrients 2020, 12, 2481. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.L.; Spinelli, A.; Lazzeri, G.; Lamberti, A.; Mazzarella, G.; Nardone, P.; Pilato, V.; Buoncristiano, M.; Caroli, M. Severe Obesity Prevalence in 8- to 9-Year-Old Italian Children: A Large Population-Based Study. Eur. J. Clin. Nutr. 2014, 69, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Beynon, C.; Bailey, L. Prevalence of Severe Childhood Obesity in Wales UK. J. Public Health 2019, 42, e435–e439. [Google Scholar] [CrossRef] [PubMed]
- Cadenas-Sanchez, C.; Intemann, T.; Labayen, I.; Artero, E.G.; Alvarez-Bueno, C.; Sanchis-Moysi, J.; Benito, P.J.; Beltran-Valls, M.R.; Pérez-Bey, A.; Sanchez-Delgado, G.; et al. Prevalence of Severe/Morbid Obesity and Other Weight Status and Anthropometric Reference Standards in Spanish Preschool Children: The PREFIT Project. Pediatr. Res. 2019, 87, 501–510. [Google Scholar] [CrossRef]
- Rulkiewicz, A.; Pilchowska, I.; Lisik, W.; Pruszczyk, P.; Ciurzyński, M.; Domienik-Karłowicz, J. Prevalence of Obesity and Severe Obesity among Professionally Active Adult Population in Poland and Its Strong Relationship with Cardiovascular Co-Morbidities-POL-O-CARIA 2016–2020 Study. J. Clin. Med. 2022, 11, 3720. [Google Scholar] [CrossRef]
- Correa-Burrows, P.; Rogan, J.; Blanco, E.; East, P.; Lozoff, B.; Gahagan, S.; Burrows, R. Resolving Early Obesity Leads to a Cardiometabolic Profile within Normal Ranges at 23 Years Old in a Two-Decade Prospective Follow-up Study. Sci. Rep. 2021, 11, 18927. [Google Scholar] [CrossRef]
- Seo, Y.-G.; Lim, H.; Kim, Y.; Ju, Y.-S.; Lee, H.-J.; Jang, H.; Park, S.; Park, K. The Effect of a Multidisciplinary Lifestyle Intervention on Obesity Status, Body Composition, Physical Fitness, and Cardiometabolic Risk Markers in Children and Adolescents with Obesity. Nutrients 2019, 11, 137. [Google Scholar] [CrossRef]
- Mierzwa, M.; Bik-Multanowski, M.; Ranke, M.B.; Brandt, S.; Flehmig, B.; Małecka-Tendera, E.; Mazur, A.; Petriczko, E.; Wabitsch, M.; Wójcik, M.; et al. Clinical, Genetic, and Epidemiological Survey of Polish Children and Adolescents with Severe Obesity: A Study Protocol of the Polish–German Study Project on Severe Early-Onset Obesity. Front. Endocrinol. 2022, 13, 972174. [Google Scholar] [CrossRef]
- Pediatric Z-Score Calculator. Available online: https://zscore.research.chop.edu/calcbmi.php (accessed on 12 September 2023).
- MetS Z-Score Calculator. Available online: https://metscalc.org/ (accessed on 28 January 2024).
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef]
- WHO. Waist circumference and waist-hip ratio: Report of a WHO expert consultation. In Proceedings of the WHO Conference, Geneva, Switzerland, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011; Volume 39. Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 1 February 2024).
- Eslami, M.; Pourghazi, F.; Khazdouz, M.; Tian, J.; Pourrostamim, K.; Esmaeili-Abdar, Z.; Ejtahed, H.-S.; Qorbani, M. Optimal Cut-Off Value of Waist Circumference-to-Height Ratio to Predict Central Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis of Diagnostic Studies. Front. Nutr. 2023, 9, 985319. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-G. Waist-To-Height Ratio as a Screening Tool for Obesity and Cardiometabolic Risk. Korean J. Pediatr. 2016, 59, 425. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.J.E.; Byrne, C.D.; Wilson, J.F.; Wild, S.H. Evaluation of Bioelectrical Impedance Analysis for Identifying Overweight Individuals at Increased Cardiometabolic Risk: A Cross-Sectional Study. PLoS ONE 2014, 9, e106134. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Xanthakos, S.A.; Hornung, L.; Arce-Clachar, C.; Siegel, R.; Kalkwarf, H.J. Relative Accuracy of Bioelectrical Impedance Analysis for Assessing Body Composition in Children with Severe Obesity. J. Pediatr. Gastroenterol. Nutr. 2020, 70, e129–e135. [Google Scholar] [CrossRef]
- Bredella, M.A. Sex Differences in Body Composition. Sex Gend. Factors Affect. Metab. Homeost. Diabetes Obes. 2017, 1043, 9–27. [Google Scholar] [CrossRef]
- Wells, J.C.K. Body Composition in Childhood: Effects of Normal Growth and Disease. Proc. Nutr. Soc. 2003, 62, 521–528. [Google Scholar] [CrossRef]
- Lee, L.-W.; Yen, J.-B.; Lu, H.-K.; Liao, Y.-S. Prediction of Nonalcoholic Fatty Liver Disease by Anthropometric Indices and Bioelectrical Impedance Analysis in Children. Child. Obes. 2021, 17, 551–558. [Google Scholar] [CrossRef]
- Muhanna, R.G.; Aljuraiban, G.S.; Almadani, N.K.; Alquraishi, M.; El-Sharkawy, M.S.; Abulmeaty, M.M. Value of Adding Bioelectrical Impedance Analysis to Anthropometric Indices in the Diagnosis of Metabolic Syndrome in 10–16 Years Old Schoolgirls. Healthcare 2022, 10, 419. [Google Scholar] [CrossRef]
- Reinehr, T.; Tittel, S.R.; Holle, R.; Wiegand, S.; Gellhaus, I.; Hebebrand, J.; Greber-Platzer, S.; Denzer, C.; Linke, S.; Kieß, W.; et al. Comparison of Cardiovascular Risk Factors between Children and Adolescents with Classes III and IV Obesity: Findings from the APV Cohort. Int. J. Obes. 2021, 45, 1061–1073. [Google Scholar] [CrossRef]
- Bojanic, D.; Ljubojevic, M.; Gontarev, S.; Georgiev, G.; Aleksovska Velickovska, L. First Body Fat Reference Curves for Macedonian Children and Adolescents: The MAKFIT Study. Nutr. Hosp. 2024, 41, 560–566. [Google Scholar] [CrossRef]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference Ranges of HOMA-IR in Normal-Weight and Obese Young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; White, B.; Barrett, T.; David, C.A.C.; Gibson, P.; Gregory, J.; Matyka, K.; Ong, K.K.; Roche, E.; Rudolf, M.C.J.; et al. Assessment of Childhood Obesity in Secondary Care: OSCA Consensus Statement. Arch. Dis. Child.—Educ. Pract. 2012, 97, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Horsley, L. AAP Clinical Report on Lipid Screening in Children. Am. Fam. Physician 2009, 79, 703–705. Available online: https://www.aafp.org/pubs/afp/issues/2009/0415/p703.html (accessed on 2 February 2024).
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef]
- Shaunak, M.; Byrne, C.D.; Davis, N.; Afolabi, P.; Faust, S.N.; Davies, J.H. Non-Alcoholic Fatty Liver Disease and Childhood Obesity. Arch. Dis. Child. 2020, 106, 3–8. [Google Scholar] [CrossRef]
- Marcinkiewicz, K.; Horodnicka-Józwa, A.; Jackowski, T.; Strączek, K.; Biczysko-Mokosa, A.; Walczak, M.; Petriczko, E. Nonalcoholic Fatty Liver Disease in Children with Obesity–Observations from One Clinical Centre in the Western Pomerania Region. Front. Endocrinol. 2022, 13, 992264. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Falkner, B.; Gidding, S.S.; Baker-Smith, C.M.; Brady, T.M.; Flynn, J.T.; Malle, L.M.; South, A.M.; Tran, A.H.; Urbina, E.M. Pediatric Primary Hypertension: An Underrecognized Condition: A Scientific Statement from the American Heart Association. Hypertension 2023, 80, e101–e111. [Google Scholar] [CrossRef]
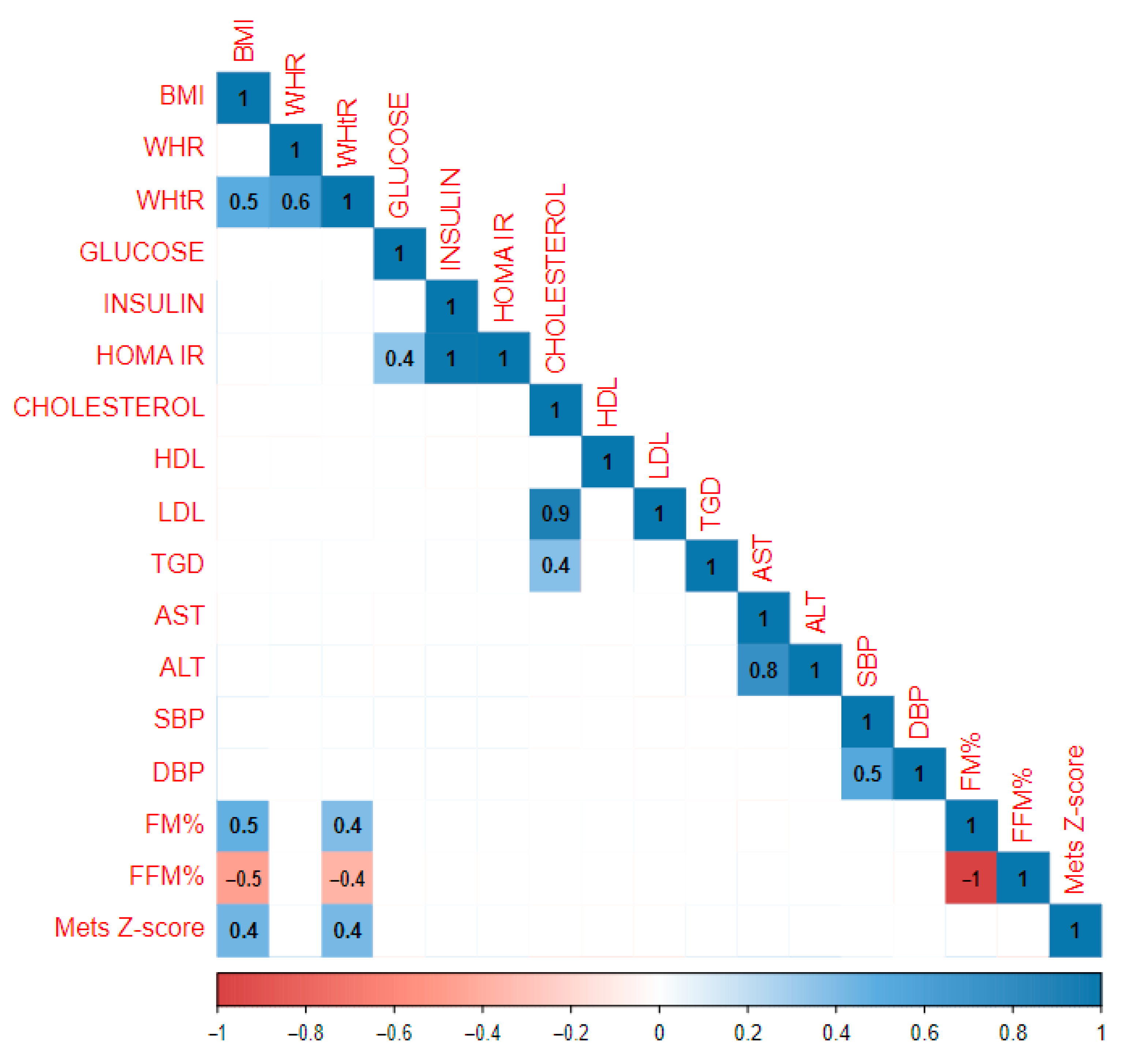
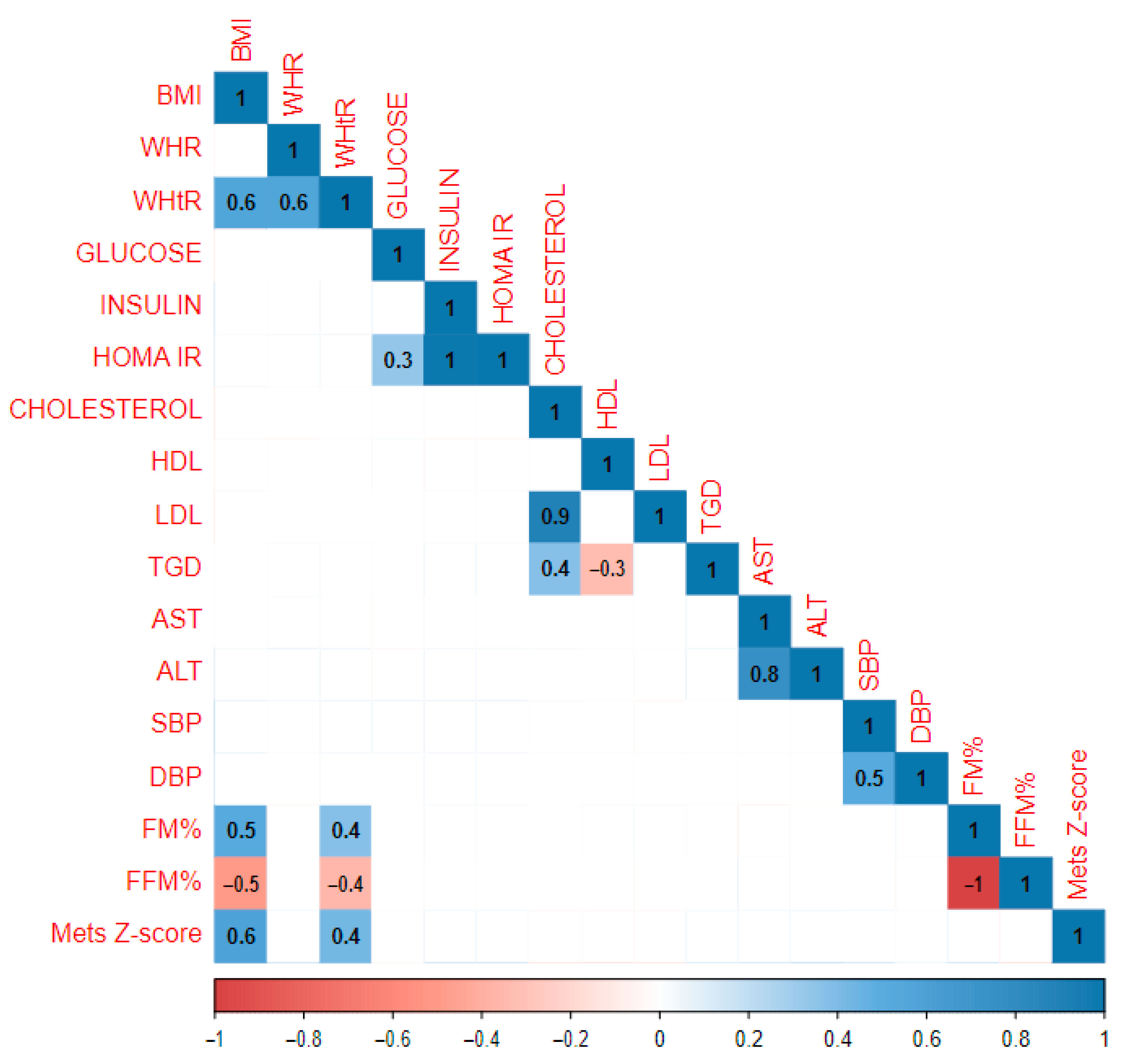
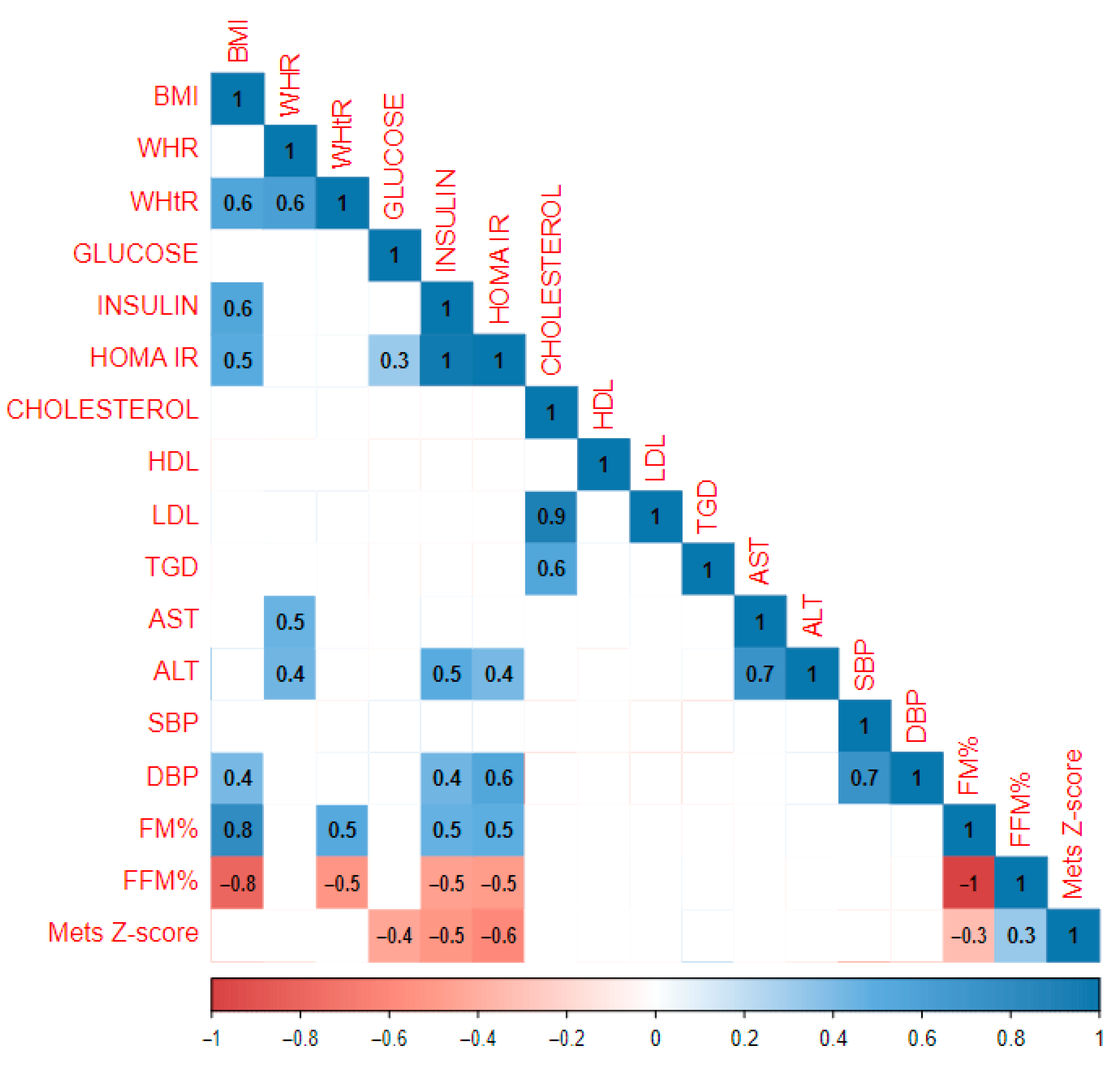
| Whole Study Population | Gender | Pubertal Period | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Available Data | Overall n = 347 1 | Girls n = 185 1 | Boys n = 162 1 | p-Value 2 | Puberty n = 301 1 | Prepuberty n = 46 1 | p-Value 2 |
| Age | 347 | 0.8–18.9 (13.4/3.4) | 0.8–18.9 (13.7/3.4) | 0.8–18.6 (13.1/3.4) | 0.034 | 7.8–18.9 (14.4/2.0) | 0.8–14.3 (7.0/3.7) | <0.001 |
| BMI | 347 | 20.7–65.8 (40.1/5.9) | 20.7–57.1 (40.1/5.7) | 24.0–65.8 (40.1/6.1) | 0.5 | 25.5–65.8 (40.9/5.3) | 20.7–62.1 (34.9/6.9) | <0.001 |
| BMI Z-score | 340 | 1.7–5.5 (2.7/0.4) | 1.7–4.9 (2.6/0.3) | 2.1–5.5 (2.8/0.4) | <0.001 | 1.7–3.4 (2.6/0.2) | 2.5–5.5 (3.1/0.7) | <0.001 |
| Unknown | 7 | 4 | 3 | 0 | 7 | |||
| Mets Z-score | 310 | 1.7–5.5 (2.7/0.3) | 1.7–3.2 (2.5/0.2) | 2.1–5.5 (2.8/0.4) | <0.001 | 1.7–3.4 (2.6/0.2) | 2.5–5.5 (3.0/0.6) | <0.001 |
| Unknown | 37 | 19 | 18 | 25 | 12 | |||
| WHR | 340 | 0.5–4.5 (0.9/0.2) | 0.7–4.5 (0.9/0.3) | 0.5–1.2 (0.9/0.1) | <0.001 | 0.5–4.5 (0.9/0.2) | 0.5–1.2 (1.0/0.1) | <0.001 |
| Unknown | 7 | 3 | 4 | 6 | 1 | |||
| WHtR | 340 | 0.3–1.0 (0.7/0.1) | 0.5–1.0 (0.7/0.1) | 0.3–1.0 (0.7/0.1) | 0.2 | 0.3–1.0 (0.7/0.1) | 0.4–0.9 (0.7/0.1) | 0.005 |
| Unknown | 7 | 3 | 4 | 6 | 1 | |||
| HOMA IR | 319 | 0.5–19.1 (6.4/3.6) | 1.2–19.1 (6.2/3.8) | 0.5–17.3 (6.7/3.5) | 0.057 | 1.2–19.1 (6.7/3.7) | 0.5–15.3 (4.8/2.9) | 0.002 |
| Unknown | 28 | 14 | 14 | 23 | 5 | |||
| Glucose (mg/dL) | 338 | 63.0–123.3 (88.6/9.2) | 64.0–122.4 (87.6/9.0) | 63.0–123.3 (89.7/9.3) | 0.027 | 64.0–123.3 (88.8/8.9) | 63.0–122.4 (87.4/11.0) | 0.2 |
| Unknown | 9 | 3 | 6 | 8 | 1 | |||
| Insulin (µIU/mL) | 319 | 2.5–98.5 (29.0/15.6) | 5.9–98.5 (28.2/16.0) | 2.5–83.0 (30.0/15.0) | 0.093 | 6.1–98.5 (30.1/15.7) | 2.5–63.2 (21.8/12.3) | 0.001 |
| Unknown | 28 | 14 | 14 | 23 | 5 | |||
| Cholesterol (mg/dL) | 336 | 82.5–307.0 (163.5/32.0) | 89.0–307.0 (163.7/31.1) | 82.5–237.0 (163.2/33.0) | 0.8 | 82.5–248.7 (162.8/31.1) | 103.0–307.0 (168.0/37.6) | 0.5 |
| Unknown | 11 | 6 | 5 | 8 | 3 | |||
| HDL (mg/dL) | 335 | 25.0–64.0 (41.8/8.3) | 26.0–64.0 (42.6/8.5) | 25.0–59.2 (40.9/8.1) | 0.089 | 25.0–64.0 (41.8/8.3) | 25.3–58.0 (42.1/8.4) | 0.7 |
| Unknown | 12 | 6 | 6 | 9 | 3 | |||
| LDL (mg/dL) | 334 | 34.9–225.0 (97.0/27.0) | 34.9–225.0 (97.6/26.8) | 44.8–161.0 (96.4/27.3) | 0.4 | 34.9–161.0 (96.5/25.9) | 35.9–225.0 (100.3/33.9) | 0.7 |
| Unknown | 13 | 8 | 5 | 10 | 3 | |||
| TGD (mg/dL) | 332 | 10.5–597.0 (134.5/69.5) | 10.5–361.4 (124.0/52.5) | 40.0–597.0 (146.7/83.8) | 0.032 | 10.5–597.0 (134.6/70.4) | 46.7–335.0 (133.6/63.8) | >0.9 |
| Unknown | 15 | 6 | 9 | 12 | 3 | |||
| ALT (U/L) | 341 | 7.5–194.0 (33.5/22.0) | 7.5–104.0 (28.8/15.9) | 10.9–194.0 (38.8/26.4) | <0.001 | 7.5–194.0 (33.0/21.8) | 10.9–133.0 (36.6/23.2) | 0.2 |
| Unknown | 6 | 3 | 3 | 6 | 0 | |||
| AST (U/L) | 329 | 8.9–93.8 (27.5/11.6) | 8.9–66.9 (25.1/9.9) | 14.0–93.8 (30.1/12.7) | <0.001 | 8.9–93.8 (26.5/11.1) | 16.0–84.0 (33.6/12.6) | <0.001 |
| Unknown | 18 | 11 | 7 | 16 | 2 | |||
| SBP (mmHG) | 323 | 70.0–186.0 (134.9/15.3) | 100.0–186.0 (133.1/15.4) | 70.0–174.0 (137.0/15.0) | 0.007 | 100.0–186.0 (136.2/14.7) | 70.0–174.0 (125.0/16.3) | <0.001 |
| Unknown | 24 | 13 | 11 | 15 | 9 | |||
| DBP (mmHg) | 323 | 40.0–118.0 (80.5/10.7) | 56.0–110.0 (80.9/10.0) | 40.0–118.0 (80.1/11.5) | 0.7 | 40.0–118.0 (81.1/10.2) | 40.0–116.0 (76.4/13.6) | 0.023 |
| Unknown | 24 | 13 | 11 | 15 | 9 | |||
| Fat mass (%) | 275 | 14.7–68.0 (46.8/7.1) | 32.7–68.0 (48.5/6.1) | 14.7–62.2 (44.8/7.7) | <0.001 | 14.7–68.0 (46.7/7.1) | 25.2–61.5 (48.2/8.1) | 0.2 |
| Unknown | 72 | 38 | 34 | 50 | 22 | |||
| Children in Pubertal Period | Gender | ||||
|---|---|---|---|---|---|
| Characteristic | Available Data | Overall n = 301 1 | Girls n = 172 1 | Boys n = 129 1 | p-Value 2 |
| Age | 301 | 7.8–18.9 (14.4/2.0) | 7.8–18.9 (14.4/2.2) | 10.3–18.6 (14.4/1.8) | 0.6 |
| BMI | 301 | 25.5–65.8 (40.9/5.3) | 25.5–57.1 (40.8/5.1) | 30.0–65.8 (41.0/5.6) | 0.8 |
| BMI Z-score | 301 | 1.7–3.4 (2.6/0.2) | 1.7–3.0 (2.5/0.2) | 2.1–3.4 (2.7/0.2) | <0.001 |
| Mets Z-score | 276 | 1.7–3.4 (2.6/0.2) | 1.7–3.0 (2.5/0.2) | 2.1–3.4 (2.7/0.2) | <0.001 |
| Unknown | 25 | 13 | 12 | ||
| WHR | 295 | 0.5–4.5 (0.9/0.2) | 0.7–4.5 (0.9/0.3) | 0.5–1.2 (0.9/0.1) | <0.001 |
| Unknown | 6 | 3 | 3 | ||
| WHtR | 295 | 0.3–1.0 (0.7/0.1) | 0.5–1.0 (0.7/0.1) | 0.3–1.0 (0.7/0.1) | 0.2 |
| Unknown | 6 | 3 | 3 | ||
| HOMA IR | 278 | 1.2–19.1 (6.7/3.7) | 1.2–19.1 (6.3/3.8) | 2.1–17.3 (7.2/3.5) | 0.007 |
| Unknown | 23 | 11 | 12 | ||
| Glucose (mg/dL) | 293 | 64.0–123.3 (88.8/8.9) | 64.0–117.0 (87.5/8.7) | 72.7–123.3 (90.5/9.0) | 0.006 |
| Unknown | 8 | 3 | 5 | ||
| Insulin (µIU/mL) | 278 | 6.1–98.5 (30.1/15.7) | 6.1–98.5 (28.7/16.1) | 9.9–83.0 (32.0/15.0) | 0.016 |
| Unknown | 23 | 11 | 12 | ||
| Cholesterol (mg/dL) | 293 | 82.5–248.7 (162.8/31.1) | 89.0–248.7 (163.4/29.6) | 82.5–237.0 (162.1/33.0) | 0.5 |
| Unknown | 8 | 5 | 3 | ||
| HDL (mg/dL) | 292 | 25.0–64.0 (41.8/8.3) | 26.0–64.0 (42.9/8.5) | 25.0–59.2 (40.4/8.0) | 0.020 |
| Unknown | 9 | 5 | 4 | ||
| LDL (mg/dL) | 291 | 34.9–161.0 (96.5/25.9) | 34.9–157.9 (97.2/25.0) | 44.8–161.0 (95.7/27.0) | 0.3 |
| Unknown | 10 | 7 | 3 | ||
| TGD (mg/dL) | 288 | 32.9–597.0 (135.0/70.2) | 32.9–361.4 (124.4/50.5) | 40.0–597.0 (149.6/88.5) | 0.047 |
| Unknown | 13 | 6 | 7 | ||
| ALT (U/L) | 295 | 7.5–194.0 (33.0/21.8) | 7.5–104.0 (28.9/16.4) | 11.8–194.0 (38.5/26.5) | <0.001 |
| Unknown | 6 | 3 | 3 | ||
| AST (U/L) | 285 | 8.9–93.8 (26.5/11.1) | 8.9–66.9 (24.7/9.6) | 14.0–93.8 (28.9/12.4) | <0.001 |
| Unknown | 16 | 10 | 6 | ||
| SBP (mmHG) | 286 | 100.0–186.0 (136.2/14.7) | 100.0–186.0 (133.8/15.3) | 111.0–174.0 (139.4/13.4) | <0.001 |
| Unknown | 15 | 8 | 7 | ||
| DBP (mmHg) | 286 | 40.0–118.0 (81.1/10.2) | 56.0–110.0 (81.1/10.0) | 40.0–118.0 (81.0/10.5) | 0.9 |
| Unknown | 15 | 8 | 7 | ||
| Fat mass (%) | 251 | 14.7–68.0 (46.7/7.1) | 32.7–68.0 (48.7/6.2) | 14.7–62.2 (44.1/7.3) | <0.001 |
| Unknown | 50 | 30 | 20 | ||
| Fat-free mass (%) | 250 | 32.0–85.3 (53.4/7.1) | 32.0–67.3 (51.3/6.2) | 37.8–85.3 (56.0/7.3) | <0.001 |
| Unknown | 51 | 31 | 20 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzeba, E.; Bik-Multanowski, M.; Brandt, S.; Małecka-Tendera, E.; Mazur, A.; Ranke, M.B.; Wabitsch, M.; Wójcik, M.; Zachurzok, A.; Przestalska-Sowa, A.; et al. Factors beyond Body Mass Index Associated with Cardiometabolic Risk among Children with Severe Obesity. J. Clin. Med. 2024, 13, 5701. https://doi.org/10.3390/jcm13195701
Kostrzeba E, Bik-Multanowski M, Brandt S, Małecka-Tendera E, Mazur A, Ranke MB, Wabitsch M, Wójcik M, Zachurzok A, Przestalska-Sowa A, et al. Factors beyond Body Mass Index Associated with Cardiometabolic Risk among Children with Severe Obesity. Journal of Clinical Medicine. 2024; 13(19):5701. https://doi.org/10.3390/jcm13195701
Chicago/Turabian StyleKostrzeba, Ewa, Mirosław Bik-Multanowski, Stephanie Brandt, Ewa Małecka-Tendera, Artur Mazur, Michael B. Ranke, Martin Wabitsch, Małgorzata Wójcik, Agnieszka Zachurzok, Anna Przestalska-Sowa, and et al. 2024. "Factors beyond Body Mass Index Associated with Cardiometabolic Risk among Children with Severe Obesity" Journal of Clinical Medicine 13, no. 19: 5701. https://doi.org/10.3390/jcm13195701
APA StyleKostrzeba, E., Bik-Multanowski, M., Brandt, S., Małecka-Tendera, E., Mazur, A., Ranke, M. B., Wabitsch, M., Wójcik, M., Zachurzok, A., Przestalska-Sowa, A., & Petriczko, E. (2024). Factors beyond Body Mass Index Associated with Cardiometabolic Risk among Children with Severe Obesity. Journal of Clinical Medicine, 13(19), 5701. https://doi.org/10.3390/jcm13195701








