Alterations of the Gut Microbiome and TMAO Levels in Patients with Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. TMAO Level Determination
2.4. Endoscopic Examination
2.5. Microbiome Analysis
2.6. Statistical Analysis and Visualization of the Results
3. Results
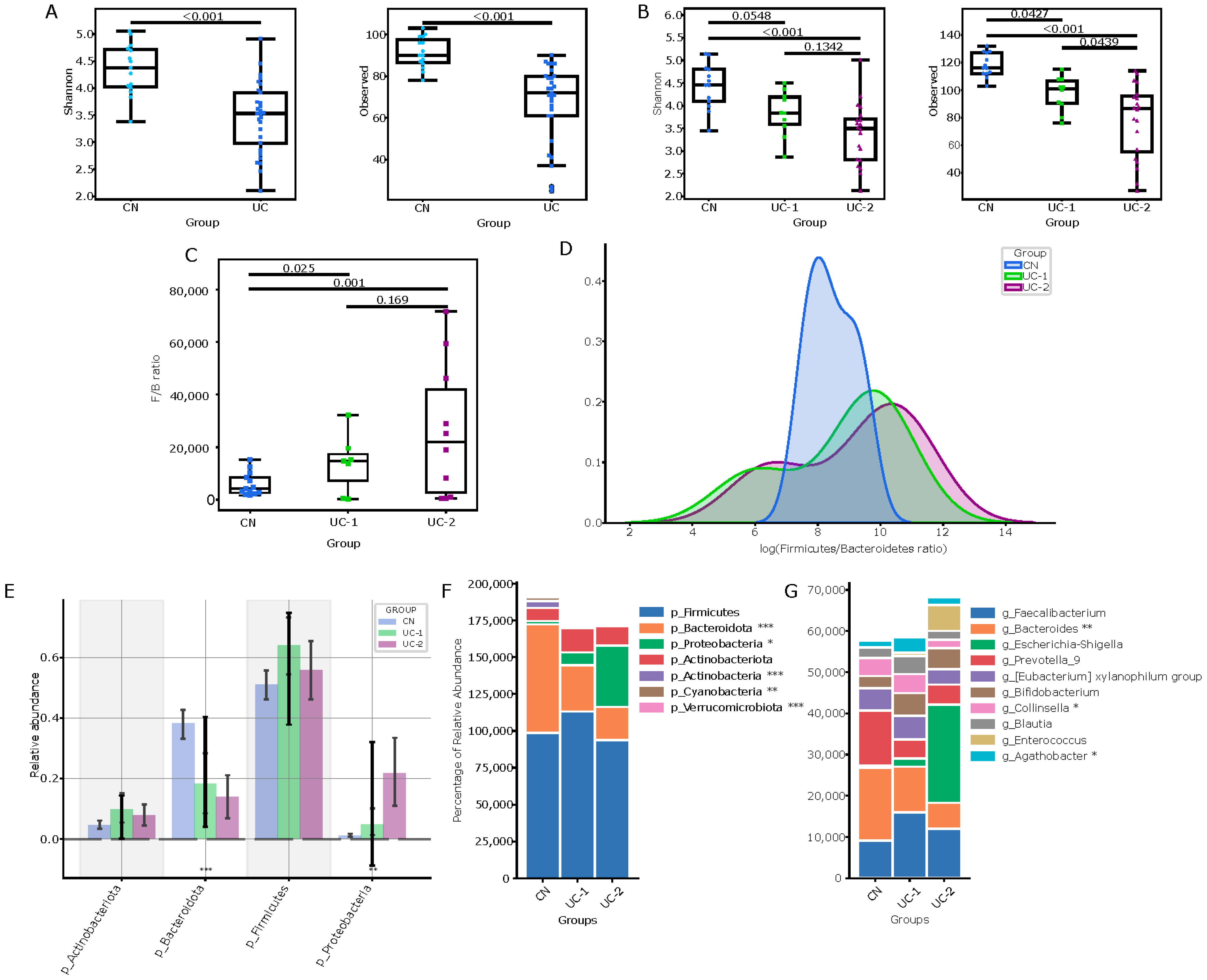
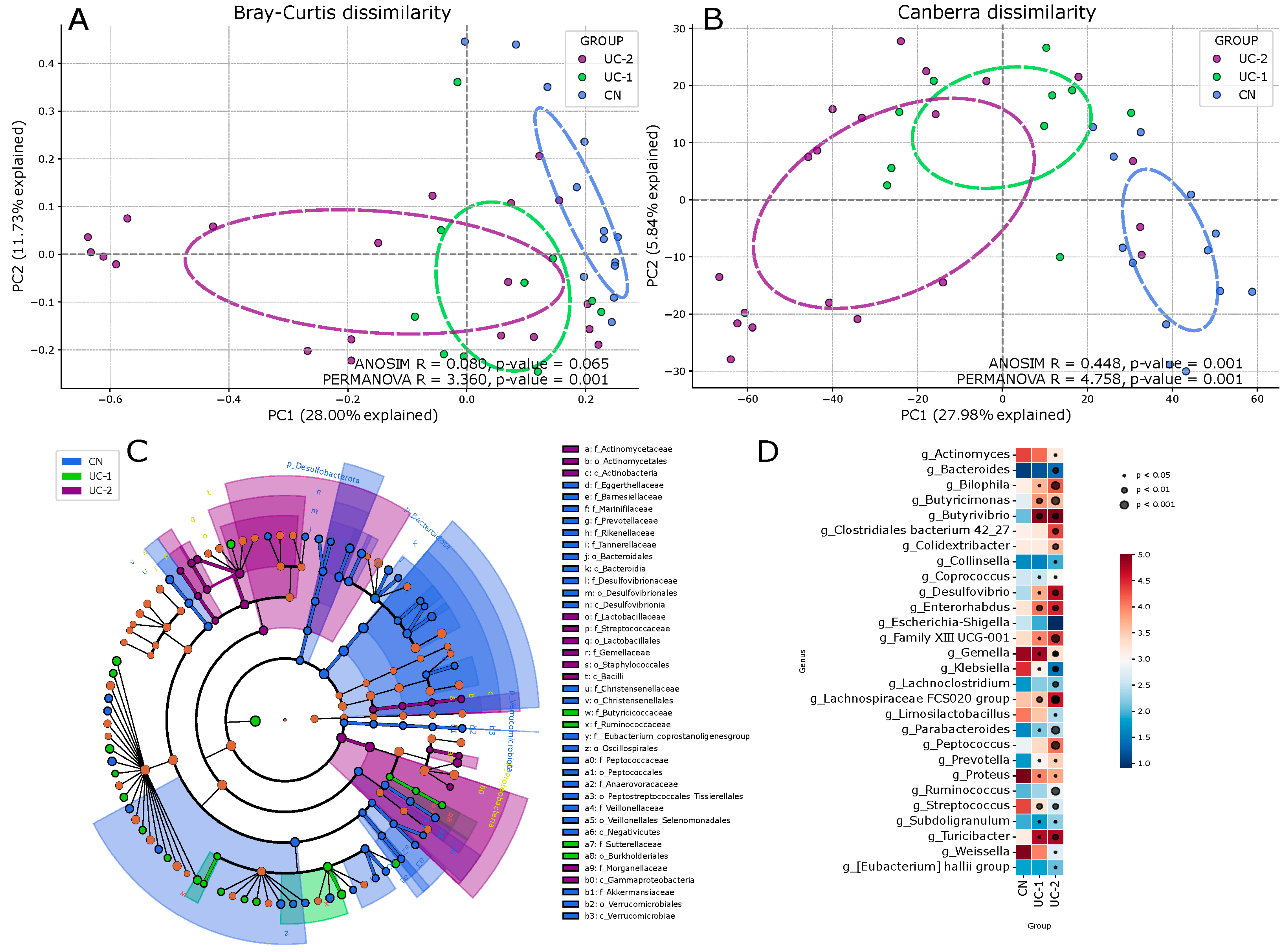
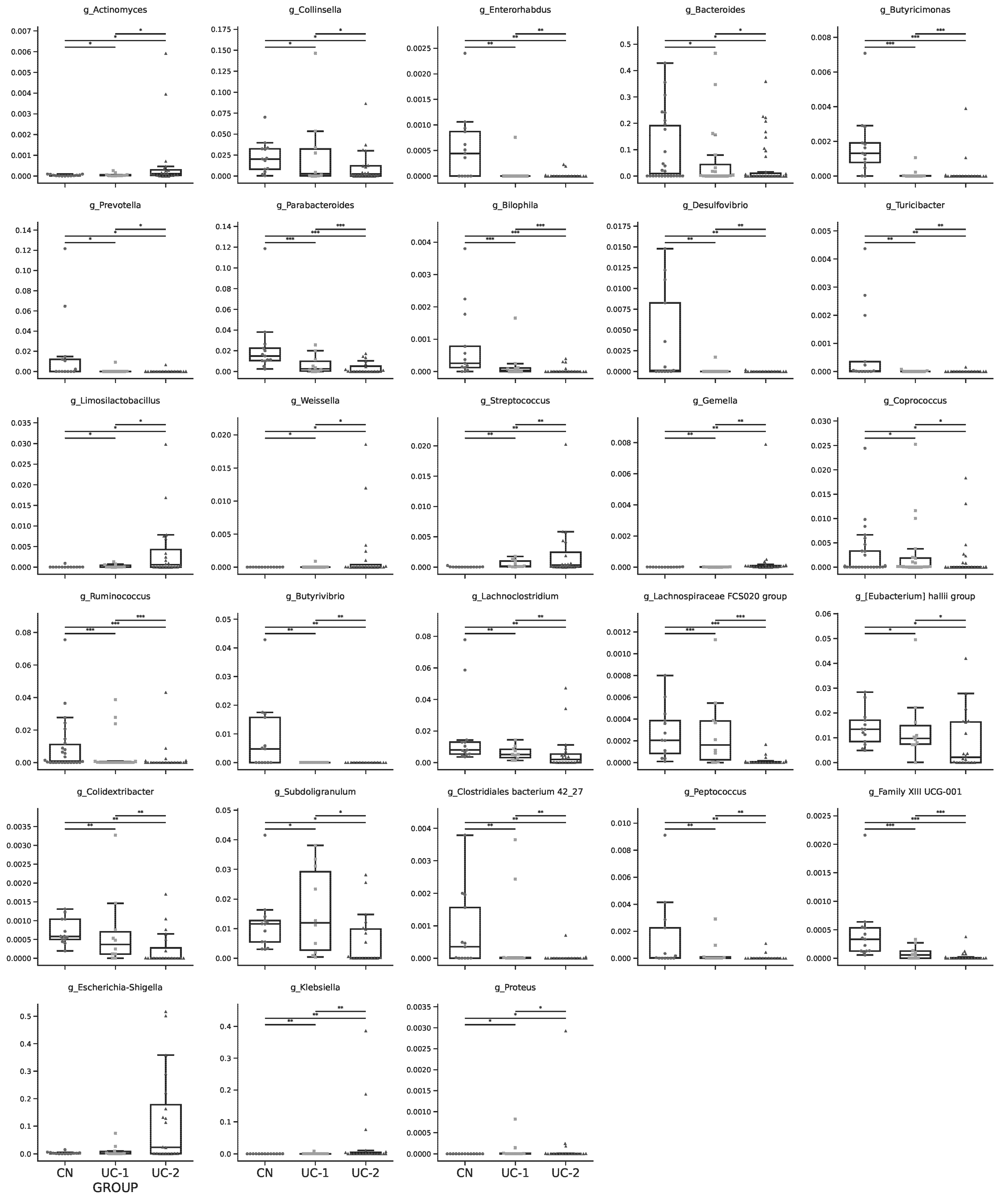
3.1. Evaluation of the Intestinal Microbiota in Patients with Ulcerative Colitis and Its Changes in Different Clinical and Endoscopic Stages
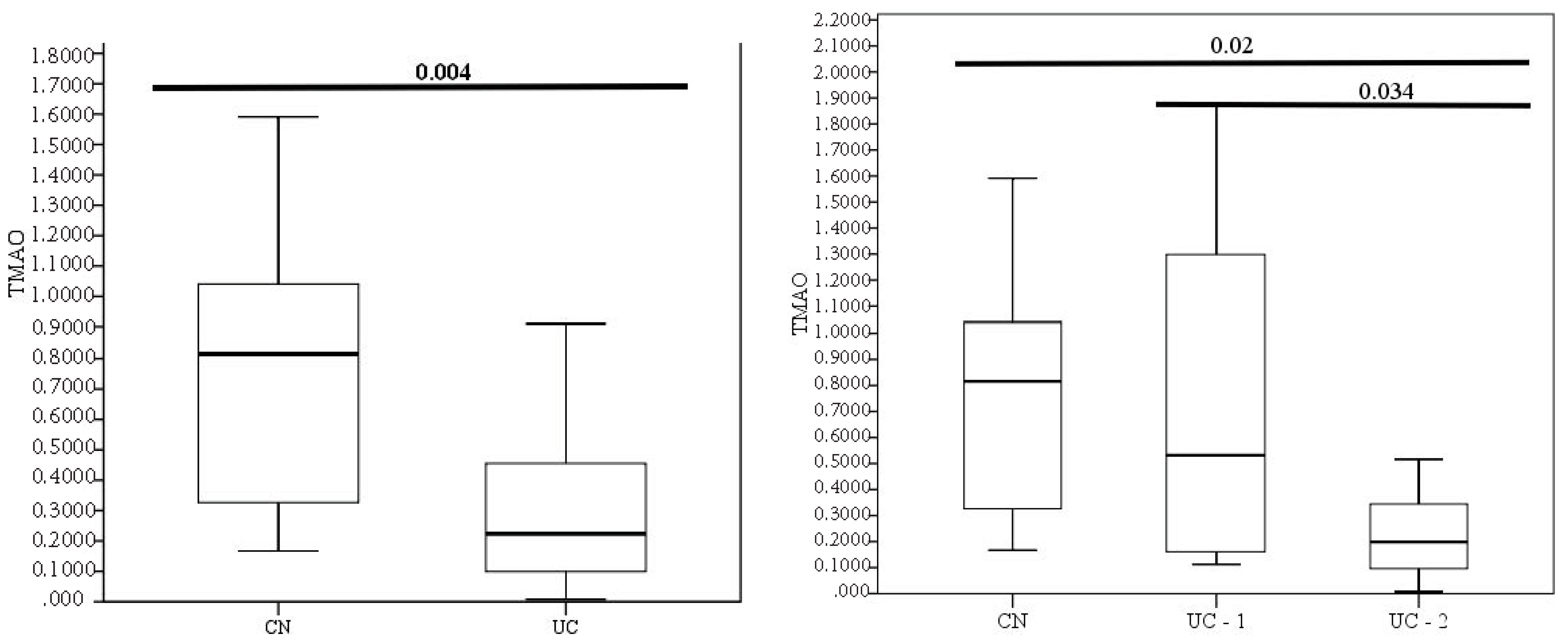
3.2. Evaluation of TMAO Levels in Patients with Different Clinical and Endoscopic Activities
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Świrkosz, G.; Szczygieł, A.; Logoń, K.; Wrześniewska, M.; Gomułka, K. The Role of the Microbiome in the Pathogenesis and Treatment of Ulcerative Colitis—A Literature Review. Biomedicines 2023, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients With vs. Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Nicoara, A.D.; Tofolean, D.E.; Herlo, A.; Nelson Twakor, A.; Tocia, C.; Trandafir, A.; Dumitru, A.; Dumitru, E.; Aftenie, C.F.; et al. Healing from Within: How Gut Microbiota Predicts IBD Treatment Success—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8451. [Google Scholar] [CrossRef]
- Malham, M.; Vestergaard, M.V.; Bataillon, T.; Villesen, P.; Dempfle, A.; Bang, C.; Engsbro, A.L.; Jakobsen, C.; Franke, A.; Wewer, V.; et al. The Composition of the Fecal and Mucosa-adherent Microbiota Varies Based on Age and Disease Activity in Ulcerative Colitis. Inflamm. Bowel Dis. 2024, izae179. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated with Gut Barrier Dysfunction in Healthy Relatives of Patients with Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e10. [Google Scholar] [CrossRef]
- Basha, O.M.; Hafez, R.A.; Salem, S.M.; Anis, R.H.; Hanafy, A.S. Impact of gut Microbiome alteration in Ulcerative Colitis patients on disease severity and outcome. Clin. Exp. Med. 2023, 23, 1763–1772. [Google Scholar] [CrossRef]
- Liao, L.; Huang, J.; Zheng, J.; Ma, X.; Huang, L.; Xu, W. Gut microbiota in Chinese and Japanese patients with cardiovascular diseases: A systematic review and meta-analysis. Ann. Saudi Med. 2023, 43, 105–114. [Google Scholar] [CrossRef]
- Lu, J.; Jin, X.; Yang, S.; Li, Y.; Wang, X.; Wu, M. Immune mechanism of gut microbiota and its metabolites in the occurrence and development of cardiovascular diseases. Front. Microbiol. 2022, 13, 1034537. [Google Scholar] [CrossRef]
- Czaja, A.J. Incorporating the Molecular Mimicry of Environmental Antigens into the Causality of Autoimmune Hepatitis. Dig. Dis. Sci. 2023, 68, 2824–2842. [Google Scholar] [CrossRef]
- Evrensel, A. Microbiome-Induced Autoimmunity and Novel Therapeutic Intervention. Adv. Exp. Med. Biol. 2023, 1411, 71–90. [Google Scholar] [CrossRef]
- Zhang, X.; Ha, S.; Lau, H.C.; Yu, J. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin. Cancer Biol. 2023, 92, 16–27. [Google Scholar] [CrossRef]
- Qin, L.; Wu, J.; Sun, X.; Huang, X.; Huang, W.; Weng, C.; Cai, J. The regulatory role of metabolic organ-secreted factors in the nonalcoholic fatty liver disease and cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1119005. [Google Scholar] [CrossRef]
- Wilson, A.; Teft, W.A.; Morse, B.L.; Choi, Y.H.; Woolsey, S.; DeGorter, M.K.; Hegele, R.A.; Tirona, R.G.; Kim, R.B. Trimethylamine-N-oxide: A Novel Biomarker for the Identification of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 3620–3630. [Google Scholar] [CrossRef]
- Kul, S.; Caliskan, Z.; Guvenc, T.S.; Celik, F.B.; Sarmis, A.; Atici, A.; Konal, O.; Akıl, M.; Cumen, A.S.; Bilgic, N.M.; et al. Gut microbiota-derived metabolite trimethylamine N-oxide and biomarkers of inflammation are linked to endothelial and coronary microvascular function in patients with inflammatory bowel disease. Microvasc. Res. 2023, 146, 104458. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Sun, Y.; Limsrivilai, J.; Abdullah, M.; Kaibullayeva, J.; Balderramo, D.; Vergara, B.I.; Paudel, M.S.; Banerjee, R.; Hilmi, I.; et al. Development of the global inflammatory bowel disease visualization of epidemiology studies in the 21st century (GIVES-21). BMC Med. Res. Methodol. 2023, 23, 129. [Google Scholar] [CrossRef]
- Marchenko, A.B.; Ivasenko, S.A.; Laryushina, E.M.; Turmukhambetova, A.A. A Method for Quantifying the Level of Trimethylamine N-Oxide in Blood Plasma. Patent of the Republic of Kazakhstan No. 33624, 4 May 2019. [Google Scholar]
- Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Pola, S.; McDonald, J.W.; Rutgeerts, P.; Munkholm, P.; Mittmann, U.; King, D.; Wong, C.J.; et al. The Role of Centralized Reading of Endoscopy in a Randomized Controlled Trial of Mesalamine for Ulcerative Colitis. Gastroenterology 2013, 145, 149–157.e2. [Google Scholar] [CrossRef]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Sanat, Z.M.; Vahedi, H.; Malekzadeh, R.; Fanni, Z. Epidemiologic profile of inflammatory bowel disease in Eastern Mediterranean Region (EMRO) countries: A systematic review and meta-analysis. BMC Public Health 2024, 24, 1395. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of Inflammatory Bowel Diseases: A Population Study in a Healthcare District of North-West Italy. J. Clin. Med. 2023, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.J.; Lee, S.W.; Park, S.; Koh, S.J.; Im, J.P.; Kim, B.G.; Han, K.D.; Kim, J.S. Recent trends in the epidemiology and clinical outcomes of inflammatory bowel disease in South Korea, 2010–2018. World J. Gastroenterol. 2024, 30, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Saberzadeh-Ardestani, B.; Khosravi, A.A.; Mansour-Ghanaei, F.; Vahedi, H.; Baniasadi, N.; Seyyedmajidi, M.; Parhizkar, B.; Hormati, A.; Naghshbandi, S.J.; Matin, S.; et al. Clinical Phenotype and Disease Course of Inflammatory Bowel Disease in Iran: Results of the Iranian Registry of Crohn’s and Colitis (IRCC). Arch. Iran. Med. 2024, 27, 183–190. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Nowak, J.K.; Bergemalm, D.; Vatn, S.; Ventham, N.T.; Kennedy, N.A.; Ricanek, P.; Lindstrom, J.; IBD-Character Consortium; et al. Analysis of Systemic Epigenetic Alterations in Inflammatory Bowel Disease: Defining Geographical, Genetic and Immune-Inflammatory influences on the Circulating Methylome. J. Crohns Colitis 2023, 17, 170–184. [Google Scholar] [CrossRef]
- Inciuraite, R.; Gedgaudas, R.; Lukosevicius, R.; Tilinde, D.; Ramonaite, R.; Link, A.; Kasetiene, N.; Malakauskas, M.; Kiudelis, G.; Jonaitis, L.V.; et al. Constituents of stable commensal microbiota imply diverse colonic epithelial cell reactivity in patients with ulcerative colitis. Gut Pathog. 2024, 16, 16. [Google Scholar] [CrossRef]
- Xu, X.; Ocansey, D.K.W.; Hang, S.; Wang, B.; Amoah, S.; Yi, C.; Zhang, X.; Liu, L.; Mao, F. The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 2022, 14, 26. [Google Scholar] [CrossRef]
- Zhu, S.; Han, M.; Liu, S.; Fan, L.; Shi, H.; Li, P. Composition and diverse differences of intestinal microbiota in ulcerative colitis patients. Front. Cell. Infect. Microbiol. 2022, 12, 953962. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yang, L.; Feng, J.; Tian, S.; Chen, L.; Huang, W.; Liu, J.; Wang, X. Integrated 16S rRNA sequencing and metagenomics insights into microbial dysbiosis and distinct virulence factors in inflammatory bowel disease. Front. Microbiol. 2024, 15, 1375804. [Google Scholar] [CrossRef]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef]
- Manandhar, I.; Alimadadi, A.; Aryal, S.; Munroe, P.B.; Joe, B.; Cheng, X. Gut microbiome-based supervised machine learning for clinical diagnosis of inflammatory bowel diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G328–G337. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Zhou, Y.L.; Sun, H.; Zhang, Y.; Shen, C.; Wang, Z.; Xuan, B.; Zhao, Y.; Ma, Y.; Yan, Y.; et al. Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat. Commun. 2023, 14, 7135. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Chen, L.; Guo, R.; Ma, S.; Li, S.; Zhang, Y.; Jiang, H.; Shi, H.; Zhang, P. Altered gut mycobiome in patients with end-stage renal disease and its correlations with serum and fecal metabolomes. J. Transl. Med. 2024, 22, 202. [Google Scholar] [CrossRef]
- Saadh, M.J.; Ahmed, H.M.; Alani, Z.K.; Al Zuhairi, R.A.H.; Almarhoon, Z.M.; Ahmad, H.; Ubaid, M.; Alwan, N.H. The Role of Gut-derived Short-Chain Fatty Acids in Multiple Sclerosis. Neuromol. Med. 2024, 26, 14. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Wan, G.; Yang, D.; Zhu, D.; Jia, L.; Zheng, J. Mechanism of intestinal microbiota disturbance promoting the occurrence and development of esophageal squamous cell carcinoma-based on microbiomics and metabolomics. BMC Cancer 2024, 24, 245. [Google Scholar] [CrossRef]
- Zhou, P.; Feng, P.; Liao, B.; Fu, L.; Shan, H.; Cao, C.; Luo, R.; Peng, T.; Liu, F.; Li, R. Role of polyphenols in remodeling the host gut microbiota in polycystic ovary syndrome. J. Ovarian Res. 2024, 17, 69. [Google Scholar] [CrossRef]
- Chen, Y.; Patel, N.A.; Crombie, A.; Scrivens, J.H.; Murrell, J.C. Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc. Natl. Acad. Sci. USA 2011, 108, 17791–17796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Tang, W.H.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Martínez-del Campo, A.; Bodea, S.; Hamer, H.A.; Marks, J.A.; Haiser, H.J.; Turnbaugh, P.J.; Balskus, E.P. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio 2015, 6, e00042-15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jameson, E.; Crosatti, M.; Schäfer, H.; Rajakumar, K.; Bugg, T.D.; Chen, Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 4268–4273. [Google Scholar] [CrossRef] [PubMed]
- Massmig, M.; Reijerse, E.; Krausze, J.; Laurich, C.; Lubitz, W.; Jahn, D.; Moser, J. Carnitine metabolism in the human intestine: Characterization of the two-component carnitine monooxygenase CntAB from Acinetobacter baumannii. J. Biolog. Chem. 2020, 295, 13065–13078. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef]
| Variables | Ulcerative Colitis (UC) | Control (CN) | p-Level |
|---|---|---|---|
| Male/female, N | 31 (16/5) | 15 (5/10) | 0.238 |
| Age, me (Q25;Q75) | 38 (33;52) | 42 (33;51) | 0.907 |
| Occupation (urb/vil), N | 20/11 | 9/6 | 0.767 |
| Feeding Characteristics (in mL or gram per 1 week) | |||
| Meat/meat products | 1183 | 1232 | 0.356 |
| Fatty fish (1 serving of 100 g) | 136 | 145.6 | 0.765 |
| Dairy products | 611 | 653 | 0.493 |
| Eggs (1 egg = 64 g.) | 364 | 384 | 0.612 |
| Cereals/Bread and bakery products | 504 | 546.2 | 0.32 |
| Fruit | 331.3 | 688.3 | <0.001 |
| Vegetables | 434 | 539 | 0.031 |
| Alcohol (1 portion = 250 mL of beer, 100 mL of wine or 30 mL of spirits) | 6 portions | 1 portion | <0.001 |
| Laboratory characteristics | |||
| Me (Q25;Q75) | p-level | ||
| Hb, g/L | 131 (114;144) | 134 (116;143) | 0.844 |
| ESR, mm/h | 15 (4;28) | 11 (8;20) | 0.163 |
| Albumin, g/L | 40.8 (36.1;46.7) | 44.6 (43.6;45.9) | 0.016 |
| CRP, g/L | 2.3 (1;8.7) | 1.1 (0.4;2.8) | 0.054 |
| Fecal calprotectin, mcg/kg | 305.9 (91.1;1202) | 18 (12;23) | <0.001 |
| Clinical characteristics | |||
| Extent, N/%: | - | - | |
| E1 (proctitis) | 3/9.7 | ||
| E2 (left-sided colitis) | 13/41.9 | ||
| E3 (pan-colitis) | 15/48.4 | ||
| Mayo activity, N/%: | - | - | |
| Mayo ≤ 5 points (UC1) | 8/25.8 | ||
| Mayo > 6 points (UC2) | 23/74.2 | ||
| r (Spearman) | p-Level | r (Spearman) | p-Level | ||
|---|---|---|---|---|---|
| Age | 0.29 | 0.113 | ESR | −0.210 | 0.258 |
| Defecation frequency | −0.208 | 0.269 | CRP | −0.114 | 0.542 |
| Extension | −0.018 | 0.924 | Albumin | 0.451 | 0.011 |
| Hb | 0.111 | 0.554 | Fecal calprotectin | −0.458 | 0.010 |
| RBC | 0.198 | 0.285 | Mayo UC-1, UC-2 | −0.387 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laryushina, Y.; Samoilova-Bedych, N.; Turgunova, L.; Kozhakhmetov, S.; Alina, A.; Suieubayev, M.; Mukhanbetzhanov, N. Alterations of the Gut Microbiome and TMAO Levels in Patients with Ulcerative Colitis. J. Clin. Med. 2024, 13, 5794. https://doi.org/10.3390/jcm13195794
Laryushina Y, Samoilova-Bedych N, Turgunova L, Kozhakhmetov S, Alina A, Suieubayev M, Mukhanbetzhanov N. Alterations of the Gut Microbiome and TMAO Levels in Patients with Ulcerative Colitis. Journal of Clinical Medicine. 2024; 13(19):5794. https://doi.org/10.3390/jcm13195794
Chicago/Turabian StyleLaryushina, Yelena, Nadezhda Samoilova-Bedych, Lyudmila Turgunova, Samat Kozhakhmetov, Assel Alina, Maxat Suieubayev, and Nurislam Mukhanbetzhanov. 2024. "Alterations of the Gut Microbiome and TMAO Levels in Patients with Ulcerative Colitis" Journal of Clinical Medicine 13, no. 19: 5794. https://doi.org/10.3390/jcm13195794
APA StyleLaryushina, Y., Samoilova-Bedych, N., Turgunova, L., Kozhakhmetov, S., Alina, A., Suieubayev, M., & Mukhanbetzhanov, N. (2024). Alterations of the Gut Microbiome and TMAO Levels in Patients with Ulcerative Colitis. Journal of Clinical Medicine, 13(19), 5794. https://doi.org/10.3390/jcm13195794






