Novel Immune Checkpoint Inhibitor Targets in Advanced or Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives
Abstract
1. Introduction
2. Methods
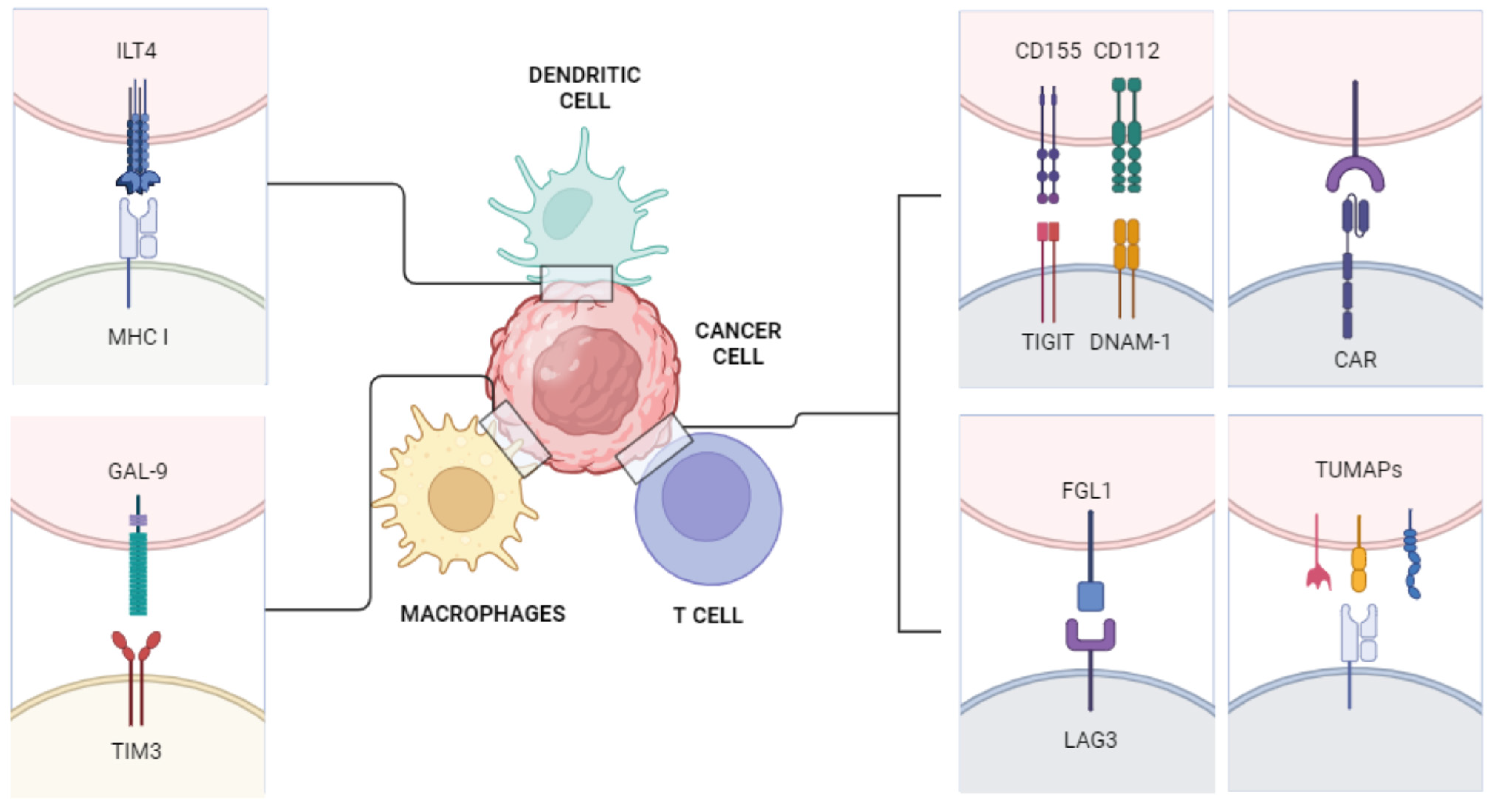
3. New Immune Pathways
3.1. T Cell Immunoglobulin and ITIM Domain (TIGIT)
3.2. Immunoglobulin-like Transcript 4 (ILT4)
3.3. Chimeric Antigen Receptor-T (CAR-T)
3.4. Lymphocytes Activation Gene 3 (LAG3)
3.5. T Cell Immunoglobulin and Mucin Domain 3 (TIM-3)
3.6. Vaccines
3.7. Immunosuppressive Cells and Resistance
4. Trials Ongoing
4.1. Phase I–II Targeting TIGIT
4.2. Phase I–II Targeting ILT4
4.3. Phase I–II of CAR-T
4.4. Phase I–II Targeting LAG3
4.5. Phase I–II Targeting TIM-3
4.6. Phase I–II of Vaccines
4.7. Overview on Ongoing Trials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. International Agency for Research on Cancer. World Health Organization. 2020. Available online: https://gco.iarc.fr/ (accessed on 30 March 2021).
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, C.; Brunelli, M.; Montironi, R.; Fiorentino, M.; Iacovelli, R.; Heng, D.; Tortora, G.; Massari, F. The prospect of precision therapy for renal cell carcinoma. Cancer Treat. Rev. 2016, 49, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, M.; Marchetti, A.; Mollica, V.; Rizzo, A.; Santoni, M.; Massari, F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 2023, 20, 133–157. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef]
- Massari, F.; Rizzo, A.; Mollica, V.; Rosellini, M.; Marchetti, A.; Ardizzoni, A.; Santoni, M. Immune-based combinations for the treatment of metastatic renal cell carcinoma: A meta-analysis of randomised clinical trials. Eur. J. Cancer 2021, 154, 120–127. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Hansen, A.S.; Park, J.; Lemaitre, L.; Lai, I.; Adeniji, N.; Kuruvilla, S.; Suresh, A.; Zhang, J.; Swamy, V.; et al. MYC Overexpression Drives Immune Evasion in Hepatocellular Carcinoma That Is Reversible through Restoration of Proinflammatory Macrophages. Cancer Res. 2023, 83, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers 2021, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Fu, B.M. Resistance Mechanisms of Anti-angiogenic Therapy and Exosomes-Mediated Revascularization in Cancer. Front. Cell Dev. Biol. 2020, 8, 610661. [Google Scholar] [CrossRef]
- Albiges, L.; Schmidinger, M.; Taguieva-Pioger, N.; Perol, D.; Grünwald, V.; Guemas, E. CaboPoint: A phase II study of cabozantinib as second-line treatment in patients with metastatic renal cell carcinoma. Futur. Oncol. 2021, 18, 915–926. [Google Scholar] [CrossRef]
- Procopio, G.; Claps, M.; Pircher, C.; Porcu, L.; Sepe, P.; Guadalupi, V.; De Giorgi, U.; Bimbatti, D.; Nolè, F.; Carrozza, F.; et al. A multicenter phase 2 single arm study of cabozantinib in patients with advanced or unresectable renal cell carcinoma pre-treated with one immune-checkpoint inhibitor: The BREAKPOINT trial (Meet-Uro trial 03). Tumori J. 2023, 109, 129–137. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Bracarda, S.; Grande, E.; Matrana, M.R.; Rizzo, M.; De Giorgi, U.; Basso, U.; Aurilio, G.; Incorvaia, L.; et al. Cabozantinib in Patients with Advanced Renal Cell Carcinoma Primary Refractory to First-line Immunocombinations or Tyrosine Kinase Inhibitors. Eur. Urol. Focus 2022, 8, 1696–1702. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Buti, S.; Olivari, A.; Masini, C.; Bimbatti, D.; Sartori, D.; Ermacora, P.; Cattrini, C.; Vitale, M.G.; Rossi, E.; Mucciarini, C.; et al. Assessing the effectiveness and safety of lenvatinib and everolimus in advanced renal cell carcinoma: Insights from the RELIEVE study’s analysis of heavily pretreated patients. Ther. Adv. Urol. 2024, 16, 17562872241244574. [Google Scholar] [CrossRef]
- Albiges, L.; Rini, B.; Peltola, K.; Oria, G.D.V.; Burotto, M.; Rodriguez, C.S.; Ghatalia, P.; Iacovelli, R.; Lam, E.; Verzoni, E.; et al. LBA88 Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): Randomized open-label phase III LITESPARK-005 study. Ann. Oncol. 2023, 34, S1329–S1330. [Google Scholar] [CrossRef]
- Choueiri, T.K.; McDermott, D.F.; Merchan, J.; Bauer, T.M.; Figlin, R.; Heath, E.I.; Michaelson, M.D.; Arrowsmith, E.; D’SOuza, A.; Zhao, S.; et al. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: An open-label, single-arm, phase 2 study. Lancet Oncol. 2023, 24, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Albiges, L.; Tomczak, P.; Suárez, C.; Voss, M.H.; de Velasco, G.; Chahoud, J.; Mochalova, A.; Procopio, G.; Mahammedi, H.; et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): A multicentre, randomised, open-label, phase 3 trial. Lancet 2023, 402, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, M.; Tassinari, E.; Marchetti, A.; Mollica, V.; Massari, F. Re: Atezolizumab Plus Cabozantinib Versus Cabozantinib Monotherapy for Patients with Renal Cell Carcinoma After Progression with Previous Immune Checkpoint Inhibitor Treatment (CONTACT-03): A Multicentre, Randomised, Open-label, Phase 3 Trial. Eur. Urol. 2024, 85, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting Edge: TIGIT Has T Cell-Intrinsic Inhibitory Functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314. [Google Scholar] [CrossRef]
- Nagumo, Y.; Iguchi-Manaka, A.; Yamashita-Kanemaru, Y.; Abe, F.; Bernhardt, G.; Shibuya, A.; Shibuya, K. Increased CD112 Expression in Methylcholanthrene-Induced Tumors in CD155-Deficient Mice. PLoS ONE 2014, 9, e112415. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8 + T Cell Effector Function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef]
- Rousseau, A.; Parisi, C.; Barlesi, F. Anti-TIGIT therapies for solid tumors: A systematic review. ESMO Open 2023, 8, 101184. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wang, X.; Wang, T.; Zhang, X. Correlation of T Cell Immunoglobulin and ITIM Domain (TIGIT) and Programmed Death 1 (PD-1) with Clinicopathological Characteristics of Renal Cell Carcinoma May Indicate Potential Targets for Treatment. Med. Sci. Monit. 2018, 24, 6861–6872. [Google Scholar] [CrossRef] [PubMed]
- Nuvola, G.; Mollica, V.; Massari, F.; Suárez, C. The Future of Immunotherapy in Advanced Renal Cell Carcinoma: Beyond PD-1/PD-L1 Inhibitors. Immunotherapy 2023, 15, 1429–1433. [Google Scholar] [CrossRef]
- Gao, A.; Sun, Y.; Peng, G. ILT4 functions as a potential checkpoint molecule for tumor immunotherapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.L.; Wang, D.; Hilton, J.; Geva, R.; Rasco, D.; Perets, R.; Abraham, A.K.; Wilson, D.C.; Markensohn, J.F.; Lunceford, J.; et al. First-in-Class Anti-immunoglobulin–like Transcript 4 Myeloid-Specific Antibody MK-4830 Abrogates a PD-1 Resistance Mechanism in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Riddell, S.R. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015, 36, 494–502. [Google Scholar] [CrossRef]
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical development of CAR T cells—Challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Afrough, A.; Abraham, P.R.; Turer, L.; Kaur, G.; Sannareddy, A.; Hansen, D.K.; Anderson, L.D., Jr. Toxicity of CAR T-Cell Therapy for Multiple Myeloma. Acta Haematol. 2024, 1–15. [Google Scholar] [CrossRef]
- Mansouri, V.; Yazdanpanah, N.; Rezaei, N. The immunologic aspects of cytokine release syndrome and graft versus host disease following CAR T cell therapy. Int. Rev. Immunol. 2022, 41, 649–668. [Google Scholar] [CrossRef]
- Gong, Y.; Wolterink, R.G.J.K.; Wang, J.; Bos, G.M.J.; Germeraad, W.T.V. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Lipson, E.J.; Dummer, R.; Larkin, J.; Long, G.V.; Sanborn, R.E.; Chiarion-Sileni, V.; Dréno, B.; Dalle, S.; Schadendorf, D.; et al. Nivolumab and Relatlimab in Patients With Advanced Melanoma That Had Progressed on Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy: Results From the Phase I/IIa RELATIVITY-020 Trial. J. Clin. Oncol. 2023, 41, 2724–2735. [Google Scholar] [CrossRef]
- Long, G.V.; Hodi, F.S.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Salman, P.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; et al. Overall Survival and Response with Nivolumab and Relatlimab in Advanced Melanoma. NEJM Evid. 2023, 2, EVIDoa2200239. [Google Scholar] [CrossRef]
- Schoenfeld, D.A.; Merkin, R.D.; Moutafi, M.; Martinez, S.; Adeniran, A.; Kumar, D.; Jilaveanu, L.; Hurwitz, M.; Rimm, D.L.; Kluger, H.M. Location matters: LAG3 levels are lower in renal cell carcinoma metastatic sites compared to primary tumors, and expression at metastatic sites only may have prognostic importance. Front. Oncol. 2022, 12, 990367. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Kamimura, Y.; Akiba, H.; Yagita, H.; Okumura, K.; Takahashi, H.; Zeniya, M.; Tajiri, H.; Azuma, M. Preferential Involvement of Tim-3 in the Regulation of Hepatic CD8+ T Cells in Murine Acute Graft-versus-Host Disease. J. Immunol. 2006, 177, 4281–4287. [Google Scholar] [CrossRef]
- Li, H.; Wu, K.; Tao, K.; Chen, L.; Zheng, Q.; Lu, X.; Liu, J.; Shi, L.; Liu, C.; Wang, G.; et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012, 56, 1342–1351. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, B.; Zhao, H.; Huang, Q. Prognostic implication of TIM-3 in clear cell renal cell carcinoma. Neoplasma 2014, 61, 35–40. [Google Scholar] [CrossRef]
- Rammensee, H.G.; Falk, K.; Rötzschke, O. Peptides naturally presented by MHC class I molecules. Annu. Rev. Immunol. 1993, 11, 213–244. [Google Scholar] [CrossRef] [PubMed]
- Kirner, A.; Mayer-Mokler, A.; Reinhardt, C. IMA901: A multi-peptide cancer vaccine for treatment of renal cell cancer. Hum. Vaccines Immunother. 2014, 10, 3179–3189. [Google Scholar] [CrossRef]
- Walter, S.; Weinschenk, T.; Stenzl, A.; Zdrojowy, R.; Pluzanska, A.; Szczylik, C.; Staehler, M.; Brugger, W.; Dietrich, P.-Y.; Mendrzyk, R.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012, 18, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Stenzl, A.; Zdrojowy, R.; Kogan, M.; Shkolnik, M.; Oudard, S.; Weikert, S.; Bracarda, S.; Crabb, S.J.; Bedke, J.; et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 1599–1611. [Google Scholar] [CrossRef]
- Figlin, R.A. Personalized immunotherapy (AGS-003) when combined with sunitinib for the treatment of metastatic renal cell carcinoma. Expert Opin. Biol. Ther. 2015, 15, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; Van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune-checkpoint inhibition—Novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Sato, Y.; Karasaki, T.; Nakagawa, T.; Kume, H.; Ogawa, S.; Homma, Y.; Kakimi, K. Neoantigen Load, Antigen Presentation Machinery, and Immune Signatures Determine Prognosis in Clear Cell Renal Cell Carcinoma. Cancer Immunol. Res. 2016, 4, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, J.; Lu, D.; Xu, X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lu, D.; Wei, X.; Wang, J.; Xu, X. Heterogeneous responses in hepatocellular carcinoma: The achilles heel of immune checkpoint inhibitors. Am. J. Cancer Res. 2020, 10, 1085–1102. [Google Scholar]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef] [PubMed]
| Trial | Treatment | Median Follow Up, Months | OS HR | mOS, Months | PFS HR | mPFS, Months | ORR, % | Reference |
|---|---|---|---|---|---|---|---|---|
| Checkmate 214 | Nivolumab + ipilimumab (n = 550) vs. sunitinib (n = 546) | 96 | 0.72 | 52.7 vs. 37.8 | 0.88 | 12.4 vs. 12.3 | 39 vs. 32 CR 12% | Motzer RJ et al., N Engl J Med. 2018 [8] |
| Keynote-426 | Pembrolizumab + axitinib (n = 432) vs. sunitinib (n = 429) | 67 | 0.84 | 47.2 vs. 40.8 | 0.69 | 15.7 vs. 11.1 | 61 vs. 40 CR 2% | Rini BI et al., N Engl J Med. 2019 [9] |
| Checkmate-9ER | Nivolumab + cabozantinib (n = 323) vs. sunitinib (n = 328) | 55 | 0.77 | 46.5 vs. 36.0 | 0.58 | 16.4 vs. 8.4 | 56 vs. 28 CR 13.6% | Choueiri TK et al., N Engl J Med. 2021 [10] |
| Clear | Pembrolizumab + lenvatinib (n = 355) vs. sunitinib (n = 357) | 48 | 0.79 | 53.7 vs. 54.3 | 0.47 | 23.9 vs. 9.2 | 71 vs. 37 CR 18% | Motzer RJ et al., N Engl J Med. 2021 [11] |
| ClinicalTrials.gov ID | Phase | Setting | Drug | Primary Endpoints and Phase | Estimated Primary Completion Date |
|---|---|---|---|---|---|
| NCT05805501 | II | Untreated, unresectable locally advanced or metastatic RCC | Tobemstomig (RO7247669) plus axitinib with or without tiragolumab versus pembrolizumab plus axitinib | Efficacy, safety, and pharmacokinetics | September 2024 |
| NCT05259319 | I | Second-line therapy after an anti-angiogenic plus immunotherapy or immunotherapy alone | Atezolizumab and tiragolumab, with concomitant or sequential stereotactic body radiation therapy | Safety and efficacy | December 2024 |
| NCT04626479 | Ib–II | First-line in untreated patient with advanced or metastatic RCC | Vibostolimab/ pembrolizumab | Safety and efficacy | May 2026 |
| NCT05788484 | I | Relapsed, locally advanced or metastatic setting after standard treatment | CDX-585 | Dose escalation | December 2024 |
| NCT04626518 Substudy 03B MK-3475-03B | Ib–II | Second and later lines | Pembrolizumab + MK-4830 | Safety and efficacy | September 2025 |
| NCT05420519 | I | Advanced or metastatic RCC | CD70 CAR-T cells | Safety and tolerability | December 2024 |
| NCT04969354 | I | Advanced or metastatic RCC | CAIX-targeted CAR-T Cells | Safety and efficacy | September 2026 |
| NCT03393936 | I–II | Advanced or metastatic RCC | CCT301-38 CCT301-59 CART-T Cells | Safety, tolerability and anti-tumor activity | June 2023 |
| NCT06182735 | I | Advanced or metastatic RCC | Cyclophosphamide plus fludarabine plus infusion of CAR-NKT Cells | Safety, tolerability, PK, and preliminary efficacy | January 2025 |
| NCT04696731 | I | Advanced or metastatic RCC | Cyclophosphamide, fludarabine, ALLO-647, ALLO-316 | Safety and efficacy | August 2025 |
| NCT04438083 | I | Advanced, relapsed or refractory RCC | CTX130 | Safety and efficacy | February 2027 |
| NCT05176483 | Ib | Advanced or metastatic RCC | XL092, novolumab, ipilimumab, relatlimab | Safety, tolerability, PK, preliminary antitumor activity, and effect | February 2026 |
| NCT05641545 | Ib | Advanced or metastatic RCC | Personalized neoantigen vaccine plus standard of care. | Safety and clinical toxicity | December 2024 |
| NCT05269381 | I | Advanced or metastatic solid tumors (including RCC) | Cyclophosphamide, neoantigen peptide vaccine, pembrolizumab, sargramostim | Safety and tolerability | February 2025 |
| NCT05703854 | I–II | Advanced or metastatic solid tumors (including RCC) | CAR.70/IL15-transduced CB-derived NK cells, fludarabine phosphate, cyclophosphamide | Safety, tolerability, and optimal cell dose | September 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compagno, S.; Casadio, C.; Galvani, L.; Rosellini, M.; Marchetti, A.; Tassinari, E.; Piazza, P.; Mottaran, A.; Santoni, M.; Schiavina, R.; et al. Novel Immune Checkpoint Inhibitor Targets in Advanced or Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. J. Clin. Med. 2024, 13, 5738. https://doi.org/10.3390/jcm13195738
Compagno S, Casadio C, Galvani L, Rosellini M, Marchetti A, Tassinari E, Piazza P, Mottaran A, Santoni M, Schiavina R, et al. Novel Immune Checkpoint Inhibitor Targets in Advanced or Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Journal of Clinical Medicine. 2024; 13(19):5738. https://doi.org/10.3390/jcm13195738
Chicago/Turabian StyleCompagno, Samuele, Chiara Casadio, Linda Galvani, Matteo Rosellini, Andrea Marchetti, Elisa Tassinari, Pietro Piazza, Angelo Mottaran, Matteo Santoni, Riccardo Schiavina, and et al. 2024. "Novel Immune Checkpoint Inhibitor Targets in Advanced or Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives" Journal of Clinical Medicine 13, no. 19: 5738. https://doi.org/10.3390/jcm13195738
APA StyleCompagno, S., Casadio, C., Galvani, L., Rosellini, M., Marchetti, A., Tassinari, E., Piazza, P., Mottaran, A., Santoni, M., Schiavina, R., Massari, F., & Mollica, V. (2024). Novel Immune Checkpoint Inhibitor Targets in Advanced or Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Journal of Clinical Medicine, 13(19), 5738. https://doi.org/10.3390/jcm13195738







