Safety of Sofosbuvir-Based Direct-Acting Antivirals for Hepatitis C Virus Infection and Direct Oral Anticoagulant Co-Administration
Abstract
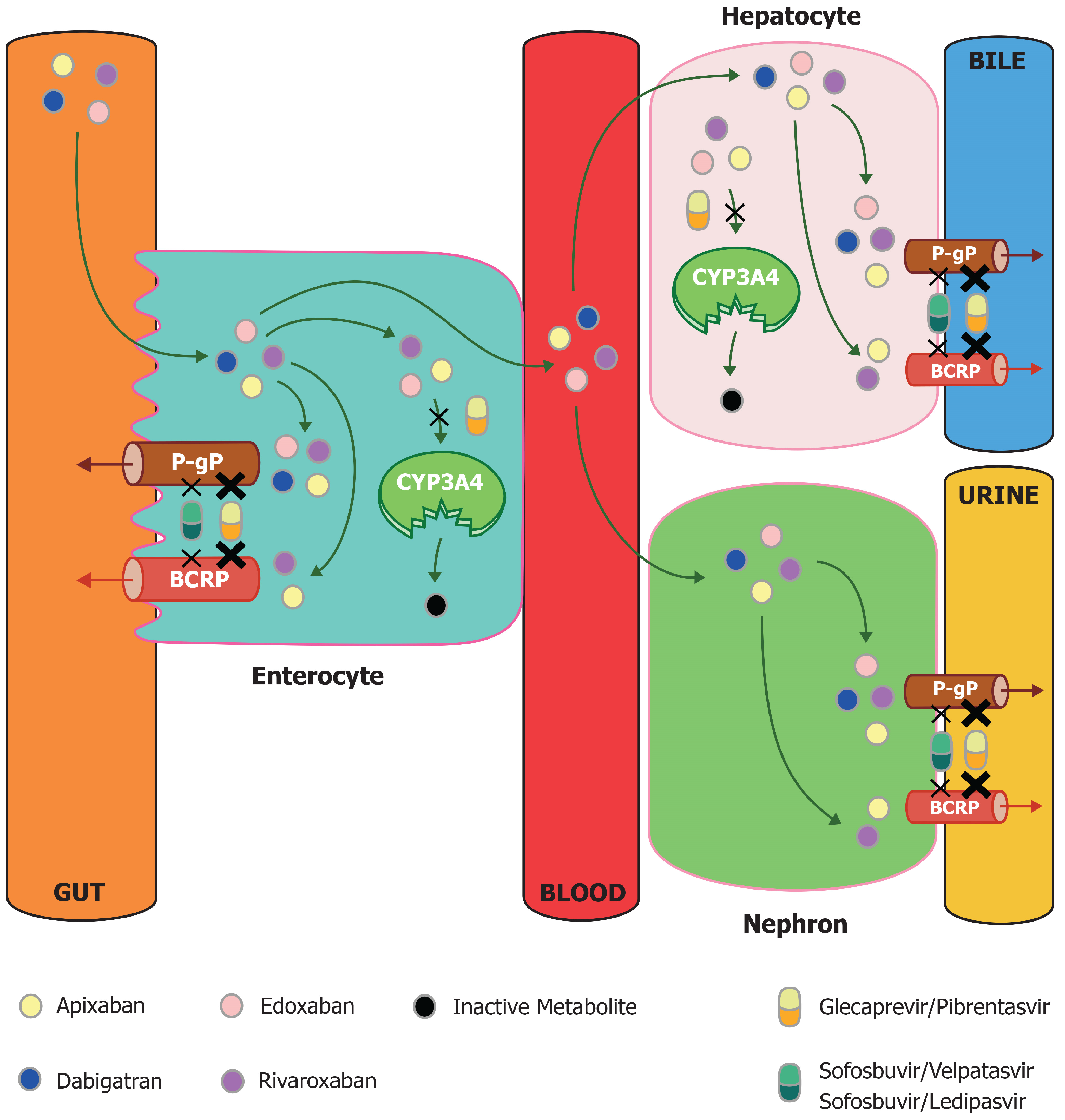
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Endpoints
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. DOAC-DAA Group
3.2. Comparison between DOACs/DAAs and VKAs/DAAs Groups
3.3. Factors Associated to Bleeding Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Blach, S.; Manzengo Mingiedi, C.; Gonzalez, M.A.; Sabry Alaama, A.; Mozalevskis, A.; Séguy, N.; Rewari, B.B.; Chan, P.L.; Le, L.V.; et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol. Hepatol. 2023, 8, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Talavera Pons, S.; Boyer, A.; Lamblin, G.; Chennell, P.; Châtenet, F.T.; Nicolas, C.; Sautou, V.; Abergel, A. Managing drug-drug interactions with new direct-acting antiviral agents in chronic hepatitis C. Br. J. Clin. Pharmacol. 2017, 83, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Colombo, E.; Tenconi, M.; Baldessin, L.; Corsini, A. Drug-Drug Interactions of Direct Oral Anticoagulants (DOACs): From Pharmacological to Clinical Practice. Pharmaceutics 2022, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Dunois, C. Laboratory Monitoring of Direct Oral Anticoagulants (DOACs). Biomedicines 2021, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Bellesini, M.; Bianchin, M.; Corradi, C.; Donadini, M.P.; Raschi, E.; Squizzato, A. Drug-Drug Interactions between Direct Oral Anticoagulants and Hepatitis C Direct-Acting Antiviral Agents: Looking for Evidence Through a Systematic Review. Clin. Drug Investig. 2020, 40, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Gelosa, P.; Castiglioni, L.; Tenconi, M.; Baldessin, L.; Racagni, G.; Corsini, A.; Bellosta, S. Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs). Pharmacol. Res. 2018, 135, 60–79. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair; EASL Governing Board Representative; Panel Members. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; He, W.; Guo, L.; Wang, X.; Hong, K. The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin. Cardiol. 2015, 38, 61–555. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; C Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 4–692. [Google Scholar] [CrossRef]
- McDaniel, K.; Utz, A.E.; Akbashev, M.; Fuller, K.G.; Boyle, A.; Davidson, K.; Marra, F.; Shah, S.; Cartwright, E.J.; Arora, A.A.; et al. Safe co-administration of direct-acting antivirals and direct oral anticoagulants among patients with hepatitis C virus infection: An international multicenter retrospective cohort study. J. Viral. Hepat. 2022, 29, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Cui, Y.; Platt, R.W.; Filion, K.B.; Sebastiani, G.; Renoux, C. Effectiveness and safety of direct oral anticoagulants among patients with non-valvular atrial fibrillation and liver disease: A multinational cohort study. Thromb. Res. 2024, 237, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Kondili, L.; Nevola, R.; Perillo, P.; Mastrocinque, D.; Aghemo, A.; Claar, E. Elimination of Hepatitis C in Southern Italy: A Model of HCV Screening and Linkage to Care among Hospitalized Patients at Different Hospital Divisions. Viruses 2022, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.H.; Lensing, A.W.A.; Prandoni, P.; Wells, P.S.; Verhamme, P.; Beyer-Westendorf, J.; Bauersachs, R.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; et al. Risk of recurrent venous thromboembolism according to baseline risk factor profiles. Blood Adv. 2018, 2, 788–796. [Google Scholar] [CrossRef] [PubMed]
- de Winter, M.A.; Büller, H.R.; Carrier, M.; Cohen, A.T.; Hansen, J.B.; Kaasjager, K.A.H.; Kakkar, A.K.; Middeldorp, S.; Raskob, G.E.; Sørensen, H.T.; et al. Recurrent venous thromboembolism and bleeding with extended anticoagulation: The VTE-PREDICT risk score. Eur. Heart J. 2023, 44, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Rahman, A.; Carrier, M.; Kearon, C.; Weitz, J.I.; Schulman, S.; Couturaud, F.; Eichinger, S.; Kyrle, P.A.; Becattini, C.; et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: Systematic review and meta-analysis. BMJ 2019, 366, l4363. [Google Scholar] [CrossRef] [PubMed]
- Canonico, M.E.; Sanna, G.D.; Siciliano, R.; Scudiero, F.; Esposito, G.; Parodi, G. Drug-drug interactions between antithrombotics and direct-acting antivirals in hepatitis C virus (HCV) patients: A brief, updated report. Front. Pharmacol. 2022, 13, 916361. [Google Scholar] [CrossRef] [PubMed]
| Total | Non-Cirrhotic | Cirrhotic | p-Value | |
|---|---|---|---|---|
| N. of pts | 104 | 66 | 38 | |
| Age, years | 80 (71–83) | 79 (73–82) | 82 (67–83) | 0.023 |
| Males | 47 (45.2) | 29 (43.9) | 18 (47.4) | 0.735 |
| Indication for anticoagulation | ||||
| Atrial Fibrillation | 79 (76) | 53 (80.3) | 26 (68.4) | 0.172 |
| Thrombosis | 14 (13.5) | 10 (15.2) | 4 (10.5) | 0.259 |
| Other | 11 (10.5) | 3 (4.5) | 8 (21.1) | 0.008 |
| DOAC | 0.220 | |||
| Rivaroxaban | 37 (35.6) | 27 (40.9) | 10 (26.3) | |
| Apixaban | 28 (26.9) | 19 (28.8) | 9 (23.7) | |
| Edoxaban | 19 (18.3) | 9 (13.6) | 10 (26.3) | |
| Dabigatran | 20 (19.2) | 11 (16.7) | 9 (23.7) | |
| Antiplatelet therapy | 9 (8.7) | 8 (12.1) | 1 (2.6) | 0.097 |
| Hypertension | 67 (64.4) | 42 (63.3) | 25 (65.8) | 0.825 |
| DAAs treatment | 0.328 | |||
| SOF/VEL | 82 (78.6) | 54 (81.8) | 28 (73.7) | |
| SOF/LDV | 22 (21.4) | 12 (18.2) | 10 (26.3) | |
| Creatinine at baseline (mg/dL) | 0.91 (0.8–1.1) | 0.92 (0.9–1.04) | 0.91 (0.76–1.11) | 0.363 |
| Platelets (103/mL) | 177 (132–226) | 182 (173–259) | 123 (91–174) | 0.252 |
| INR | 1.1 (1–1.2) | 1.1 (1–1.2) | 1.2 (1.1–1.3) | 0.576 |
| Bilirubin, mg/dL | 0.8 (0.58–1) | 0.8 (0.5–1) | 0.8 (0.7–1.2) | 0.745 |
| Albumin, g/dL | 4 (3.7–4.3) | 4.1 (3.7–4.3) | 3.9 (3.4–4.2) | 0.435 |
| Liver stiffness median, kPa | 9.2 (6.1–16.8) | 6.7 (5.3–8.9) | 17.8 (15.4–19.6) | <0.001 |
| HAS BLED > 3 | 38 (36.5) | 14 (21.1) | 24 (63.1) | <0.001 |
| SVR 12 | 103 (99) | 65 (98.5) | 38 (100) | 0.446 |
| Clinically relevant bleeding | ||||
| Major bleeding | 0 | 0 (0) | 0 (0) | - |
| Minor bleeding | 4 (3.8) | 4 (6.1) | 0 (0) | 0.122 |
| DOAC discontinuation | 0 | 0 (0) | 0 (0) | - |
| DOACs/DAAs | VKAs/DAAs | p-Value | |
|---|---|---|---|
| N. of pts | 104 | 104 | |
| Age, years | 80 (71–83) | 78 (69–81) | 0.079 |
| Males | 47 (45.2) | 46 (44.2) | 0.889 |
| Indication for anticoagulation | 0.610 | ||
| Atrial Fibrillation | 79 (76) | 83 (79.8) | |
| Thrombosis | 14 (13.5) | 14 (13.5) | |
| Other | 11 (10.6) | 7 (6.7) | |
| Antiplatelet therapy | 9 (8.7) | 14 (13.5) | 0.269 |
| Hypertension | 67(64.4) | 68 (65.4) | 0.884 |
| Cirrhosis | 38 (36.5) | 33 (31.7) | 0.465 |
| Decompensation | 2 (1.9) | 1 (1) | 0.138 |
| Esophageal varices | 13 (12.6) | 9 (8.7) | 0.356 |
| DAAs treatment | 0.460 | ||
| SOF/VEL | 81(78.6) | 86 (82.7) | |
| SOF/LDV | 22 (21.4) | 18 (17.3) | |
| Creatinine at baseline (mg/dL) | 0.91 (0.79–1.1) | 0.9 (0.77–1.08) | 0.873 |
| Platelets (103/mL) | 177(132–226) | 174 (142–199) | 0.244 |
| INR | 1.1 (1–1.2) | 2.1 (1.53–2.56) | <0.001 |
| Bilirubin, mg/dL | 0.8 (0.5–1) | 0.9 (0.7–1) | 0.389 |
| Albumin, g/dL | 4 (3.7–4.3) | 3.9 (3.5–4.1) | 0.061 |
| Liver stiffness median, kPa | 9.2 (6.1–16.8) | 9.3 (7.1–14.7) | 0.979 |
| HAS BLED score > 3 | 38 (36.5) | 32 (30.8) | 0.379 |
| SVR 12 | 103 (99) | 103 (99) | 1 |
| Clinically relevant bleeding | 4 (3.8) | 5 (4.8) | 0.316 |
| Major bleeding | 0 (0) | 1 (0,9) | |
| Non-major bleeding | 4 (3.8) | 4 (3.9) | |
| DOAC or VKA discontinuation | 0 (0) | 1 (1) | 0.316 |
| DOACs/DAAs | ||||||||||||
| Pts n. | Age | Sex | Cirrhosis | DOACs | DAAs | Antiplatelet therapy | Creatinine | Platelets (103/mcl) | HAS BLED | DOAC stopped | SVR 12 | Bleeding event |
| 1 | 85 | M | No | Dabigatran | SOF/VEL | Aspirin | 0.79 | 141 | 4 | No | Yes | Minor |
| 2 | 84 | F | No | Rivaroxaban | SOF/VEL | no | 0.96 | 112 | 3 | No | Yes | Minor |
| 3 | 78 | F | No | Edoxaban | SOF/LDV | no | 1.63 | 174 | 3 | No | Yes | Minor |
| 4 | 77 | M | No | Apixaban | SOF/LDV | Aspirin | 0.62 | 293 | 2 | No | Yes | Minor |
| VKAs/DAAs | ||||||||||||
| Pts n. | Age | Sex | Cirrhosis | Warfarin (INR at BL) | DAAs | Antiplatelet therapy | Creatinine | Platelets (103/mcl) | HAS BLED | Warfarin stopped | SVR 12 | Bleeding event |
| 1 | 60 | F | No | 2.1 | SOF/LDV | No | 0.7 | 165 | 3 | No | Yes | Minor |
| 2 | 40 | F | Yes | 2.7 | SOF/LDV | No | 1 | 115 | 3 | No | Yes | Minor |
| 3 | 78 | F | Yes | 2.16 | SOF/VEL | No | 1.37 | 160 | 4 | No | Yes | Minor |
| 4 | 73 | F | No | 1.95 | SOF/LDV | No | 0.87 | 225 | 2 | Yes | Yes | Major |
| 5 | 69 | M | Yes | 1.46 | SOF/VEL | No | 1.1 | 152 | 2 | No | Yes | Minor |
| DOACs | VKAs | p-Value | |
|---|---|---|---|
| N. of pts | 104 | 104 | |
| Clinically relevant bleeding | 4 (3.8) | 5 (4.8) | 0.316 |
| Major bleeding | 0 (0) | 1 (0.9) | |
| Non-major bleeding | 4 (3.8) | 4 (3.9) | |
| Clinically relevant thrombotic event | 1 (0.9) | 0 (0) | 0.248 |
| Arterial thromboembolism | 1 * (0.9) | 0 | 0.248 |
| Venous thromboembolism | 0 | 0 | |
| Venous thrombosis | 0 | 0 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Age, year | 1.071 (0.935–1.226) | 0.324 | - | |
| Male gender | 1.222 (0.166–9.024) | 0.844 | - | |
| LSM, kPa | 0.923 (0.757–1.126) | 0.431 | - | |
| Platelets, (103/mL) | 0.999 (0.986–1.012) | 0.863 | - | |
| INR | 0.809 (0.002–280.727) | 0.943 | - | |
| HAS BLED > 3 | 5.571 (0.558–55.580) | 0.143 | - | |
| Antiplatelet therapy | 13.286 (1.619–109.051) | 0.016 | 20.815 (1.506–287.593) | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosato, V.; Nevola, R.; Dallio, M.; Di Micco, P.; Spinetti, A.; Zeneli, L.; Ciancio, A.; Milella, M.; Colombatto, P.; D’Adamo, G.; et al. Safety of Sofosbuvir-Based Direct-Acting Antivirals for Hepatitis C Virus Infection and Direct Oral Anticoagulant Co-Administration. J. Clin. Med. 2024, 13, 5807. https://doi.org/10.3390/jcm13195807
Rosato V, Nevola R, Dallio M, Di Micco P, Spinetti A, Zeneli L, Ciancio A, Milella M, Colombatto P, D’Adamo G, et al. Safety of Sofosbuvir-Based Direct-Acting Antivirals for Hepatitis C Virus Infection and Direct Oral Anticoagulant Co-Administration. Journal of Clinical Medicine. 2024; 13(19):5807. https://doi.org/10.3390/jcm13195807
Chicago/Turabian StyleRosato, Valerio, Riccardo Nevola, Marcello Dallio, Pierpaolo Di Micco, Angiola Spinetti, Laert Zeneli, Alessia Ciancio, Michele Milella, Piero Colombatto, Giuseppe D’Adamo, and et al. 2024. "Safety of Sofosbuvir-Based Direct-Acting Antivirals for Hepatitis C Virus Infection and Direct Oral Anticoagulant Co-Administration" Journal of Clinical Medicine 13, no. 19: 5807. https://doi.org/10.3390/jcm13195807
APA StyleRosato, V., Nevola, R., Dallio, M., Di Micco, P., Spinetti, A., Zeneli, L., Ciancio, A., Milella, M., Colombatto, P., D’Adamo, G., Rosselli Del Turco, E., Gallo, P., Falcomatà, A., De Nicola, S., Pugliese, N., D’Ambrosio, R., Soria, A., Colella, E., Federico, A., ... Claar, E. (2024). Safety of Sofosbuvir-Based Direct-Acting Antivirals for Hepatitis C Virus Infection and Direct Oral Anticoagulant Co-Administration. Journal of Clinical Medicine, 13(19), 5807. https://doi.org/10.3390/jcm13195807










