Amplitude of Lower Limb Muscle Activation in Different Phases of the Illinois Test in Parkinson’s Disease Patients: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Electrical Activity Registration
2.3. Statistical Analysis
2.4. Ethical Considerations
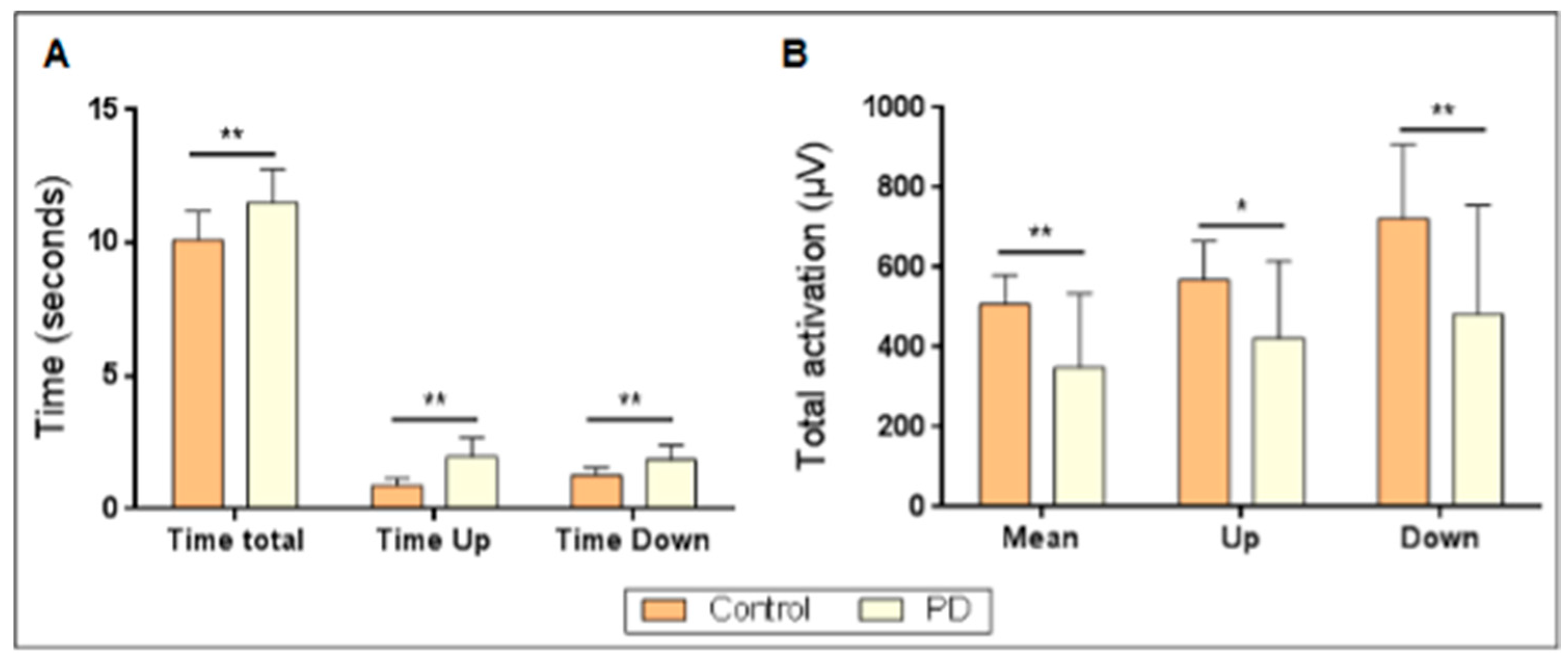
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Control | PD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Sex | Age | ||||||
| rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value | ||
| ARF-R (MA) | –0.174 | 0.631 | –0.711 * | 0.021 | ARF-R (MA) | 0.087 | 0.649 | 0.257 | 0.170 |
| ARF-L (MA) | 0.244 | 0.497 | –0.571 | 0.084 | ARF-L (MA) | –0.032 | 0.867 | 0.361 * | 0.050 |
| BCF-R (MA) | 0.244 | 0.497 | –0.195 | 0.590 | BCF-R (MA) | 0.214 | 0.256 | 0.110 | 0.562 |
| BCF-L (MA) | –0.383 | 0.275 | –0.043 | 0.907 | BCF-L (MA) | 0.077 | 0.684 | 0.220 | 0.243 |
| ATB-R (MA) | –0.035 | 0.924 | –0.286 | 0.424 | ATB-R (MA) | 0.278 | 0.137 | 0.305 | 0.102 |
| ATB-L (MA) | –0.244 | 0.497 | 0.274 | 0.444 | ATB-L (MA) | 0.296 | 0.112 | 0.404 * | 0.027 |
| GNM-R (MA) | –0.870 ** | 0.001 | 0.158 | 0.663 | GNM-R (MA) | 0.032 | 0.867 | 0.155 | 0.413 |
| GNM-L (MA) | –0.870 ** | 0.001 | 0.146 | 0.688 | GNM-L (MA) | –0.032 | 0.867 | –0.015 | 0.938 |
| ARF-R (Up) | –0.661 * | 0.037 | –0.267 | 0.455 | ARF-R (Up) | 0.114 | 0.549 | 0.287 | 0.123 |
| ARF-L (Up) | –0.453 | 0.189 | –0.419 | 0.228 | ARF-L (Up) | 0.041 | 0.830 | 0.310 | 0.095 |
| BCF-R (Up) | –0.174 | 0.631 | 0.170 | 0.638 | BCF-R (Up) | 0.114 | 0.549 | 0.043 | 0.822 |
| BCF-L (Up) | –0.244 | 0.497 | 0.170 | 0.638 | BCF-L (Up) | –0.005 | 0.981 | 0.131 | 0.489 |
| ATB-R (Up) | 0.244 | 0.497 | –0.322 | 0.364 | ATB-R (Up) | 0.260 | 0.166 | 0.261 | 0.163 |
| ATB-L (Up) | 0.592 | 0.071 | 0.097 | 0.789 | ATB-L (Up) | 0.260 | 0.166 | 0.440 * | 0.015 |
| GNM-R (Up) | –0.592 | 0.071 | 0.176 | 0.626 | GNM-R (Up) | –0.100 | 0.598 | 0.075 | 0.693 |
| GNM-L (Up) | –0.522 | 0.122 | 0.316 | 0.374 | GNM-L (Up) | –0.178 | 0.348 | –0.219 | 0.244 |
| ARF-R (Down) | –0.453 | 0.189 | –0.267 | 0.455 | ARF-R (Down) | 0.041 | 0.830 | 0.220 | 0.242 |
| ARF-L (Down) | –0.870 ** | 0.001 | –0.067 | 0.854 | ARF-L (Down) | –0.132 | 0.487 | 0.336 | 0.069 |
| BCF-R (Down) | 0.104 | 0.774 | –0.383 | 0.275 | BCF-R (Down) | 0.087 | 0.649 | 0.096 | 0.613 |
| BCF-L (Down) | –0.522 | 0.122 | –0.085 | 0.815 | BCF-L (Down) | 0.096 | 0.615 | 0.151 | 0.426 |
| ATB-R (Down) | –0.313 | 0.378 | –0.109 | 0.763 | ATB-R (Down) | 0.232 | 0.217 | 0.298 | 0.110 |
| ATB-L (Down) | –0.383 | 0.275 | 0.328 | 0.354 | ATB-L (Down) | 0.159 | 0.400 | 0.405 * | 0.026 |
| GNM-R (Down) | –0.453 | 0.189 | –0.061 | 0.868 | GNM-R (Down) | –0.005 | 0.981 | 0.101 | 0.596 |
| GNM-L (Down) | –0.035 | 0.924 | 0.304 | 0.393 | GNM-L (Down) | –0.141 | 0.457 | –0.067 | 0.725 |
| ARF-R (Max) | 0.035 | 0.924 | –0.723 * | 0.018 | ARF-R (Max) | 0.141 | 0.457 | 0.134 | 0.480 |
| ARF-L (Max) | –0.244 | 0.497 | –0.565 | 0.089 | ARF-L (Max) | 0.132 | 0.487 | 0.310 | 0.095 |
| BCF-R (Max) | 0.870 ** | 0.001 | –0.292 | 0.413 | BCF-R (Max) | 0.178 | 0.348 | 0.203 | 0.282 |
| BCF-L (Max) | –0.174 | 0.631 | 0.140 | 0.700 | BCF-L (Max) | 0.187 | 0.323 | 0.292 | 0.118 |
| ATB-R (Max) | 0.035 | 0.924 | –0.705 * | 0.023 | ATB-R (Max) | 0.250 | 0.182 | 0.288 | 0.123 |
| ATB-L (Max) | 0.592 | 0.071 | –0.164 | 0.650 | ATB-L (Max) | 0.260 | 0.166 | 0.407 * | 0.026 |
| GNM-R (Max) | –0.870 ** | 0.001 | 0.292 | 0.413 | GNM-R (Max) | 0.096 | 0.615 | 0.175 | 0.355 |
| GNM-L (Max) | –0.731 * | 0.016 | 0.006 | 0.987 | GNM-L (Max) | –0.032 | 0.867 | 0.041 | 0.831 |
References
- Pavese, N.; Ledingham, D. Parkinson’s, where are we heading? Br. J. Hosp. Med. 2024, 85, 7. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Durstine, J.L.; Painter, P.L. ACSM’s Exercise Management for Persons With Chronic Diseases and Disabilities, 4th ed.; Human Kinetics: Champaign, IL, USA, 2016. [Google Scholar]
- Hart, A.; Cordova-Rivera, L.; Barker, F.; Sayer, A.A.; Granic, A.; Yarnall, A.J. The prevalence of sarcopenia in Parkinson’s disease and related disorders- a systematic review. Neurol. Sci. 2023, 44, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Berardelli, A.; Bhattacharya, A.; Bologna, M.; Chen, K.-H.S.; Fasano, A.; Helmich, R.C.; Hutchison, W.D.; Kamble, N.; Kühn, A.A.; et al. Clinical neurophysiology of Parkinson’s disease and parkinsonism. Clin. Neurophysiol. Pract. 2022, 7, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, A.; Fasano, A.; Kalia, S.K. Another Step Forward for Freezing of Gait in Parkinson’s Disease. J. Park. Dis. 2024, 14, 353–355. [Google Scholar] [CrossRef]
- Marusic, U.; Peskar, M.; Šömen, M.M.; Kalc, M.; Holobar, A.; Gramann, K.; Wollesen, B.; Wunderlich, A.; Michel, C.; Miladinović, A.; et al. Neuromuscular assessment of force development, postural, and gait performance under cognitive-motor dual-tasking in healthy older adults and people with early Parkinson’s disease: Study protocol for a cross-sectional Mobile Brain/Body Imaging (MoBI) study. Open Res. Eur. 2023, 3, 58. [Google Scholar] [CrossRef]
- Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle synergies in Parkinson’s Disease. Sensors 2020, 20, 3209. [Google Scholar] [CrossRef]
- Boebinger, S.; Payne, A.; Martino, G.; Kerr, K.; Mirdamadi, J.; McKay, J.L.; Borich, M.; Ting, L. Precise cortical contributions to sensorimotor feedback control during reactive balance. PLoS Comput. Biol. 2024, 20, e1011562. [Google Scholar] [CrossRef]
- Falaki, A.; Jo, H.J.; Lewis, M.M.; O’Connell, B.; De Jesus, S.; McInerney, J.; Huang, X.; Latash, M.L. Systemic effects of deep brain stimulation on synergic control in Parkinson’s disease. Clin. Neurophysiol. 2018, 129, 1320–1332. [Google Scholar] [CrossRef]
- Allen, J.L.; McKay, J.L.; Sawers, A.; Hackney, M.E.; Ting, L.H. Increased neuromuscular consistency in gait and balance after partnered dance-based rehabilitation in Parkinson’s disease. J. Neurophysiol. 2017, 118, 363–373. [Google Scholar] [CrossRef]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Impaired synergic control of posture in Parkinson’s patients without postural instability. Gait Posture 2016, 44, 209–215. [Google Scholar] [CrossRef]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Motor equivalence and structure of variance: Multi-muscle postural synergies in Parkinson’s disease. Exp. Brain Res. 2017, 235, 2243–2258. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.L.; Roemmich, R.T.; Cam, B.; Fregly, B.J.; Hass, C.J. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 2013, 124, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, S.; Hao, M.; Xiao, Q.; Lan, N. The impact of evoked cutaneous afferents on voluntary reaching movement in patients with Parkinson’s disease. J. Neural. Eng. 2019, 16, 036029. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Xu, S.; Hu, Z.; Xu, F.; Niu, C.M.; Xiao, Q.; Lan, N. Inhibition of Parkinsonian tremor with cutaneous afferent evoked by transcutaneous electrical nerve stimulation. J. Neuroeng. Rehabil. 2017, 14, 75. [Google Scholar] [CrossRef]
- Chung, C.L.; Mak, M.K.; Hallett, M. Transcranial Magnetic Stimulation Promotes Gait Training in Parkinson Disease. Ann. Neurol. 2020, 88, 933–945. [Google Scholar] [CrossRef]
- Wang, R.; Wang, F.; Huang, S.; Yang, Y. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: A double-blinded randomized controlled pilot trial. Gait Posture 2019, 68, 382–387. [Google Scholar] [CrossRef]
- Weersink, J.B.; de Jong, B.M.; Maurits, N.M. Neural coupling between upper and lower limb muscles in Parkinsonian gait. Clin. Neurophysiol. 2022, 134, 65–72. [Google Scholar] [CrossRef]
- Yokote, A.; Hayashi, Y.; Yanamoto, S.; Fujioka, S.; Higa, K.; Tsuboi, Y. Leg Muscle Strength Correlates with Gait Performance in Advanced Parkinson Disease. Intern. Med. 2022, 61, 633–638. [Google Scholar] [CrossRef]
- Kim, J.; Byeon, J.; Yang, H.; Oh, J.; Lee, J.; Choi, M.; Lee, H.; Jeon, J.Y. Neuromuscular characteristics and physical function in participants with parkinson’s disease. Exerc. Sci. 2021, 30, 318–326. [Google Scholar] [CrossRef]
- Bailo, G.; Saibene, F.L.; Bandini, V.; Arcuri, P.; Salvatore, A.; Meloni, M.; Castagna, A.; Navarro, J.; Lencioni, T.; Ferrarin, M.; et al. Characterization of Walking in Mild Parkinson’s Disease: Reliability, Validity and Discriminant Ability of the Six-Minute Walk Test Instrumented with a Single Inertial Sensor. Sensors 2024, 24, 662. [Google Scholar] [CrossRef]
- Weston, A.R.; Antonellis, P.; Fino, P.C.; Hoppes, C.W.; Lester, M.E.; Weightman, M.M.; Dibble, L.E.; King, L.A. Quantifying Turning Tasks with Wearable Sensors: A Reliability Assessment. Phys. Ther. 2023, 104, pzad134. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Bastidas, P.; Gómez, B.; Aqueveque, P.; Luarte-Martínez, S.; Cano-de-la-Cuerda, R. Instrumented Timed Up and Go Test (iTUG)-More Than Assessing Time to Predict Falls: A Systematic Review. Sensors 2023, 23, 3426. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; Choi, J.; Motl, R.W.; Foley, F.W.; Picone, M.A.; Lipton, M.L.; Izzetoglu, M.; Hernandez, M.; Wagshul, M.E. Individual reserve in aging and neurological disease. J. Neurol. 2023, 270, 3179–3191. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.R.; Kuroda, M.H.; Moreno, V.C.; Zámuner, A.R.; Barbieri, F.A. Effects of automatic mechanical peripheral stimulation on gait biomechanics in older adults with Parkinson’s disease: A randomized crossover clinical trial. Aging Clin. Exp. Res. 2022, 34, 1323–1331. [Google Scholar] [CrossRef]
- Giardini, M.; Nardone, A.; Godi, M.; Guglielmetti, S.; Arcolin, I.; Pisano, F.; Schieppati, M. Instrumental or Physical-Exercise Rehabilitation of Balance Improves Both Balance and Gait in Parkinson’s Disease. Neural Plast. 2018, 2018, 5614242. [Google Scholar] [CrossRef]
- King, L.A.; Horak, F.B. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys. Ther. 2009, 89, 384–393. [Google Scholar] [CrossRef]
- Hubble, R.P.; Naughton, G.; Silburn, P.A.; Cole, M.H. Trunk Exercises Improve Gait Symmetry in Parkinson Disease: A Blind Phase II Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2018, 97, 151–159. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Liguori, G.; Feito, Y.; Fountaine, C. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Roy, B.A., Ed.; Wolters Kluwer: Philadelpia, PA, USA, 2022. [Google Scholar]
- SENIAM. Sensor Locations. Available online: http://seniam.org/sensor_location.htm (accessed on 22 July 2024).
- Criswell, E. Cram’s Introduction to Surface Electromyography, 2nd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2010. [Google Scholar]
- Filibeck, U.; Del Vecchio, A.; Galliccia, F. Good Clinical Practice Principles: Legal Background and Applicability. Anal. Tech. Clin. Chem. Methods Appl. 2012, 1–27. [Google Scholar]
- Tutus, N.; Ozdemir, F. The effects of gastrocnemius muscle spasticity on gait symmetry and trunk control in chronic stroke patients. Gait Posture 2023, 105, 45–50. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, Q.; Nong, K.; Li, S.; Yue, J.; Huang, J.; Dong, B.; Beauchamp, M.; Hao, Q. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1199–1211. [Google Scholar] [CrossRef]
- Alarcón-Jimenez, J.; de la Rubia Ortí, J.E.; Ruiz, J.M.; de Bernardo, N.; Proaño, B.; Villarón-Casales, C. Muscular Response in ALS Patients during Maximal Bilateral Isometric Work of the Biceps Brachii until Fatigue. Life 2022, 12, 1978. [Google Scholar] [CrossRef] [PubMed]
- Israeli-Korn, S.D.; Barliya, A.; Paquette, C.; Franzén, E.; Inzelberg, R.; Horak, F.B.; Flash, T. Intersegmental coordination patterns are differently affected in Parkinson’s disease and cerebellar ataxia. J. Neurophysiol. 2019, 121, 672–689. [Google Scholar] [CrossRef] [PubMed]
- Ghislieri, M.; Lanotte, M.; Knaflitz, M.; Rizzi, L.; Agostini, V. Muscle synergies in Parkinson’s disease before and after the deep brain stimulation of the bilateral subthalamic nucleus. Sci. Rep. 2023, 13, 6997. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.; Spolaor, F.; Sawacha, Z.; Guiotto, A.; Pavan, D.; Bakdounes, L.; Urbani, V.; Frazzitta, G.; Iansek, R. Muscular activation changes in lower limbs after underwater gait training in Parkinson’s disease: A surface emg pilot study. Gait Posture 2020, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Spolaor, F.; Romanato, M.; Annamaria, G.; Peppe, A.; Bakdounes, L.; To, D.-K.; Volpe, D.; Sawacha, Z. Relationship between Muscular Activity and Postural Control Changes after Proprioceptive Focal Stimulation (Equistasi®) in Middle-Moderate Parkinson’s Disease Patients: An Explorative Study. Sensors 2021, 21, 560. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Romagnoli, C.; Raju, M.; Padua, E. Proprioceptive Focal Stimulation (Equistasi®) for gait and postural balance rehabilitation in patients with Parkinson’s disease: A systematic review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2023, 237, 179–189. [Google Scholar] [CrossRef]
- Gupta, R.; Kumari, S.; Senapati, A.; Ambasta, R.K.; Kumar, P. New era of artificial intelligence and machine learning-based detection, diagnosis, and therapeutics in Parkinson’s disease. Ageing Res. Rev. 2023, 90, 102013. [Google Scholar] [CrossRef]
- Wu, P.; Cao, B.; Liang, Z.; Wu, M. The advantages of artificial intelligence-based gait assessment in detecting, predicting, and managing Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1191378. [Google Scholar] [CrossRef]
| Control (N = 10) | PD (N = 30) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Md | IQR | M | SD | Md | IQR | p-Value | r | |
| ARF-R (MA) | 38.11 | 14.96 | 33.2 | 8.3 | 23.62 | 19.78 | 15.6 | 24.9 | 0.014 | −0.39 |
| ARF-L (MA) | 32.87 | 9.32 | 30.4 | 14.4 | 28.50 | 20.91 | 22.3 | 29.4 | 0.150 | −0.23 |
| BCF-R (MA) | 46.52 | 12.70 | 45.9 | 17.5 | 30.42 | 23.81 | 25.4 | 27.5 | 0.005 | −0.44 |
| BCF-L (MA) | 41.02 | 11.80 | 37.4 | 18.6 | 49.66 | 38.44 | 48.5 | 40.8 | 0.574 | −0.09 |
| ATB-R (MA) | 106.72 | 26.41 | 103.5 | 34.7 | 44.84 | 57.22 | 15.8 | 63.4 | <0.001 | −0.58 |
| ATB-L (MA) | 87.02 | 20.62 | 85.2 | 11.9 | 65.46 | 49.61 | 46.5 | 66.6 | 0.042 | −0.32 |
| GNM-R (MA) | 82.86 | 36.63 | 71.4 | 74.5 | 28.23 | 30.70 | 10.0 | 50.4 | <0.001 | −0.54 |
| GNM-L (MA) | 73.87 | 21.98 | 71.8 | 39.5 | 82.42 | 72.36 | 57.7 | 85.4 | 0.472 | −0.11 |
| ARF-R (Up) | 69.45 | 25.43 | 67.7 | 46.8 | 26.67 | 21.52 | 20.5 | 28.2 | <0.001 | −0.61 |
| ARF-L (Up) | 62.57 | 26.48 | 53.1 | 34.3 | 40.74 | 41.96 | 24.7 | 46.4 | 0.013 | −0.39 |
| BCF-R (Up) | 37.77 | 15.89 | 37.2 | 26.7 | 27.46 | 29.66 | 19.2 | 17.4 | 0.013 | −0.39 |
| BCF-L (Up) | 27.92 | 11.82 | 23.0 | 14.7 | 46.38 | 38.88 | 35.8 | 39.9 | 0.118 | −0.25 |
| ATB-R (Up) | 180.24 | 73.47 | 161.8 | 57.7 | 50.99 | 61.88 | 19.7 | 78.2 | <0.001 | −0.64 |
| ATB-L (Up) | 129.89 | 48.50 | 123.6 | 77.0 | 87.40 | 68.13 | 68.2 | 68.7 | 0.020 | −0.37 |
| GNM-R (Up) | 30.39 | 34.70 | 20.0 | 10.2 | 28.33 | 33.79 | 11.8 | 54.4 | 0.169 | −0.22 |
| GNM-L (Up) | 31.59 | 14.96 | 32.2 | 26.2 | 126.66 | 129.75 | 76.7 | 150.6 | 0.004 | −0.44 |
| ARF-R (Down) | 86.83 | 42.92 | 78.8 | 54.9 | 41.88 | 57.27 | 21.4 | 38.3 | <0.001 | −0.53 |
| ARF-L (Down) | 69.91 | 24.32 | 75.2 | 33.2 | 38.26 | 38.25 | 23.6 | 35.6 | 0.008 | −0.41 |
| BCF-R (Down) | 55.37 | 39.63 | 40.5 | 52.6 | 32.59 | 37.12 | 25.0 | 19.3 | 0.008 | −0.41 |
| BCF-L (Down) | 51.66 | 61.56 | 31.0 | 28.2 | 53.36 | 43.29 | 46.8 | 38.5 | 0.365 | −0.14 |
| ATB-R (Down) | 209.83 | 62.57 | 204.3 | 108.5 | 67.83 | 92.70 | 21.5 | 88.3 | <0.001 | −0.59 |
| ATB-L (Down) | 165.19 | 69.11 | 151.8 | 149.1 | 103.70 | 100.21 | 61.0 | 108.5 | 0.016 | −0.38 |
| GNM-R (Down) | 45.65 | 31.65 | 40.6 | 32.6 | 29.09 | 32.80 | 11.1 | 45.6 | 0.075 | −0.28 |
| GNM-L (Down) | 38.72 | 24.04 | 36.5 | 32.0 | 110.20 | 98.50 | 181.1 | 183.5 | 0.004 | −0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarón-Casales, C.; de Bernardo, N.; Alarcón-Jiménez, J.; López-Malo, D.; Proaño, B.; Martín-Ruiz, J.; de la Rubia Ortí, J.E. Amplitude of Lower Limb Muscle Activation in Different Phases of the Illinois Test in Parkinson’s Disease Patients: A Pilot Study. J. Clin. Med. 2024, 13, 5792. https://doi.org/10.3390/jcm13195792
Villarón-Casales C, de Bernardo N, Alarcón-Jiménez J, López-Malo D, Proaño B, Martín-Ruiz J, de la Rubia Ortí JE. Amplitude of Lower Limb Muscle Activation in Different Phases of the Illinois Test in Parkinson’s Disease Patients: A Pilot Study. Journal of Clinical Medicine. 2024; 13(19):5792. https://doi.org/10.3390/jcm13195792
Chicago/Turabian StyleVillarón-Casales, Carlos, Nieves de Bernardo, Jorge Alarcón-Jiménez, Daniel López-Malo, Belén Proaño, Julio Martín-Ruiz, and José Enrique de la Rubia Ortí. 2024. "Amplitude of Lower Limb Muscle Activation in Different Phases of the Illinois Test in Parkinson’s Disease Patients: A Pilot Study" Journal of Clinical Medicine 13, no. 19: 5792. https://doi.org/10.3390/jcm13195792
APA StyleVillarón-Casales, C., de Bernardo, N., Alarcón-Jiménez, J., López-Malo, D., Proaño, B., Martín-Ruiz, J., & de la Rubia Ortí, J. E. (2024). Amplitude of Lower Limb Muscle Activation in Different Phases of the Illinois Test in Parkinson’s Disease Patients: A Pilot Study. Journal of Clinical Medicine, 13(19), 5792. https://doi.org/10.3390/jcm13195792










