Adenosquamous Carcinoma of the Lung: Survival, Radiologic Findings, PD-L1, and Driver Mutations
Abstract
1. Introduction
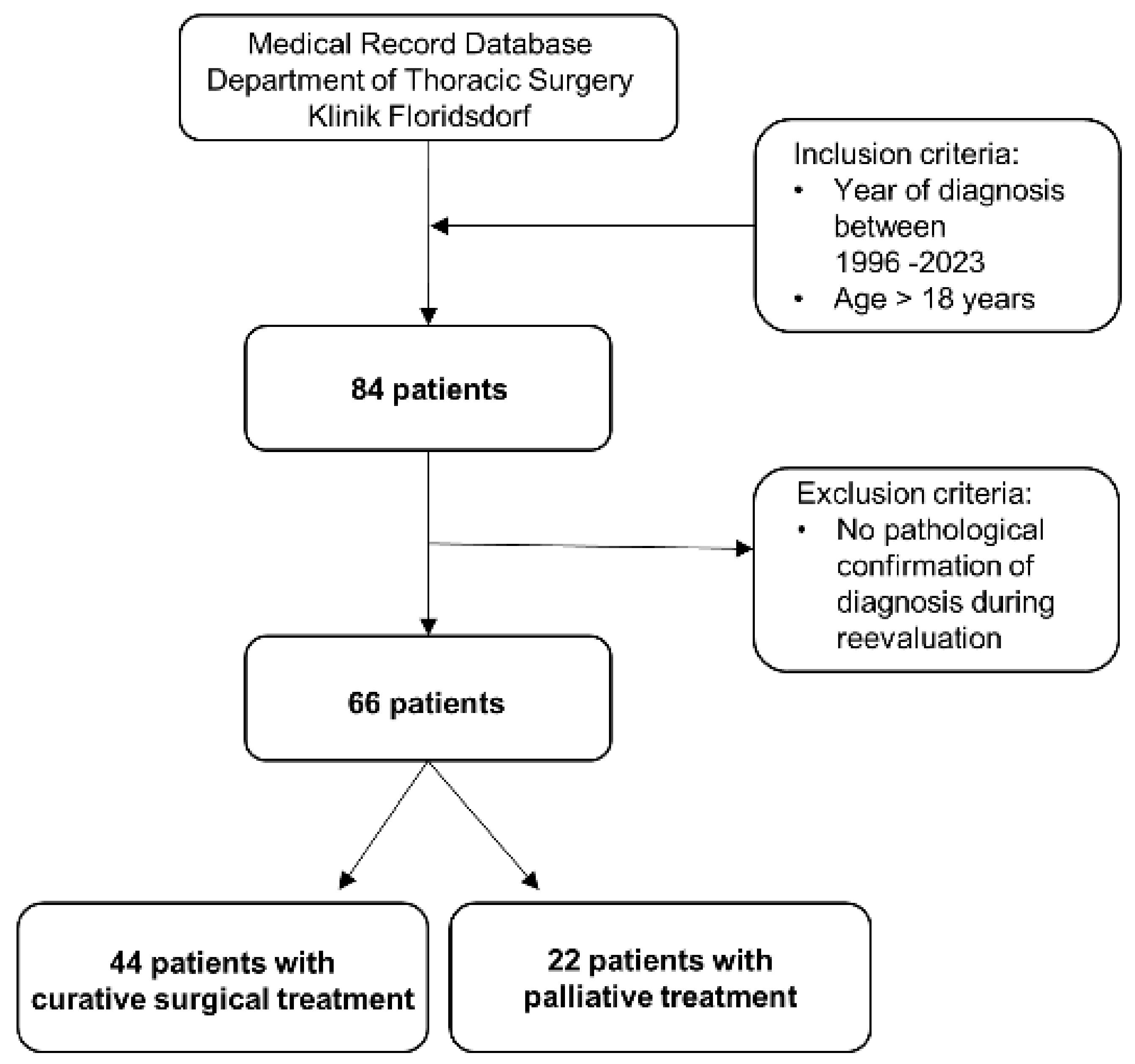
2. Patients and Methods
2.1. Study Design
2.2. Patients
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
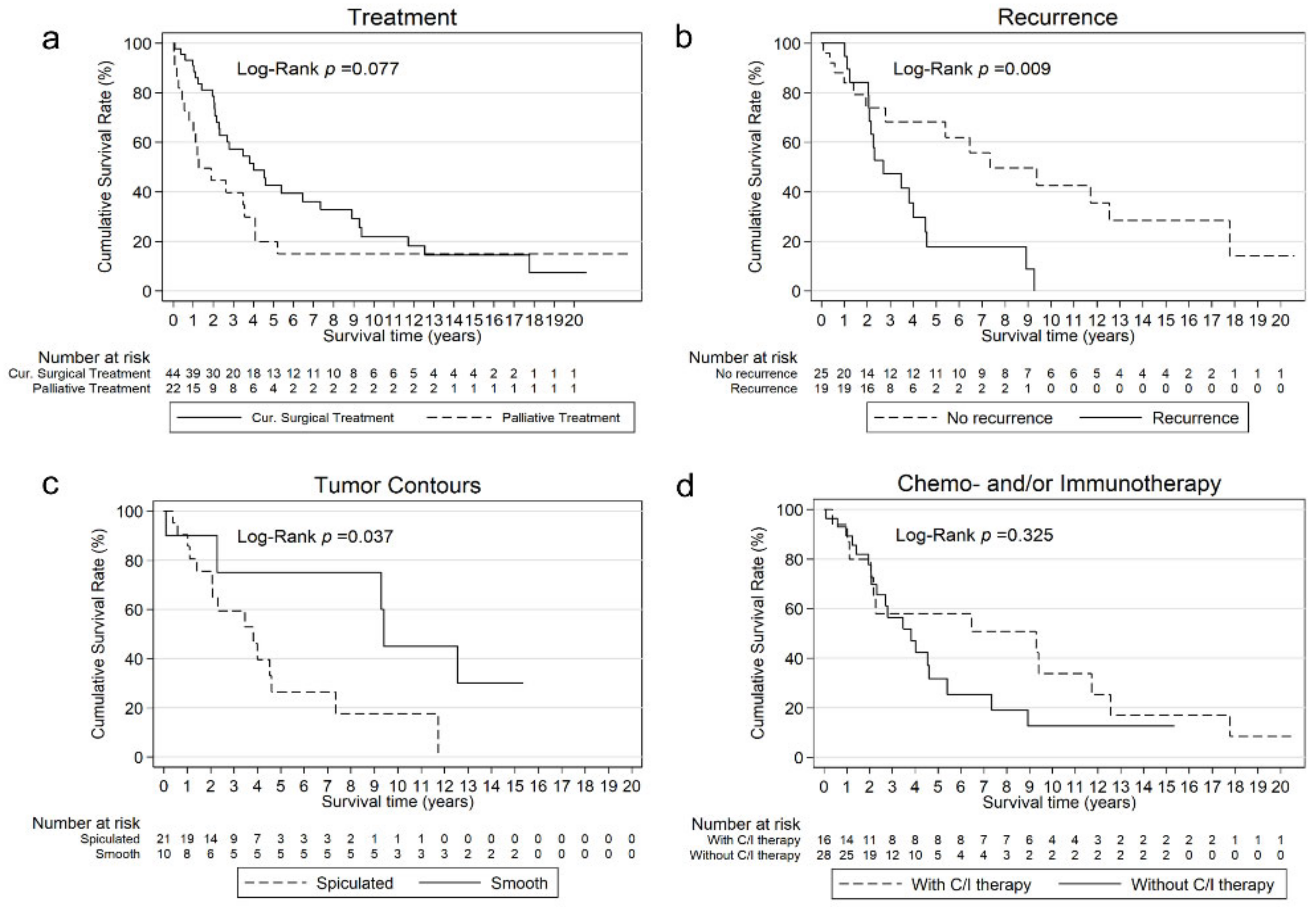
3.2. Recurrence
3.3. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenocarcinoma |
| AC-ASC | Adenocarcinoma-predominant adenosquamous carcinoma |
| ALK | Anaplastic lymphoma kinase |
| ASC | Adenosquamous carcinoma |
| BAL | Structurally balanced adenosquamous carcinoma |
| CEA | Carcinoembryonic antigen |
| CYFRA21-1 | Cytokeratin-19-fragment |
| CI | Confidence interval |
| EGFR | Epidermal growth factor receptor |
| Del19 | Exon 19 deletion |
| HR | Hazard Ratio |
| Ins20 | Exon 20 insertion |
| L858R | Exon 21 Leu858Arg substitution |
| NSCLC | Non-small cell lung cancer |
| NSE | Neuron-specific enolase |
| OS | Overall survival |
| PD-L1 | Programmed death-ligand 1 |
| PL 1-3 | Invasion of the pleura visceralis |
| ROS-1 | ROS protooncogene 1 |
| SCC | Squamous cell carcinoma |
| SCC-ASC | Squamous cell carcinoma predominant adenosquamous carcinoma |
| TTF-1 | Thyroid transcription factor 1 |
| V1/2 | Vascular invasion |
| WHO | World Health Organization |
References
- Li, C.; Lu, H. Adenosquamous Carcinoma of the Lung. OncoTargets Ther. 2018, 11, 4829–4835. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.; Ikeda, T.; Hanaki, H.; Miyata, Y.; Mukaida, H.; Okada, M. Clinicopathologic Study of Stage I Adenosquamous Carcinoma of the Lung. Jpn. J. Clin. Oncol. 2023, 53, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Mordant, P.; Grand, B.; Cazes, A.; Foucault, C.; Dujon, A.; Le Pimpec Barthes, F.; Riquet, M. Adenosquamous Carcinoma of the Lung: Surgical Management, Pathologic Characteristics, and Prognostic Implications. Ann. Thorac. Surg. 2013, 95, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Krawczyk, P.; Ramlau, R.; Sura, S.; Wojas-Krawczyk, K.; Kucharczyk, T.; Walczyna, B.; Szumiło, J.; Szyszka-Barth, K.; Milecki, P.; et al. EGFR Gene Mutations in Patients with Adenosquamous Lung Carcinoma: EGFR Mutations in ADSQ Cell Carcinoma. Asia Pac. J. Clin. Oncol. 2014, 10, 340–345. [Google Scholar] [CrossRef]
- Vassella, E.; Langsch, S.; Dettmer, M.S.; Schlup, C.; Neuenschwander, M.; Frattini, M.; Gugger, M.; Schäfer, S.C. Molecular Profiling of Lung Adenosquamous Carcinoma: Hybrid or Genuine Type? Oncotarget 2015, 6, 23905–23916. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Shimizu, J.; Oda, M.; Hayashi, Y.; Nonomura, A.; Watanabe, Y. A Clinicopathologic Study of Resected Cases of Adenosquamous Carcinoma of the Lung. Chest 1996, 109, 989–994. [Google Scholar] [CrossRef]
- Gawrychowski, J.; Brulinski, K.; Malinowski, E.; Papla, B. Prognosis and Survival after Radical Resection of Primary Adenosquamous Lung Carcinoma. Eur. J. Cardiothorac. Surg. 2005, 27, 686–692. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yasumitu, T.; Fukuhara, K.; Shiono, H.; Kikui, M. Poor Prognosis after Lung Resection for Patients with Adenosquamous Carcinoma of the Lung. Ann. Thorac. Surg. 2003, 75, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, A.; Kawabata, T.; Suito, T.; Kawashima, O.; Watanabe, T.; Okabayashi, K.; Kubota, I.; for the Japan National Hospital Organization Study Group for Lung Cancer. Adenosquamous Carcinoma of the Lung: Surgical Results as Compared with Squamous Cell and Adenocarcinoma Cases. Eur. J. Cardiothorac. Surg. 2012, 41, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Matsuzaki, Y.; Shimizu, T.; Hara, M.; Ayabe, T.; Onitsuka, T. Prognostic Determinants for Lung Cancer Patients with Preoperative High Serum Carcinoembryonic Antigen Levels. Thorac. Cardiovasc. Surg. 2005, 53, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR -Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR -Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Solomon, B.J.; Bauer, T.M.; Mok, T.S.K.; Liu, G.; Mazieres, J.; De Marinis, F.; Goto, Y.; Kim, D.-W.; Wu, Y.-L.; Jassem, J.; et al. Efficacy and Safety of First-Line Lorlatinib versus Crizotinib in Patients with Advanced, ALK-Positive Non-Small-Cell Lung Cancer: Updated Analysis of Data from the Phase 3, Randomised, Open-Label CROWN Study. Lancet Respir. Med. 2023, 11, 354–366. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.-S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in Resected ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, B.; Xu, J.; Wang, S.; Zhao, Y.; Zhang, L.; Han, B. Clinical Outcomes of Different Generations of EGFR Tyrosine Kinase Inhibitors in Advanced Lung Adenosquamous Carcinoma. Mol. Diagn. Ther. 2019, 23, 773–779. [Google Scholar] [CrossRef]
- Bell, D.W.; Lynch, T.J.; Haserlat, S.M.; Harris, P.L.; Okimoto, R.A.; Brannigan, B.W.; Sgroi, D.C.; Muir, B.; Riemenschneider, M.J.; Iacona, R.B.; et al. Epidermal Growth Factor Receptor Mutations and Gene Amplification in Non–Small-Cell Lung Cancer: Molecular Analysis of the IDEAL/INTACT Gefitinib Trials. J. Clin. Oncol. 2005, 23, 8081–8092. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, T.; Ishii, G.; Goto, K.; Nagai, K.; Mimaki, S.; Ono, S.; Niho, S.; Fujii, S.; Ohe, Y.; Tsuchihara, K.; et al. Clinicopathological Characteristics of EGFR Mutated Adenosquamous Carcinoma of the Lung. Pathol. Int. 2013, 63, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Luo, Z.; Xiong, W.; Shi, Z.; Tan, H. Epidemiology and Survival Outcomes in Adenosquamous Carcinoma: A Population-Based Study. Int. J. Color. Dis. 2022, 37, 1581–1592. [Google Scholar] [CrossRef]
- Yip, S.S.F.; Liu, Y.; Parmar, C.; Li, Q.; Liu, S.; Qu, F.; Ye, Z.; Gillies, R.J.; Aerts, H.J.W.L. Associations between Radiologist-Defined Semantic and Automatically Computed Radiomic Features in Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 3519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Bai, L.; Chen, F.; Wang, J.; Guo, Y. Adenocarcinomatous-Predominant Subtype Associated with a Better Prognosis in Adenosquamous Lung Carcinoma. BMC Cancer 2020, 20, 520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, H.; Yao, F.; Sun, Y.; Xu, J.; Gu, H.; Shen, Z. Improved Survival Associated with a Balanced Structure between Adenomatous and Squamous Components in Patients with Adenosquamous Carcinoma of the Lung. Eur. J. Surg. Oncol. EJSO 2016, 42, 1699–1706. [Google Scholar] [CrossRef]
- Desai, A.; Peters, S. Immunotherapy-Based Combinations in Metastatic NSCLC. Cancer Treat. Rev. 2023, 116, 102545. [Google Scholar] [CrossRef]
- Hlaing, A.M.; Furusato, B.; Udo, E.; Kitamura, Y.; Souda, M.; Masutani, M.; Fukuoka, J. Expression of Phosphatase and Tensin Homolog and Programmed Cell Death Ligand 1 in Adenosquamous Carcinoma of the Lung. Biochem. Biophys. Res. Commun. 2018, 503, 2764–2769. [Google Scholar] [CrossRef]
- Shi, X.; Wu, S.; Sun, J.; Liu, Y.; Zeng, X.; Liang, Z. PD-L1 Expression in Lung Adenosquamous Carcinomas Compared with the More Common Variants of Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 46209. [Google Scholar] [CrossRef]
- Li, C.; Zheng, X.; Li, P.; Wang, H.; Hu, J.; Wu, L.; Wang, Z.; Guo, H.; Wu, F.; Zhong, W.; et al. Heterogeneity of Tumor Immune Microenvironment and Real-World Analysis of Immunotherapy Efficacy in Lung Adenosquamous Carcinoma. Front. Immunol. 2022, 13, 944812. [Google Scholar] [CrossRef]
| Characteristics at Diagnosis | All Patients (n = 66) | Patients with Curative Surgical Treatment (n = 44) | Patients with Palliative Treatment (n = 22) |
|---|---|---|---|
| Age, years | |||
| Median (range) | 70 (37–92) | 71 (37–92) | 69 (39–82) |
| Age groups, n (%) | |||
| <65 | 23 (35) | 15 (34) | 8 (36) |
| ≥65 | 43 (65) | 29 (66) | 14 (64) |
| Gender, n (%) | |||
| Male | 35 (53) | 21 (48) | 14 (64) |
| Female | 31 (47) | 23 (52) | 8 (36) |
| Ethnicity, n (%) | |||
| Asian | 2 (3) | 1 (2) | 1 (5) |
| Non-Asian | 64 (97) | 43 (98) | 21 (95) |
| Smoking status, n (%) | (n = 62) | (n = 41) | (n = 21) |
| Never smoker | 13 (21) | 10 (24) | 3 (14) |
| Former smoker | 26 (42) | 17 (41) | 9 (43) |
| Current smoker | 23 (37) | 14 (34) | 9 (43) |
| Pack years, n (%) | (n = 36) | (n = 23) | (n = 13) |
| Smoker (<30 py) | 8 (22) | 6 (26) | 2 (15) |
| Heavy smoker (≥30 py) | 28 (78) | 17 (74) | 11 (85) |
| Stage at initial diagnosis, n (%) | |||
| Stage I | 16 (24) | 14 (32) | 2 (9) |
| Stage Ia | 6 (9) | 4 (9) | 2 (9) |
| Stage Ib | 10 (15) | 10 (23) | 0 (0) |
| Stage II | 10 (15) | 7 (16) | 3 (14) |
| Stage IIa | 2 (3) | 2 (5) | 0 (0) |
| Stage IIb | 8 (12) | 5 (11) | 3 (14) |
| Stage III | 24 (36) | 23 (52) | 1 (5) |
| Stage IIIa | 18 (27) | 17 (39) | 1 (5) |
| Stage IIIb | 6 (9) | 6 (14) | 0 (0) |
| Stage IV | 16 (24) | 0 (0) | 16 (73) |
| Stage IVa | 14 (21) | 0 (0) | 14 (64) |
| Stage IVb | 2 (3) | 0 (0) | 2 (9) |
| Pathological characteristics | All patients (n = 66) | Patients with curative surgical treatment (n = 44) | Patients with palliative treatment (n = 22) |
| PD-L1 status, n (%) | |||
| Negative (<1%) | 25 (38) | 16 (36) | 9 (41) |
| 1–49% | 30 (45) | 21 (48) | 9 (41) |
| 50–89% | 6 (9) | 3 (7) | 3 (14) |
| ≥90% | 5 (8) | 4 (9) | 1 (5) |
| EGFR-mutation 1, n (%) | |||
| Positive | 11 (17) | 7 (16) | 4 (18) |
| Negative | 55 (83) | 37 (84) | 18 (82) |
| ALK-fusion, n (%) | |||
| Positive | 1 (2) | 1 (2) | 0 (0) |
| Negative | 65 (98) | 43 (98) | 22 (100) |
| ROS-1-fusion, n (%) | |||
| Positive | 0 (0) | 0 (0) | 0 (0) |
| Radiological characteristics | All patients (n = 50) | Patients with curative surgical treatment (n = 33) | Patients with palliative treatment (n = 17) |
| Tumor location, n (%) | |||
| Peripheral | 33 (66) | 22 (67) | 11 (65) |
| Central | 17 (34) | 11 (33) | 6 (35) |
| Cavitation, n (%) | |||
| Yes | 4 (8) | 2 (6) | 2 (12) |
| No | 46 (92) | 31 (94) | 15 (88) |
| Cysts, n (%) | |||
| Yes | 2 (4) | 1 (3) | 1 (6) |
| No | 48 (96) | 32 (97) | 16 (94) |
| Tumor contours, n (%) | |||
| Spiculated | 32 (64) | 21 (64) | 11 (65) |
| Smooth | 15 (30) | 10 (30) | 5 (29) |
| Lobulated | 3 (6) | 2 (6) | 1 (6) |
| Air bronchogram, n (%) | |||
| Yes | 9 (18) | 7 (21) | 2 (12) |
| No | 41 (82) | 26 (79) | 15 (88) |
| Inflammatory changes, n (%) | |||
| Yes | 15 (30) | 10 (30) | 5 (29) |
| No | 35 (70) | 23 (70) | 12 (71) |
| Pleural or pericardial effusion, n (%) | |||
| Yes | 8 (16) | 2 (6) | 6 (35) |
| No | 42 (84) | 31 (94) | 11 (65) |
| Pleura tag, n (%) | |||
| Yes | 24 (48) | 15 (45) | 9 (53) |
| No | 26 (52) | 18 (55) | 8 (47) |
| Pathological Characteristics | (n = 44) |
|---|---|
| Structural components, n (%) | |
| AC-ASC | 17 (39) |
| SCC-ASC | 26 (59) |
| BAL-ASC | 1 (2) |
| T-stage, n (%) | |
| T1 | 12 (27) |
| T1a 1 | 2 (5) |
| T1b | 7 (16) |
| T1c | 3 (7) |
| T2 | 21 (48) |
| T2a | 14 (32) |
| T2b | 7 (16) |
| T3 | 7 (16) |
| T4 | 4 (9) |
| N-stage, n (%) | |
| N0 | 24 (55) |
| N1 | 8 (18) |
| N2 | 11 (25) |
| N3 | 1 (2) |
| Tumor size (cm) | |
| Median (Range) | 3.5 (0–10.0) |
| Groups, n (%) | |
| <5 cm | 37 (84) |
| ≥5 cm | 7 (16) |
| Vascular invasion, n (%) | |
| V0 | 35 (80) |
| V1 | 9 (20) |
| Invasion of the pleura visceralis, n (%) | |
| PL0 | 31 (70) |
| PL1 | 10 (23) |
| PL2/PL3 | 3 (7) |
| Resection margin, n (%) | |
| R0 | 42 (95) |
| R1 | 2 (5) |
| Perioperative Treatment Characteristics | (n = 44) |
|---|---|
| Chemotherapy and/or immunotherapy, n (%) | |
| Neoadjuvant | 5 (11) |
| Adjuvant | 5 (11) |
| Neoadjuvant and Adjuvant | 6 (14) |
| No chemotherapy | 28 (64) |
| Treatment with targeted therapy, n (%) 1 | |
| Neoadjuvant | 1 (2) |
| Adjuvant | 4 (9) |
| Neoadjuvant and Adjuvant | 1 (2) |
| No targeted therapy | 38 (86) |
| Radiation therapy, n (%) | |
| Adjuvant | 7 (16) |
| Type of operation, n (%) | |
| (Bi-)Lobectomy | 36 (82) |
| Pneumonectomy | 6 (14) |
| Segmentectomy | 2 (5) |
| Surgical technique, n (%) | |
| Thoracotomy | 34 (77) |
| VATS | 10 (23) |
| Lymphadenectomy, n (%) | |
| Yes | 41 (93) |
| No | 3 (7) |
| Variable | N (%) | Median Survival Time (95% CI) | p-Value |
|---|---|---|---|
| n = 44 | |||
| Age, years | |||
| <65 | 15 (34) | 24.8 (12.3; 41.7) | 0.013 |
| ≥65 | 29 (66) | 64.7 (32.4; 140.4) | |
| Gender | 0.766 | ||
| Male | 21 (48) | 45.8 (23.4; 77.4) | |
| Female | 23 (52) | 55.1 (24.8; 112.6) | |
| Smoking status | 27.7 (12.3; NA) 64.7 (32.4; 112.6) | 0.708 | |
| Never smoker | 10 (23) | ||
| Smoking history | 31 (70) | ||
| Unknown (excluded) | 3 (7) | ||
| Structural components | 0.471 | ||
| AC-ASC | 17 (39) | 45.8 (26.0; 88.0) | |
| SCC-ASC | 26 (59) | 54.4 (24.6; 112.6) | |
| BAL-ASC (excluded) | 1 (2) | ||
| TNM-stage | 0.715 | ||
| Stage I | 14 (32) | 55.1 (27.7; 77.4) | |
| Stage II | 7 (16) | 45.8 (4.5; NA) | |
| Stage III | 23 (52) | 32.4 (14.9; 112.6) | |
| Lymph nodes | 0.459 | ||
| N0 | 24 (55) | 55.1 (27.2; 88.0) | |
| N1 | 8 (18) | 106.9 (24.6; NA) | |
| N2 | 11 (25) | 17.0 (11.8; 33.5) | |
| N3 | 1 (2) | ||
| Size of tumor | 0.876 | ||
| <5 cm | 37 (84) | 54.4 (27.3; 106.9) | |
| ≥5 cm | 7 (16) | 32.4 (7.1; NA) | |
| Vascular invasion | 0.428 | ||
| V0 | 35 (80) | 41.7 (24.8; 88) | |
| V1 | 9 (20) | 55.1 (27.7; NA) | |
| Visceral pleural invasion | 0.740 | ||
| PL0 | 31 (70) | 64.7 (25.0; 111.2) | |
| PL1 | 10 (23) | 45.8 (12.3; NA) | |
| PL2/PL3 | 3 (7) | 27.3 (4.5; NA) | |
| PD-L1 status | 0.884 | ||
| Negative | 16 (36) | 33.5 (13.4; 213.0) | |
| 1–49% | 21 (48) | 48.1 (24.6; 106.9) | |
| ≥50% | 7 (16) | 111.2 (26.0; NA) | |
| Target mutation (EGFR, ALK) | 0.636 | ||
| Positive | 8 (18) | 41.7 (11.8; NA) | |
| Negative | 36 (82) | 54.4 (27.0; 88.0) | |
| EGFR status | 0.416 | ||
| Positive | 7 (16) | 41.7 (11.8; NA) | |
| Negative | 37 (84) | 48.1 (26; 88.0) | |
| Diagnosis | 0.425 | ||
| Prior 2016 | 30 (68) | 33.5 (24.8; 88.0) | |
| Since 2016 | 14 (32) | 45.8 (27.3; NA) | |
| Recurrence | 0.009 | ||
| Yes | 19 (43) | 32.4 (24.8; 54.4) | |
| No | 25 (57) | 88.0 (33.5; 213) | |
| Chemotherapy and/or immunotherapy | |||
| Yes | 16 (36) | 111.2 (13.4; 150.3) | |
| No | 28 (64) | 45.8 (24.9; 64.7) | 0.325 |
| n = 33 | |||
| Tumor location | 0.793 | ||
| Peripheral | 22 (67) | 48.1 (24.8; 112.6) | |
| Central | 11 (33) | 33.5 (13.4; NA) | |
| Inflammatory changes | 0.823 | ||
| Yes | 10 (30) | 41.7 (1.1; 112.6) | |
| No | 23 (70) | 48.1 (24.6; 140.4) | |
| Pleura tag | 0.177 | ||
| Yes | 15 (45) | 140.4 (17.0; NA) | |
| No | 18 (55) | 41.7 (24.6; 111.2) | |
| Tumor contours | 45.8 (24.8; 55.1) 112.6 (1.1; NA) | 0.037 | |
| Spiculated | 21 (64) | ||
| Smooth | 10 (30) | ||
| Lobulated (excluded) | 2 (6) | ||
| Carcinoembryonic antigen (CEA) (µg/L) | (n = 23) | ||
| <5 µg/L | 14 (61) | 106.8 (32.4; 140.5) | |
| ≥5 µg/L | 9 (39) | 33.5 (4.5; 111.2) | 0.195 |
| Cytokeratin-19-fragment (CYFRA21-1) (µg/L) | (n = 21) | ||
| <3.3 µg/L | 17 (81) | 55.1 (13.4; 111.2) | |
| ≥3.3 µg/L | 4 (19) | 54.4 (32.4; NA) | 0.214 |
| Neuron-Specific Enolase | (n = 18) | 0.191 | |
| (NSE) (µg/L) | |||
| <12.5 µg/L | 14 (78) | 54.4 (13.4; 111.3) | |
| ≥12.5 µg/L | 4 (22) | 112.6 (55.1; NA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illini, O.; Fabikan, H.; Fischer, E.; Lang-Stöberl, A.S.; Krenbek, D.; Jarius, C.; Azarnia-Medan, S.; Gasser, S.; Hochmair, M.J.; Weinlinger, C.; et al. Adenosquamous Carcinoma of the Lung: Survival, Radiologic Findings, PD-L1, and Driver Mutations. J. Clin. Med. 2024, 13, 5711. https://doi.org/10.3390/jcm13195711
Illini O, Fabikan H, Fischer E, Lang-Stöberl AS, Krenbek D, Jarius C, Azarnia-Medan S, Gasser S, Hochmair MJ, Weinlinger C, et al. Adenosquamous Carcinoma of the Lung: Survival, Radiologic Findings, PD-L1, and Driver Mutations. Journal of Clinical Medicine. 2024; 13(19):5711. https://doi.org/10.3390/jcm13195711
Chicago/Turabian StyleIllini, Oliver, Hannah Fabikan, Eva Fischer, Anna Sophie Lang-Stöberl, Dagmar Krenbek, Christa Jarius, Shokoufa Azarnia-Medan, Stefan Gasser, Maximilian Johannes Hochmair, Christoph Weinlinger, and et al. 2024. "Adenosquamous Carcinoma of the Lung: Survival, Radiologic Findings, PD-L1, and Driver Mutations" Journal of Clinical Medicine 13, no. 19: 5711. https://doi.org/10.3390/jcm13195711
APA StyleIllini, O., Fabikan, H., Fischer, E., Lang-Stöberl, A. S., Krenbek, D., Jarius, C., Azarnia-Medan, S., Gasser, S., Hochmair, M. J., Weinlinger, C., Valipour, A., & Watzka, S. (2024). Adenosquamous Carcinoma of the Lung: Survival, Radiologic Findings, PD-L1, and Driver Mutations. Journal of Clinical Medicine, 13(19), 5711. https://doi.org/10.3390/jcm13195711







