Capillary Refill Time as a Part of Routine Physical Examination in Critically Ill Patients Undergoing Vasoactive Therapy: A Prospective Study
Abstract
1. Introduction
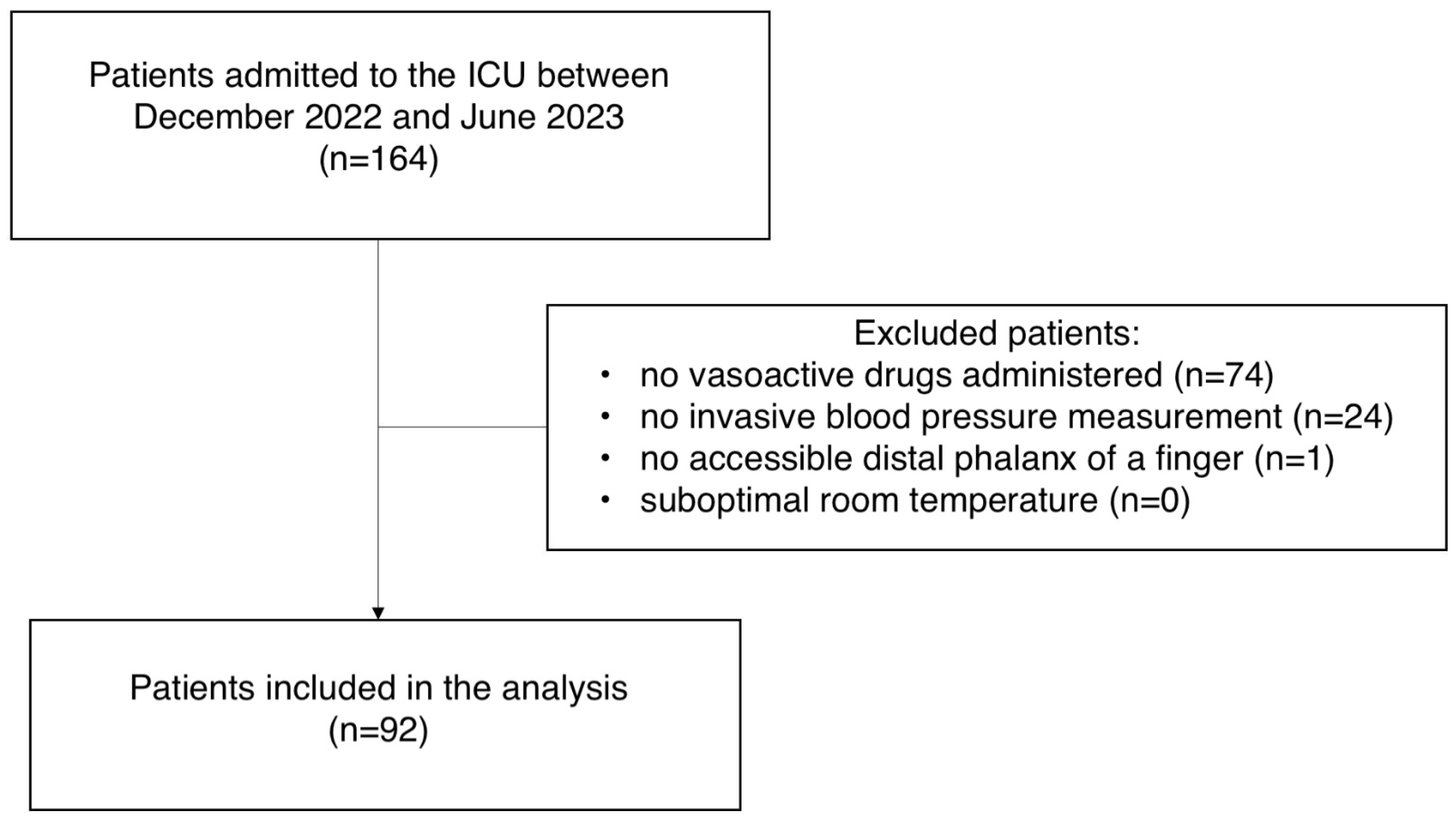
2. Material and Methods
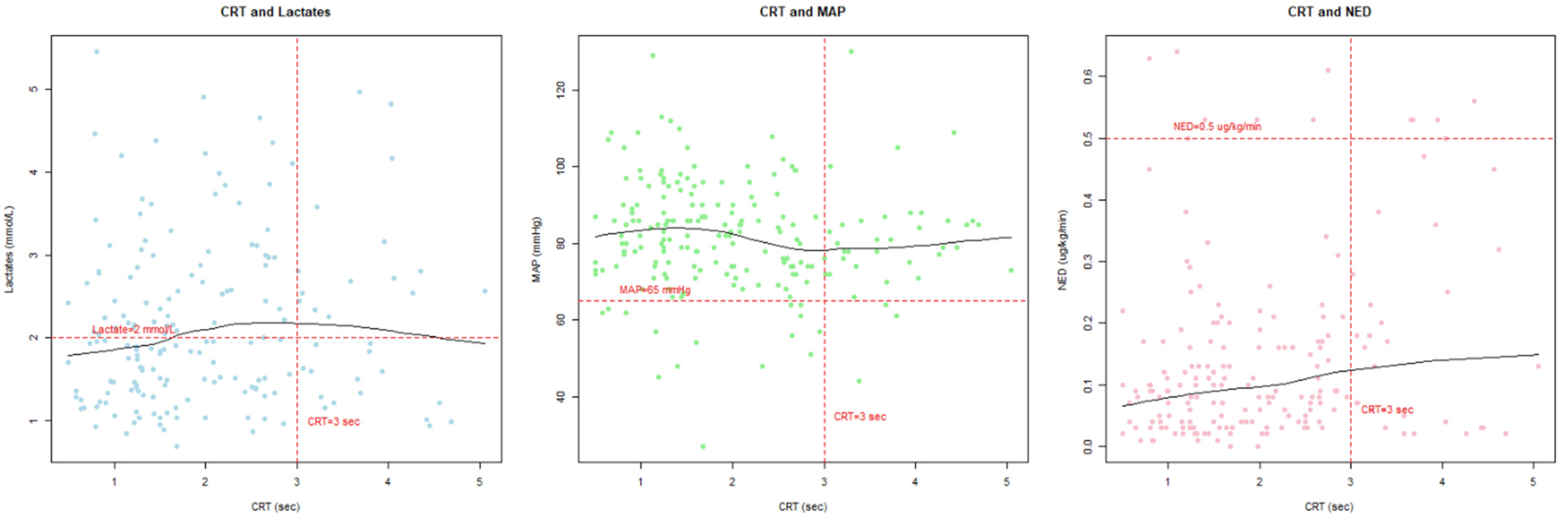
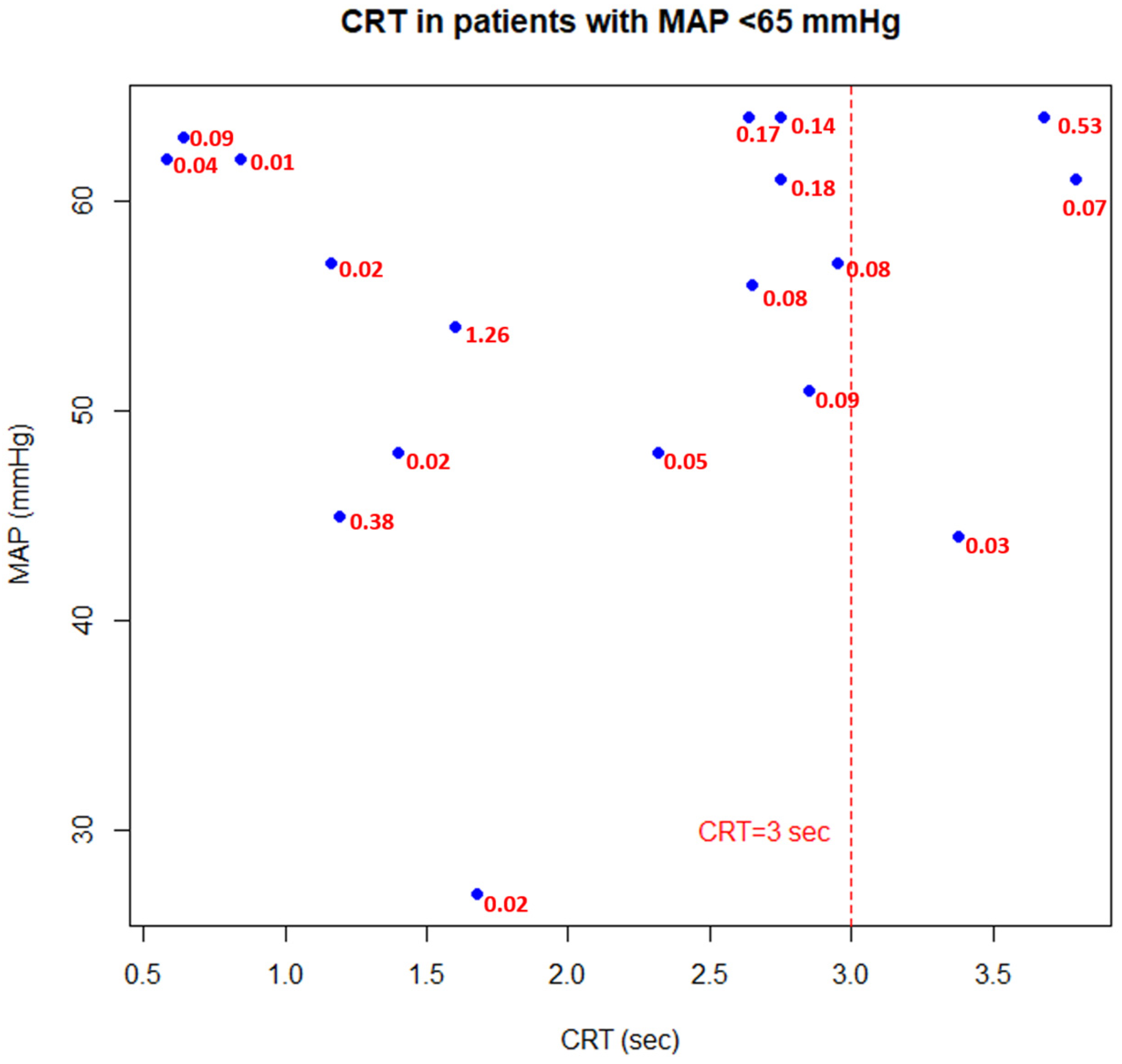
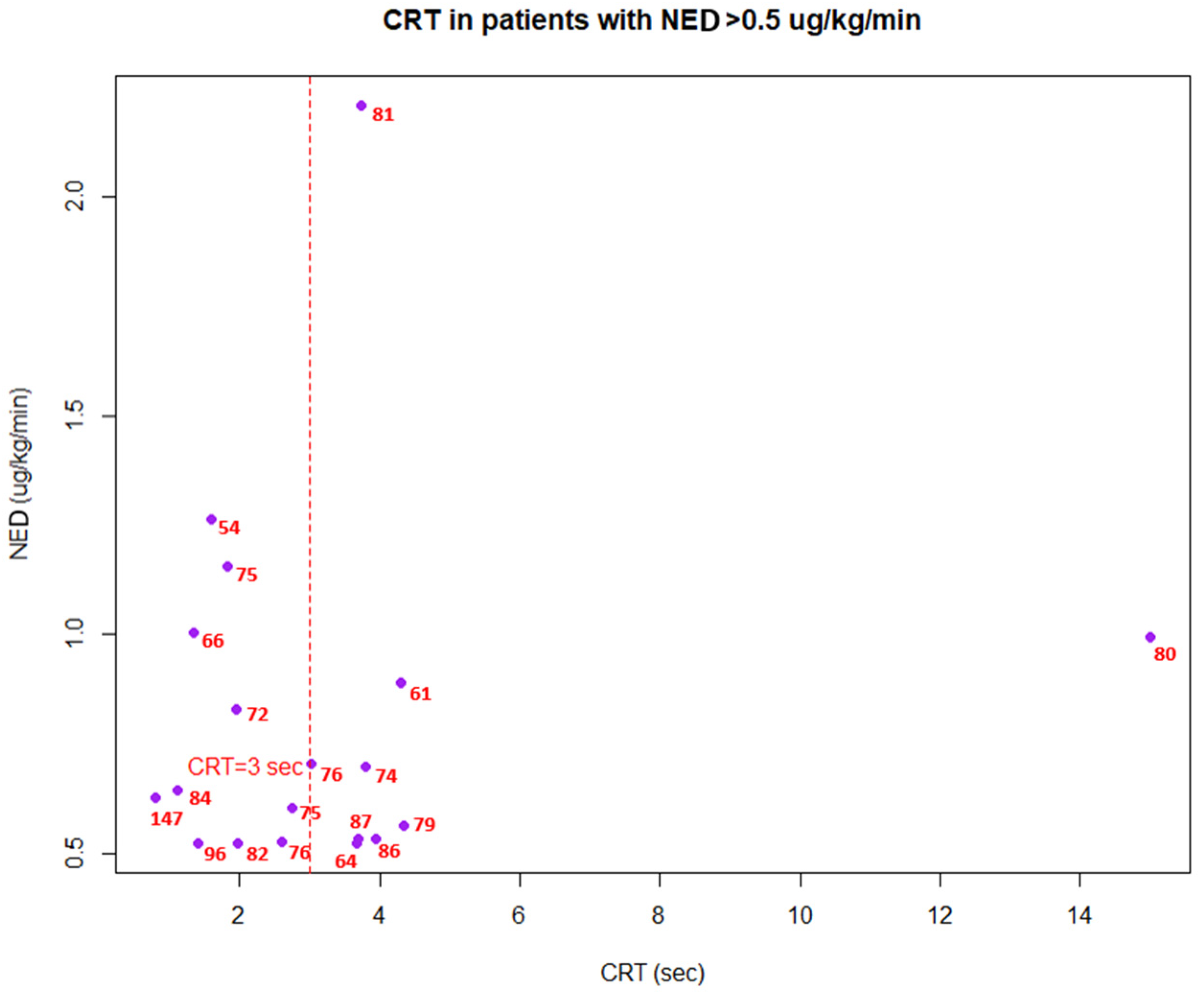
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAP | Mean Arterial Pressure |
| CRT | Capillary Refill Time |
| NED | Norepinephrine Equivalent Dose |
| SSC | Surviving Sepsis Campaign |
| ICU | Intensive Care Unit |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| IQR | Interquartile Range |
| SAH | Subarachnoid Hemorrhage |
| PLR | Passive Leg Raising |
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Barman, S.M.; Brooks, H.L.; Yuan, J.X.J.; Ganong, W.F. Ganong’s Review of Medical Physiology, 26th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Kato, R.; Pinsky, M.R. Personalizing blood pressure management in septic shock. Ann. Intensive Care 2015, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients with Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Misango, D.; Pattnaik, R.; Baker, T.; Dünser, M.W.; Dondorp, A.M.; Schultz, M.J. Haemodynamic assessment and support in sepsis and septic shock in resource-limited settings. Trans. R Soc. Trop. Med. Hyg. 2017, 111, 483–489. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Bige, N.; Boelle, P.Y.; Pichereau, C.; Alves, M.; Bertinchamp, R.; Baudel, J.L.; Galbois, A.; Maury, E.; Guidet, B. Capillary refill time exploration during septic shock. Intensive Care Med. 2014, 40, 958–964. [Google Scholar] [CrossRef]
- Brunauer, A.; Koköfer, A.; Bataar, O.; Gradwohl-Matis, I.; Dankl, D.; Bakker, J.; Dünser, M.W. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: A pilot study. J. Crit. Care 2016, 35, 105–109. [Google Scholar] [CrossRef]
- Morocho, J.P.; Martínez, A.F.; Cevallos, M.M.; Vasconez-Gonzalez, J.; Ortiz-Prado, E.; Barreto-Grimaldos, A.; Vélez-Páez, J.L. Prolonged Capillary Refilling as a Predictor of Mortality in Patients With Septic Shock. J. Intensive Care Med. 2022, 37, 423–429. [Google Scholar] [CrossRef]
- Kattan, E.; Hernández, G.; Ospina-Tascón, G.; Valenzuela, E.D.; Bakker, J.; Castro, R. A lactate-targeted resuscitation strategy may be associated with higher mortality in patients with septic shock and normal capillary refill time: A post hoc analysis of the ANDROMEDA-SHOCK study. Ann. Intensive Care 2020, 10, 114. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Damiani, L.P.; Bakker, J.; Ospina-Tascón, G.A.; Castro, R.; Cavalcanti, A.B.; Hernandez, G. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels among Patients with Septic Shock. A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 423–429. [Google Scholar] [CrossRef]
- Lara, B.; Enberg, L.; Ortega, M.; Leon, P.; Kripper, C.; Aguilera, P.; Kattan, E.; Castro, R.; Bakker, J.; Hernandez, G. Capillary refill time during fluid resuscitation in patients with sepsis-related hyperlactatemia at the emergency department is related to mortality. PLoS ONE 2017, 12, e0188548. [Google Scholar] [CrossRef] [PubMed]
- Kotani, Y.; Di Gioia, A.; Landoni, G.; Belletti, A.; Khanna, A.K. An updated “norepinephrine equivalent” score in intensive care as a marker of shock severity. Crit. Care 2023, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Jacquet-Lagrèze, M.; Bouhamri, N.; Portran, P.; Schweizer, R.; Baudin, F.; Lilot, M.; Fornier, W.; Fellahi, J.L. Capillary refill time variation induced by passive leg raising predicts capillary refill time response to volume expansion. Crit. Care 2019, 23, 281. [Google Scholar] [CrossRef] [PubMed]
- Raia, L.; Gabarre, P.; Bonny, V.; Urbina, T.; Missri, L.; Boelle, P.Y.; Baudel, J.L.; Guidet, B.; Maury, E.; Joffre, J.; et al. Kinetics of capillary refill time after fluid challenge. Ann. Intensive Care 2022, 12, 74. [Google Scholar] [CrossRef]
- Hernández, G.; Valenzuela, E.D.; Kattan, E.; Castro, R.; Guzmán, C.; Kraemer, A.E.; Sarzosa, N.; Alegría, L.; Contreras, R.; Oviedo, V.; et al. Capillary refill time response to a fluid challenge or a vasopressor test: An observational, proof-of-concept study. Ann. Intensive Care 2024, 14, 49. [Google Scholar] [CrossRef]
- Dubin, A.; Pozo, M.O.; Casabella, C.A.; Pálizas, F., Jr.; Murias, G.; Moseinco, M.C.; Kanoore Edul, V.S.; Pálizas, F.; Estenssoro, E.; Ince, C.; et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: A prospective study. Crit. Care. 2009, 13, R92. [Google Scholar] [CrossRef]
- Lima, A.; Jansen, T.C.; Van Bommel, J.; Ince, C.; Bakker, J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit. Care Med. 2009, 37, 934–938. [Google Scholar] [CrossRef]
- Landry, G.J.; Mostul, C.J.; Ahn, D.S.; McLafferty, B.J.; Liem, T.K.; Mitchell, E.L.; Jung, E.; Abraham, C.Z.; Azarbal, A.F.; McLafferty, R.B.; et al. Causes and outcomes of finger ischemia in hospitalized patients in the intensive care unit. J. Vasc. Surg. 2018, 68, 1499–1504. [Google Scholar] [CrossRef]
- Anderson, B.; Kelly, A.M.; Kerr, D.; Clooney, M.; Jolley, D. Impact of patient and environmental factors on capillary refill time in adults. Am. J. Emerg. Med. 2008, 26, 62–65. [Google Scholar] [CrossRef]
- Brown, L.H.; Prasad, N.H.; Whitley, T.W. Adverse lighting condition effects on the assessment of capillary refill. Am. J. Emerg. Med. 1994, 12, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Nakada, T.; Oshima, T.; Shinozaki, M.; Nakaguchi, T.; Haneishi, H.; Oda, S. Optimal pressing strength and time for capillary refilling time. Crit. Care 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Morton, R.; Bevan, C. How to use capillary refill time. Arch. Dis. Child. 2014, 99, 111–116. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (n = 92) |
|---|---|
| Age—median (IQR), years | 66 (45–72) |
| Gender—n (%) | |
| Female | 45 (49%) |
| Male | 47 (51%) |
| CRT—median (IQR), s | 1.9 (1.25–2.75) |
| MAP—median (IQR), mmHg | 82 (74–90) |
| Lactate—median (IQR), mmol/L | 2.1 (1.46–3.1) |
| NED—median (IQR), μg/kg/min | 0.01 (0.04–0.20) |
| ICU mortality—n (%) | 39 (42%) |
| Cause of admission—n(%) | |
| Acute respiratory failure | 32 (34.8%) |
| Subarachnoid hemorrhage (SAH) | 21 (22.8%) |
| Acute neurological disease (other than SAH) | 17 (18.5%) |
| Acute pancreatitis | 5 (5.4%) |
| Sudden cardiac arrest | 4 (4.3%) |
| Acute heart failure | 3 (3.3%) |
| Septic shock | 3 (3.3%) |
| Shock (other than septic) | 3 (3.3%) |
| Acute liver failure | 2 (2.2%) |
| Malignant neoplasm | 2 (2.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesołek, F.; Putowski, Z.; Staniszewska, W.; Latacz, R.; Krzych, Ł.J. Capillary Refill Time as a Part of Routine Physical Examination in Critically Ill Patients Undergoing Vasoactive Therapy: A Prospective Study. J. Clin. Med. 2024, 13, 5782. https://doi.org/10.3390/jcm13195782
Wesołek F, Putowski Z, Staniszewska W, Latacz R, Krzych ŁJ. Capillary Refill Time as a Part of Routine Physical Examination in Critically Ill Patients Undergoing Vasoactive Therapy: A Prospective Study. Journal of Clinical Medicine. 2024; 13(19):5782. https://doi.org/10.3390/jcm13195782
Chicago/Turabian StyleWesołek, Fabian, Zbigniew Putowski, Wiktoria Staniszewska, Robert Latacz, and Łukasz J. Krzych. 2024. "Capillary Refill Time as a Part of Routine Physical Examination in Critically Ill Patients Undergoing Vasoactive Therapy: A Prospective Study" Journal of Clinical Medicine 13, no. 19: 5782. https://doi.org/10.3390/jcm13195782
APA StyleWesołek, F., Putowski, Z., Staniszewska, W., Latacz, R., & Krzych, Ł. J. (2024). Capillary Refill Time as a Part of Routine Physical Examination in Critically Ill Patients Undergoing Vasoactive Therapy: A Prospective Study. Journal of Clinical Medicine, 13(19), 5782. https://doi.org/10.3390/jcm13195782






