Comparison of Efficacy of Micropulse Laser Settings for Glaucoma Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Procedure
2.3. Follow-Up
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Demographics
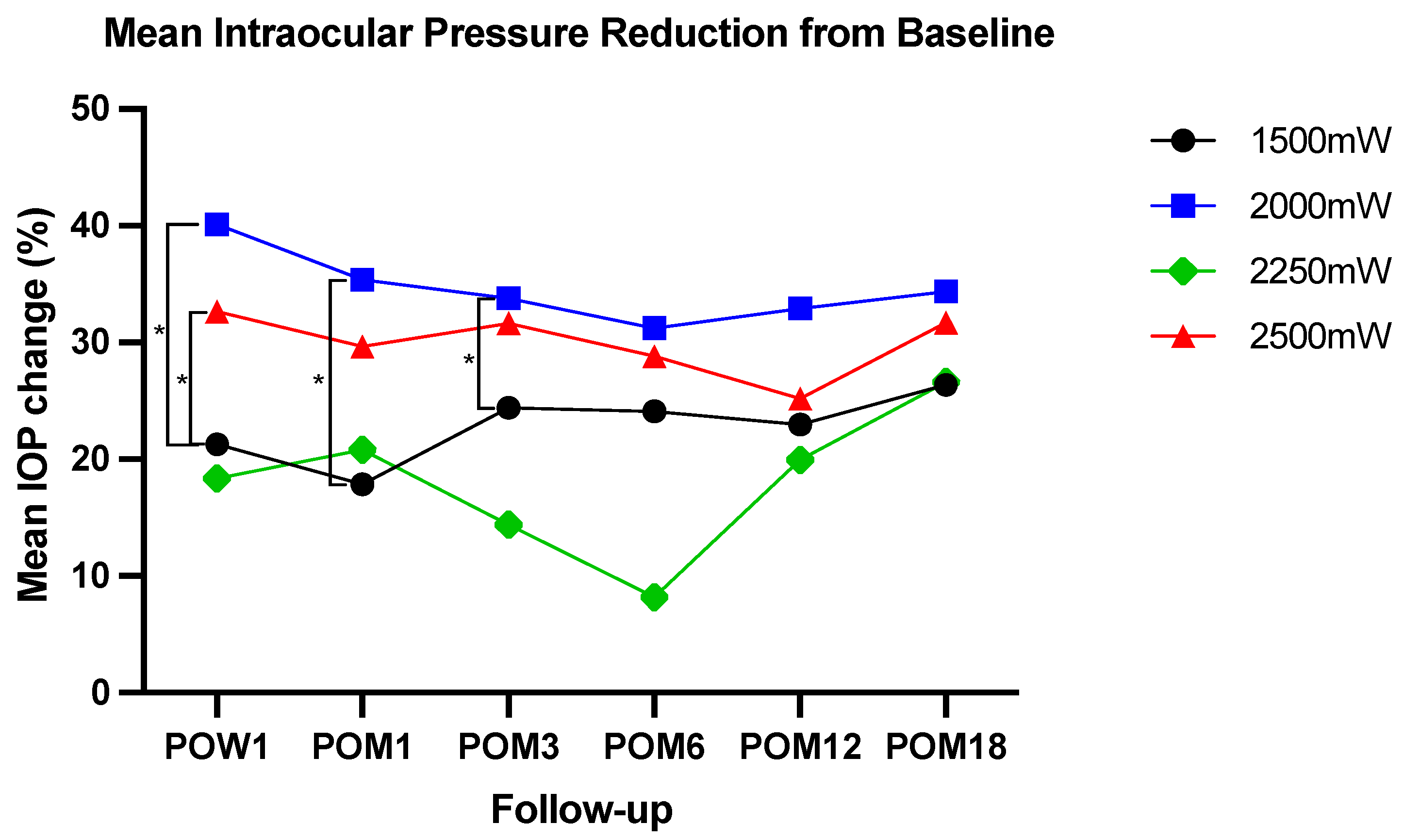
3.2. IOP
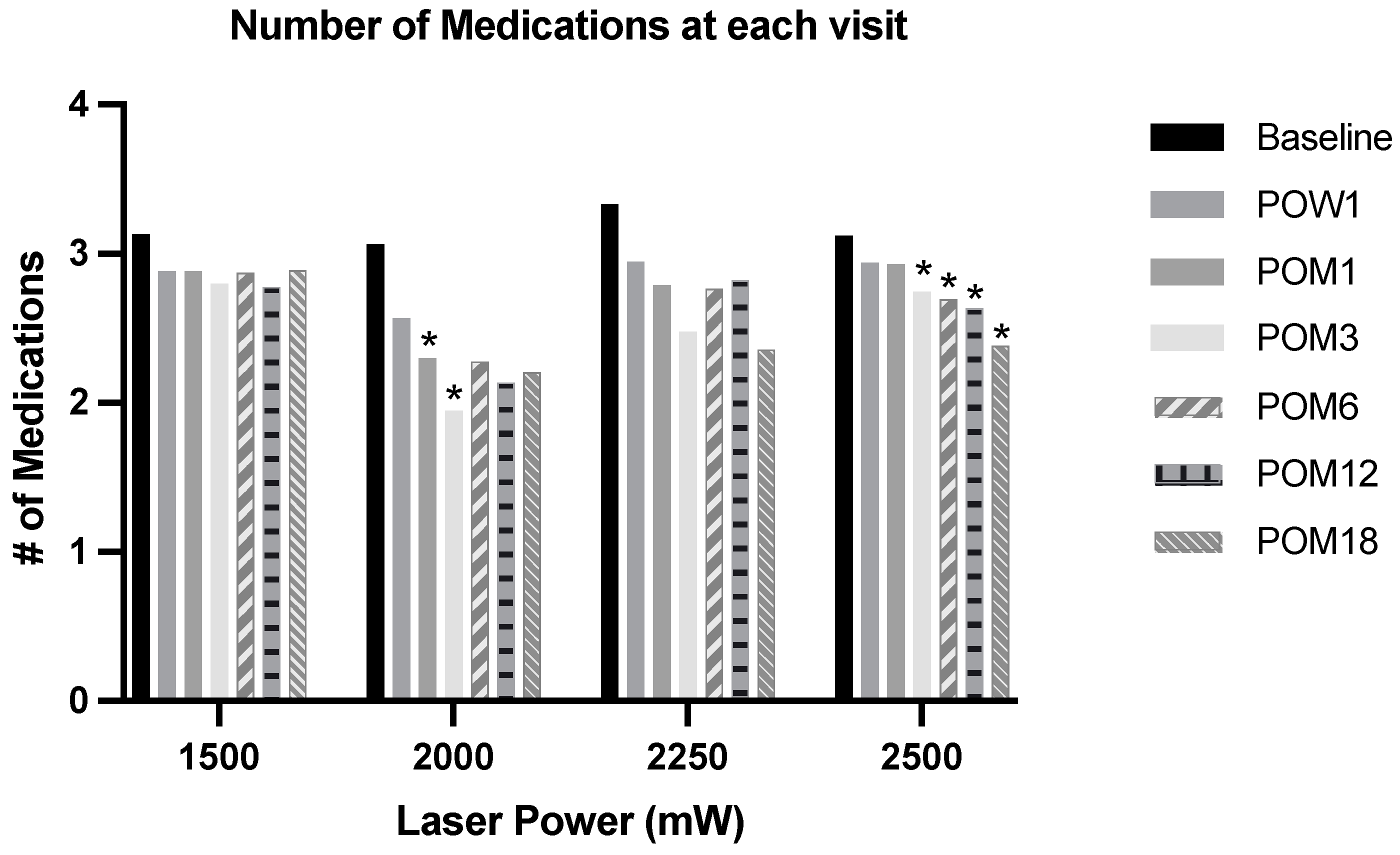
3.3. Effects on IOP-Lowering Medications
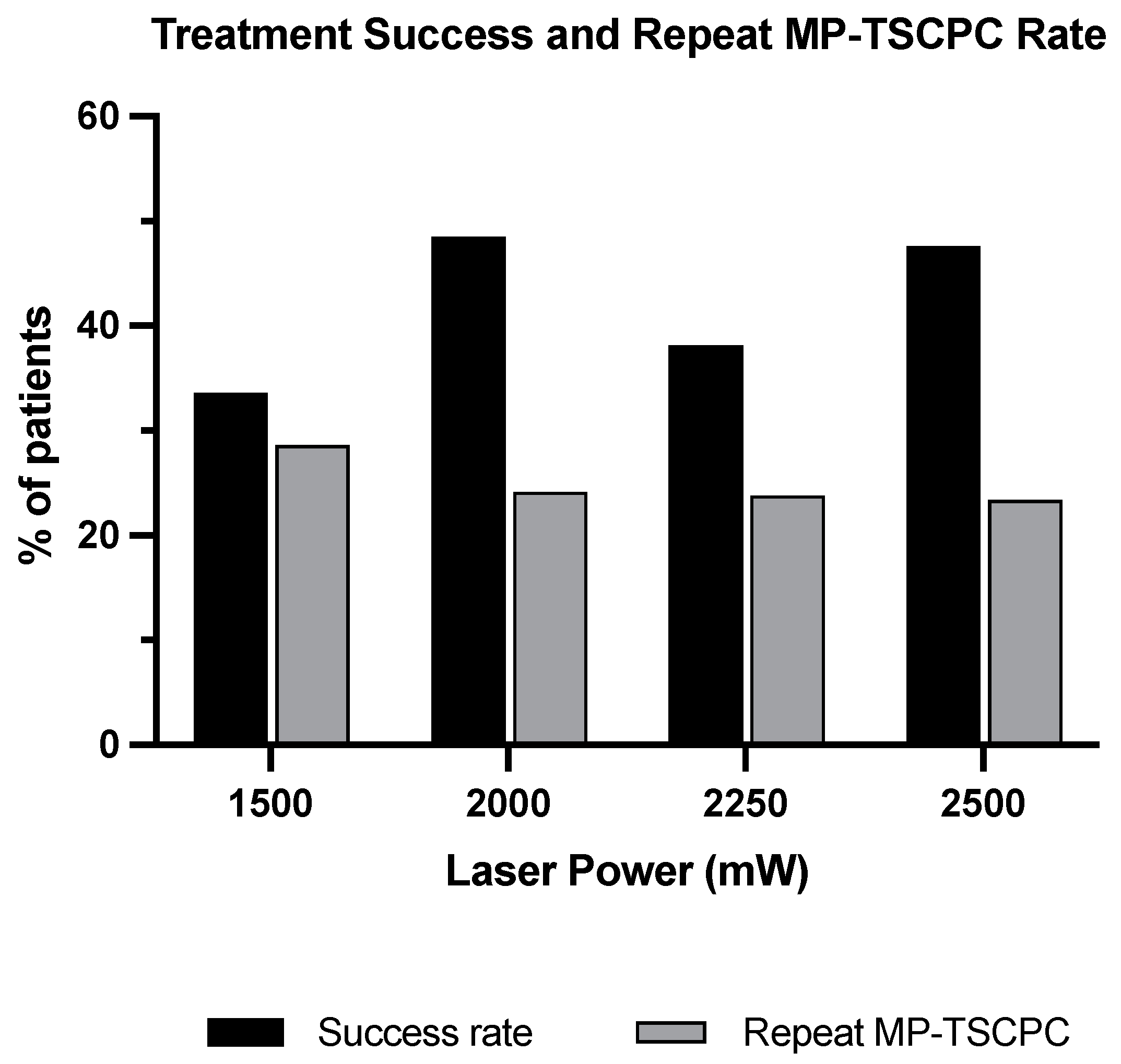
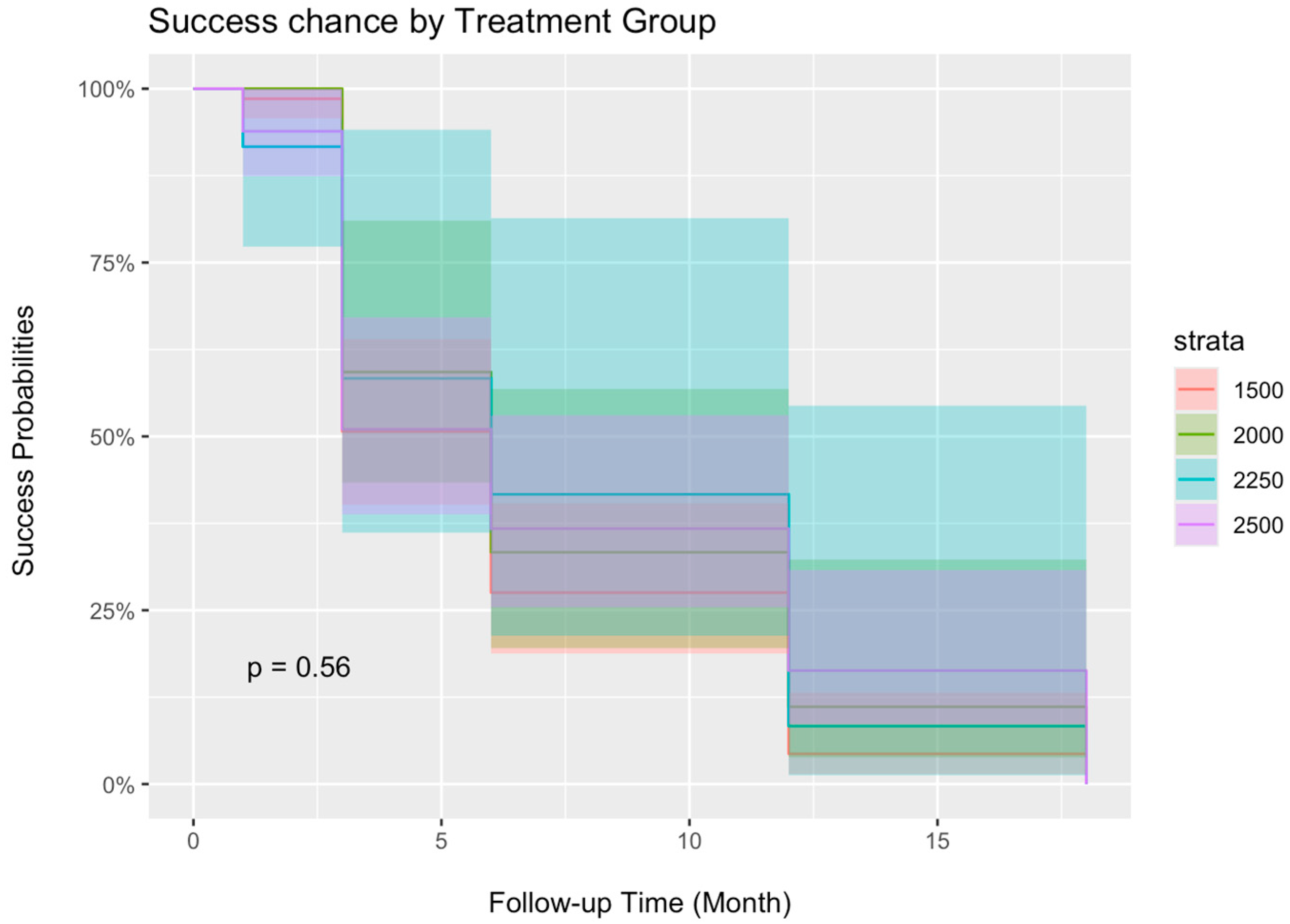
3.4. Treatment Success
3.5. Rate of Hypotony
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Peters, D.M. Pathogenesis of glaucoma: Extracellular matrix dysfunction in the trabecular meshwork—A review. Clin. Exp. Ophthalmol. 2022, 50, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial, G. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch. Arztebl. Int. 2020, 117, 225–234. [Google Scholar] [CrossRef]
- Kuchar, S.; Moster, M.R.; Reamer, C.B.; Waisbourd, M. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med. Sci. 2016, 31, 393–396. [Google Scholar] [CrossRef]
- Zemba, M.; Dumitrescu, O.M.; Vaida, F.; Dimirache, E.A.; Pistolea, I.; Stamate, A.C.; Burcea, M.; Branisteanu, D.C.; Balta, F.; Barac, I.R. Micropulse vs. continuous wave transscleral cyclophotocoagulation in neovascular glaucoma. Exp. Ther. Med. 2022, 23, 278. [Google Scholar] [CrossRef]
- Tekeli, O.; Kose, H.C. Comparative efficacy and safety of micropulse transscleral laser cyclophotocoagulation using different duration protocols in eyes with good visual acuity. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3359–3369. [Google Scholar] [CrossRef]
- Ma, A.; Yu, S.W.Y.; Wong, J.K.W. Micropulse laser for the treatment of glaucoma: A literature review. Surv. Ophthalmol. 2019, 64, 486–497. [Google Scholar] [CrossRef]
- Gambini, G.; Carla, M.M.; Caporossi, T.; De Vico, U.; Savastano, A.; Baldascino, A.; Rizzo, C.; Kilian, R.; Rizzo, S. Spotlight on MicroPulse Laser Trabeculoplasty in Open-Angle Glaucoma: What’s on? A Review of the Literature. Vision 2022, 6, 8. [Google Scholar] [CrossRef]
- Babalola, O.E. Micropulse diode laser trabeculoplasty in Nigerian patients. Clin. Ophthalmol. 2015, 9, 1347–1351. [Google Scholar] [CrossRef]
- Varikuti, V.N.V.; Shah, P.; Rai, O.; Chaves, A.C.; Miranda, A.; Lim, B.A.; Dorairaj, S.K.; Sieminski, S.F. Outcomes of Micropulse Transscleral Cyclophotocoagulation in Eyes With Good Central Vision. J. Glaucoma 2019, 28, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Al Habash, A.; AlAhmadi, A.S. Outcome Of MicroPulse((R)) Transscleral Photocoagulation In Different Types Of Glaucoma. Clin. Ophthalmol. 2019, 13, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.A.; Karancsi, O.L.; Munteanu, M.; Stanca, H.T. Clinical outcomes of micropulse transscleral cyclophotocoagulation in refractory glaucoma-18 months follow-up. Lasers Med. Sci. 2020, 35, 1487–1491. [Google Scholar] [CrossRef]
- Sarrafpour, S.; Saleh, D.; Ayoub, S.; Radcliffe, N.M. Micropulse Transscleral Cyclophotocoagulation: A Look at Long-Term Effectiveness and Outcomes. Ophthalmol. Glaucoma 2019, 2, 167–171. [Google Scholar] [CrossRef]
- Kaba, Q.; Somani, S.; Tam, E.; Yuen, D. The Effectiveness and Safety of Micropulse Cyclophotocoagulation in the Treatment of Ocular Hypertension and Glaucoma. Ophthalmol. Glaucoma 2020, 3, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chamard, C.; Bachouchi, A.; Daien, V.; Villain, M. Efficacy, Safety, and Retreatment Benefit of Micropulse Transscleral Cyclophotocoagulation in Glaucoma. J. Glaucoma 2021, 30, 781–788. [Google Scholar] [CrossRef]
- Fili, S.; Vastardis, I.; Perdikakis, G.; Kohlhaas, M. Transscleral cyclophotocoagulation with MicroPulse(R) laser versus cyclophotocoagulation with continuous diode laser in patients with open-angle glaucoma. Int. Ophthalmol. 2022, 42, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Cai, Z.; Zhang, X.; Duan, X. The efficacy and safety of micropulse transscleral laser treatment in glaucoma: A systematic review and meta-analysis. BMC Ophthalmol. 2023, 23, 263. [Google Scholar] [CrossRef]
- Aquino, M.C.; Barton, K.; Tan, A.M.; Sng, C.; Li, X.; Loon, S.C.; Chew, P.T. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin. Exp. Ophthalmol. 2015, 43, 40–46. [Google Scholar] [CrossRef]
- Tan, A.M.; Chockalingam, M.; Aquino, M.C.; Lim, Z.I.; See, J.L.; Chew, P.T. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin. Exp. Ophthalmol. 2010, 38, 266–272. [Google Scholar] [CrossRef]
- Valle, I.T.; Bazarra, S.P.; Taboas, M.F.; Cid, S.R.; Diaz, M.D.A. Medium-term Outcomes of Micropulse Transscleral Cyclophotocoagulation in Refractory Glaucoma. J. Curr. Glaucoma Pract. 2022, 16, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Souissi, S.; Baudouin, C.; Labbe, A.; Hamard, P. Micropulse transscleral cyclophotocoagulation using a standard protocol in patients with refractory glaucoma naive of cyclodestruction. Eur. J. Ophthalmol. 2021, 31, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.G.; Lerner, F.; Sampaolesi, J.; Noecker, R.; Becerra, N.; Iribarren, G.; Grippo, T.M. Efficacy and Safety of Micropulse(R) Transscleral Cyclophotocoagulation in Glaucoma. Arch. Soc. Esp. Oftalmol. Engl. Ed. 2018, 93, 573–579. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | 1500 mW 1 | 2000 mW 1 | 2250 mW 1 | 2500 mW 1 | p-Value 2 |
|---|---|---|---|---|---|
| Eyes | n = 140 | n = 66 | n = 21 | n = 124 | |
| Mean Age (years) | 64 (17) | 68 (12) | 63 (21) | 68 (15) | 0.14 |
| Glaucoma Type | |||||
| Primary open-angle glaucoma | 63 (45%) | 26 (39%) | 8 (38%) | 69 (56%) | |
| Combined-mechanism glaucoma | 1 (0.7%) | 21 (31.5%) | 6 (28.5%) | 24 (19%) | |
| Neovascular glaucoma | 18 (13%) | 12 (18%) | 1 (4.8%) | 11 (8.9%) | |
| Chronic angle-closure glaucoma | 23 (16%) | 1 (1.5%) | 2 (9.5%) | 7 (5.6%) | |
| Secondary glaucoma | 9 (6.4%) | 0 (0%) | 0 (0%) | 4 (3.2%) | |
| Congenital/juvenile glaucoma | 9 (6.4%) | 1 (1.5%) | 1 (4.8%) | 2 (1.6%) | |
| Uveitic glaucoma | 7 (5.0%) | 1 (1.5%) | 2 (9.5%) | 0 (0%) | |
| Traumatic glaucoma | 5 (3.6%) | 1 (1.5%) | 0 (0%) | 3 (0%) | |
| Pseudoexfoliation glaucoma | 4 (2.9%) | 1 (1.5%) | 0 (0%) | 1 (0.8%) | |
| Other | 1 (0.7%) | 2 (3.0%) | 1 (4.8%) | 3 (2.4%) | |
| Glaucoma Stage | |||||
| Mild | 7 (5.0%) | 0 (0%) | 0 (0%) | 5 (4.0%) | |
| Moderate | 21 (15%) | 9 (14%) | 2 (9.5%) | 8 (6.5%) | |
| Severe | 77 (55%) | 30 (45%) | 15 (71%) | 93 (75%) | |
| Indeterminate | 21 (15%) | 20 (30%) | 0 (0%) | 5 (4.0%) | |
| Absolute | 8 (5.7%) | 5 (7.6%) | 0 (0%) | 2 (1.6%) | |
| Unspecified | 6 (4.3%) | 2 (3.0%) | 4 (19%) | 11 (8.9%) | |
| Baseline mean IOP (mmHg) | 29 (11) | 30 (11) | 22 (8) | 26 (9) | <0.001 |
| Baseline number of glaucoma medications | 3.13 (1.17) | 3.06 (1.39) | 3.33 (1.15) | 3.11 (1.15) | >0.9 |
| Number of Hypotony | 1 (0.7%) | 1 (1.5%) | 0 (0%) | 0 (0%) | |
| Number of Repeat MP-TSCPC | 40 (28.6%) | 16 (24.2%) | 5 (23.8%) | 29 (23.4%) |
| Characteristics | 1500 mW 1 | 2000 mW 1 | 2250 mW 1 | 2500 mW 1 | p-Value 2 |
|---|---|---|---|---|---|
| Baseline mean IOP (mmHg) | 29 (11), n = 137 | 30(11), n = 66 | 22 (8), n = 21 | 26 (9), n = 123 | <0.001 |
| POW1 mean IOP (mmHg) | 22 (9), n = 121 | 16 (7), n = 60 | 17 (9), n = 19 | 17 (7), n = 119 | <0.001 |
| POM1 mean IOP (mmHg) | 22 (9), n = 111 | 17(7), n = 59 | 16 (7), n = 21 | 18 (7), n = 103 | <0.001 |
| POM3 mean IOP (mmHg) | 20 (9), n = 99 | 17 (6), n = 59 | 16 (7), n = 18 | 16 (5), n = 97 | 0.003 |
| POM6 mean IOP (mmHg) | 20 (7), n = 95 | 18 (8), n = 46 | 16(7), n = 15 | 18 (7), n = 92 | 0.004 |
| POM12 mean IOP (mmHg) | 20 (8), n = 93 | 16 (7), n = 36 | 15 (4), n = 13 | 18 (8), n = 90 | 0.012 |
| POM18 mean IOP (mmHg) | 20 (10), n = 81 | 15 (6), n = 24 | 15 (8), n = 12 | 16 (6), n = 81 | 0.006 |
| Characteristics | 1500 mW 1 | 2000 mW 1 | 2250 mW 1 | 2500 mW 1 | p-Value 2 |
|---|---|---|---|---|---|
| Baseline mean number of glaucoma medication | 3.13 (1.17) | 3.06 (1.39) | 3.33 (1.15) | 3.12 (1.15) | >0.9 |
| POW1 mean number of glaucoma medication | 2.89 (1.23), n = 122 | 2.57 (1.41), n = 60 | 2.95 (1.03), n = 19 | 2.94 (1.17), n = 121 | 0.7 |
| POM1 mean number of glaucoma medication | 2.88 (1.20), n = 111 | 2.30 (1.41), n = 60 | 2.79 (1.31), n = 21 | 2.93 (1.21), n = 104 | 0.065 |
| POM3 mean number of glaucoma medication | 2.80 (1.32), n = 99 | 1.95 (1.38), n = 59 | 2.48 (1.25), n = 21 | 2.75 (1.04), n = 99 | 0.001 |
| POM6 mean number of glaucoma medication | 2.97 (1.68), n = 96 | 2.28 (1.32), n = 46 | 2.76 (1.09), n = 15 | 2.69 (1.12), n = 95 | 0.054 |
| POM12 mean number of glaucoma medication | 2.78 (1.25), n = 94 | 2.14 (1.34), n = 37 | 2.82 (1.07), n = 13 | 2.63 (1.17), n = 90 | 0.06 |
| POM18 mean number of glaucoma medication | 2.89 (1.20), n = 81 | 2.21 (1.14), n = 24 | 2.36 (1.01), n = 12 | 2.38 (1.23), n = 81 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.Y.; Walker, B.D.; Hopkins, N.S.; Fowler, S.; Jerkins, B.M.; Kanner, E.M.; Wright, C.L. Comparison of Efficacy of Micropulse Laser Settings for Glaucoma Management. J. Clin. Med. 2024, 13, 5753. https://doi.org/10.3390/jcm13195753
Kim EY, Walker BD, Hopkins NS, Fowler S, Jerkins BM, Kanner EM, Wright CL. Comparison of Efficacy of Micropulse Laser Settings for Glaucoma Management. Journal of Clinical Medicine. 2024; 13(19):5753. https://doi.org/10.3390/jcm13195753
Chicago/Turabian StyleKim, Emily Y., Brooks D. Walker, Nikolas S. Hopkins, Samuel Fowler, Brian M. Jerkins, Elliott M. Kanner, and Claire L. Wright. 2024. "Comparison of Efficacy of Micropulse Laser Settings for Glaucoma Management" Journal of Clinical Medicine 13, no. 19: 5753. https://doi.org/10.3390/jcm13195753
APA StyleKim, E. Y., Walker, B. D., Hopkins, N. S., Fowler, S., Jerkins, B. M., Kanner, E. M., & Wright, C. L. (2024). Comparison of Efficacy of Micropulse Laser Settings for Glaucoma Management. Journal of Clinical Medicine, 13(19), 5753. https://doi.org/10.3390/jcm13195753






