Aortic and Mitral Valve Endocarditis—Simply Left-Sided Endocarditis or Different Entities Requiring Individual Consideration?—Insights from the CAMPAIGN Database
Abstract
1. Introduction
- Surgical treatment of MV-IE is associated with a higher mortality rate than that of AV-IE.
- MV-IE itself is an independent risk factor for mortality.
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Ethical Statement
3. Results
3.1. Characteristics of Patients with MV-IE versus AV-IE
3.2. Postoperative Outcomes after Surgery for MV-IE versus AV-IE
3.3. Independent Predictors of Mortality for Patients with Left-Sided IE
4. Discussion
4.1. Gender Distribution in MV-IE versus AV-IE
4.2. Risk of Cerebral Embolism in MV-IE Compared with AV-IE
4.3. Mortality in MV-IE versus AV-IE
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Vlasselaer, A.; Rasmussen, M.; Nilsson, J.; Olaison, L.; Ragnarsson, S. Native aortic versus mitral valve infective endocarditis: A nationwide registry study. Open Heart 2019, 6, e000926. [Google Scholar] [CrossRef]
- David, T.E.; Armstrong, S.; McCrindle, B.W.; Manlhiot, C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013, 127, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.M.T.; Frank, K.L.; Dale, J.L.; Manias, D.A.; Powers, J.L.; Dunny, G.M. Enterococcus faecalis colonizes and forms persistent biofilm microcolonies on undamaged endothelial surfaces in a rabbit endovascular infection model. FEMS Microbes 2021, 2, xtab014. [Google Scholar] [CrossRef]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.W.; Thieman, C.; Gupta, S. Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Kinney, K.J.; Stach, J.M.; Kulhankova, K.; Brown, M.; Salgado-Pabón, W. Vegetation Formation in Staphylococcus aureus Endocarditis Inversely Correlates with RNAIII and sarA Expression in Invasive Clonal Complex 5 Isolates. Front. Cell. Infect. Microbiol. 2022, 12, 925914. [Google Scholar] [CrossRef]
- Nappi, F. Current Knowledge of Enterococcal Endocarditis: A Disease Lurking in Plain Sight of Health Providers. Pathogens 2024, 13, 235. [Google Scholar] [CrossRef]
- Álvarez-Zaballos, S.; Vázquez-Alen, P.; Muñoz, P.; de Alarcón, A.; Gutiérrez Carretero, E.; Álvarez-Uría, A.; Fariñas, M.C.; Rodríguez-García, R.; Goenaga, M.; Cuervo, G.; et al. Prevalence and prognostic impact of stroke in a national cohort of infective endocarditis. Int. J. Stroke 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Gassa, A.; Rokohl, A.; Sabashnikov, A.; Deppe, A.C.; Eghbalzadeh, K.; Merkle, J.; Hamacher, S.; Liakopoulos, O.J.; Wahlers, T. Severity of presentation, not sex, increases risk of surgery for infective endocarditis. Ann. Thorac. Surg. 2018, 107, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.; Marin-Cuartas, M.; Weber, C.; De La Cuesta, M.; Lichtenberg, A.; Petrov, A.; Hagl, C.; Aubin, H.; Matschke, K.; Diab, M.; et al. Sex-related differences in patients with infective endocarditis requiring cardiac surgery: Insights from the CAMPAIGN Study Group. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2024, 66, ezae292. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Yanes, J.M.; Jimenez-Garcia, R.; De Miguel-Diez, J.; Hernández-Barrera, V.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; Noriega, C.; Lopez-de-Andres, A. Differences in Sex and the Incidence and In-Hospital Mortality among People Admitted for Infective Endocarditis in Spain, 2016–2020. J. Clin. Med. 2022, 11, 6847. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, J.; Iung, B.; de Tymoski, C.; Deconinck, L.; Para, M.; Duval, X.; Provenchere, S.; Mesnier, J.; Delhomme, C.; Haviari, S.; et al. Sex differences and outcomes in surgical infective endocarditis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2024, 65, ezae114. [Google Scholar] [CrossRef] [PubMed]
- Song, H.K.; Grab, J.D.; O’Brien, S.M.; Welke, K.F.; Edwards, F.; Ungerleider, R.M. Gender differences in mortality after mitral valve operation: Evidence for higher mortality in perimenopausal women. Ann. Thorac. Surg. 2008, 85, 2040–2044. [Google Scholar] [CrossRef]
- Mokhles, M.M.; Siregar, S.; Versteegh, M.I.; Noyez, L.; van Putte, B.; Vonk, A.B.; Roos-Hesselink, J.W.; Bogers, A.J.; Takkenberg, J.J. Male-female differences and survival in patients undergoing isolated mitral valve surgery: A nationwide cohort study in the Netherlands. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 50, 482–487. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Sambola, A.; Lozano-Torres, J.; Boersma, E.; Olmos, C.; Ternacle, J.; Calvo, F.; Tribouilloy, C.; Reskovic-Luksic, V.; Separovic-Hanzevacki, J.; Park, S.W.; et al. Predictors of embolism and death in left-sided infective endocarditis: The European Society of Cardiology EURObservational Research Programme European Infective Endocarditis registry. Eur. Heart J. 2023, 44, 4566–4575. [Google Scholar] [CrossRef] [PubMed]
- Selton-Suty, C.; Delahaye, F.; Tattevin, P.; Federspiel, C.; Le Moing, V.; Chirouze, C.; Nazeyrollas, P.; Vernet-Garnier, V.; Bernard, Y.; Chocron, S.; et al. Symptomatic and Asymptomatic Neurological Complications of Infective Endocarditis: Impact on Surgical Management and Prognosis. PLoS ONE 2016, 11, e0158522. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, D.; Vujisic-Tesic, B.; Obrenovic-Kircanski, B.; Ivanovic, B.; Kalimanovska-Ostric, D.; Petrovic, M.; Boricic-Kostic, M.; Matic, S.; Stevanovic, G.; Marinkovic, J.; et al. The relationship between causative microorganisms and cardiac lesions caused by infective endocarditis: New perspectives from the contemporary cohort of patients. J. Cardiol. 2018, 71, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, S.A.; Abrutyn, E.; Barsic, B.; Bouza, E.; Cecchi, E.; Moreno, A.; Doco-Lecompte, T.; Eisen, D.P.; Fortes, C.Q.; Fowler, V.G., Jr.; et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: An analysis from the ICE Prospective Cohort Study (ICE-PCS). Am. Heart J. 2007, 154, 1086–1094. [Google Scholar] [CrossRef]
- Ruttmann, E.; Abfalterer, H.; Wagner, J.; Grimm, M.; Müller, L.; Bates, K.; Ulmer, H.; Bonaros, N. Endocarditis-related stroke is not a contraindication for early cardiac surgery: An investigation among 440 patients with left-sided endocarditis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, I.; Hunter, M.D.; Sundheim, K.; Klein, B.; Dunn, L.; Sorabella, R.; Han, S.M.; Willey, J.; George, I.; Gutierrez, J. Clinical Risk Factors for Acute Ischemic and Hemorrhagic Stroke in Patients with Infective Endocarditis. Intern. Med. J. 2018, 48, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Franz, M.; Hagel, S.; Guenther, A.; Struve, A.; Musleh, R.; Penzel, A.; Sponholz, C.; Lehmann, T.; Kuehn, H.; et al. Impact of an In-Hospital Endocarditis Team and a State-Wide Endocarditis Network on Perioperative Outcomes. J. Clin. Med. 2021, 10, 4734. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Ochs, L.; Hohmann, C.; Baldus, S.; Michels, G.; Meyer-Schwickerath, C.; Fatkenheuer, G.; Mader, N.; Wahlers, T.; Weber, C.; et al. Surgical Procedure Time and Mortality in Patients with Infective Endocarditis Caused by Staphylococcus aureus or Streptococcus Species. J. Clin. Med. 2022, 11, 2538. [Google Scholar] [CrossRef]
- Lauten, A.; Martinović, M.; Kursawe, L.; Kikhney, J.; Affeld, K.; Kertzscher, U.; Falk, V.; Moter, A. Bacterial biofilms in infective endocarditis: An in vitro model to investigate emerging technologies of antimicrobial cardiovascular device coatings. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Galar, A.; Weil, A.A.; Dudzinski, D.M.; Munoz, P.; Siedner, M.J. Methicillin-Resistant Staphylococcus aureus Prosthetic Valve Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2019, 32, e00041-18. [Google Scholar] [CrossRef] [PubMed]
- Hoerr, V.; Franz, M.; Pletz, M.W.; Diab, M.; Niemann, S.; Faber, C.; Doenst, T.; Schulze, P.C.; Deinhardt-Emmer, S.; Loffler, B. S. aureus endocarditis: Clinical aspects and experimental approaches. Int. J. Med. Microbiol. 2018, 308, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L.; Bruun, N.E.; Voldstedlund, M.; Arpi, M.; Andersen, C.O.; Schonheyder, H.C.; Lemming, L.; Rosenvinge, F.; Valeur, N.; Sogaard, P.; et al. Prevalence of infective endocarditis in patients with positive blood cultures: A Danish nationwide study. Eur. Heart J. 2019, 40, 3237–3244. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.T.; Shrestha, N.K.; Gordon, S.M.; Houghtaling, P.L.; Blackstone, E.H.; Pettersson, G.B. Residual patient, anatomic, and surgical obstacles in treating active left-sided infective endocarditis. J. Thorac. Cardiovasc. Surg. 2014, 148, 981–988. [Google Scholar] [CrossRef] [PubMed]
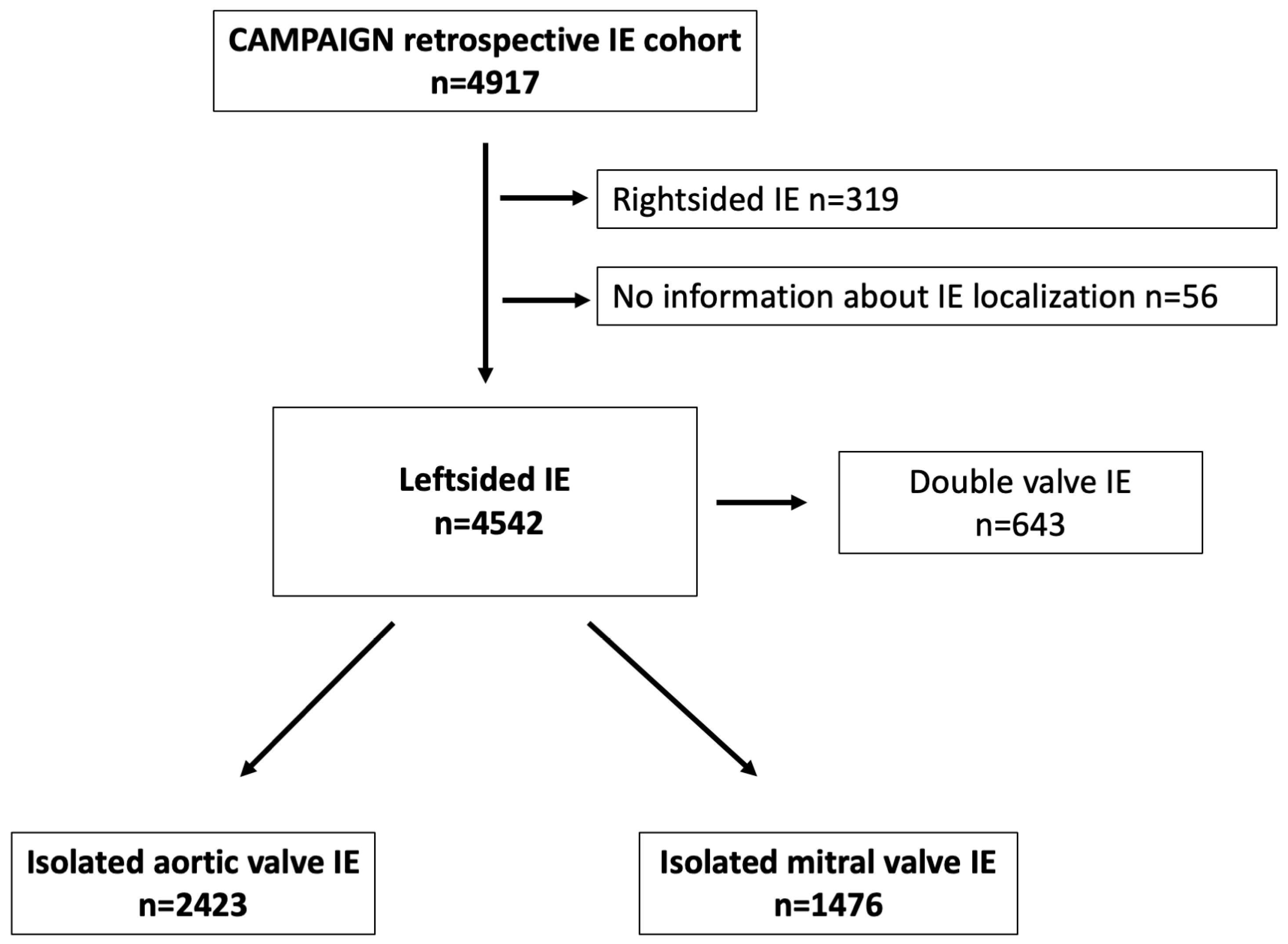
| Aortic Valve IE (n = 2423) | Mitral Valve IE (n = 1476) | p Value | |
|---|---|---|---|
| Age (years) | 65.0 [53.0–73.0] | 66.0 [56.0–74.0] | 0.013 # |
| Sex (%) | |||
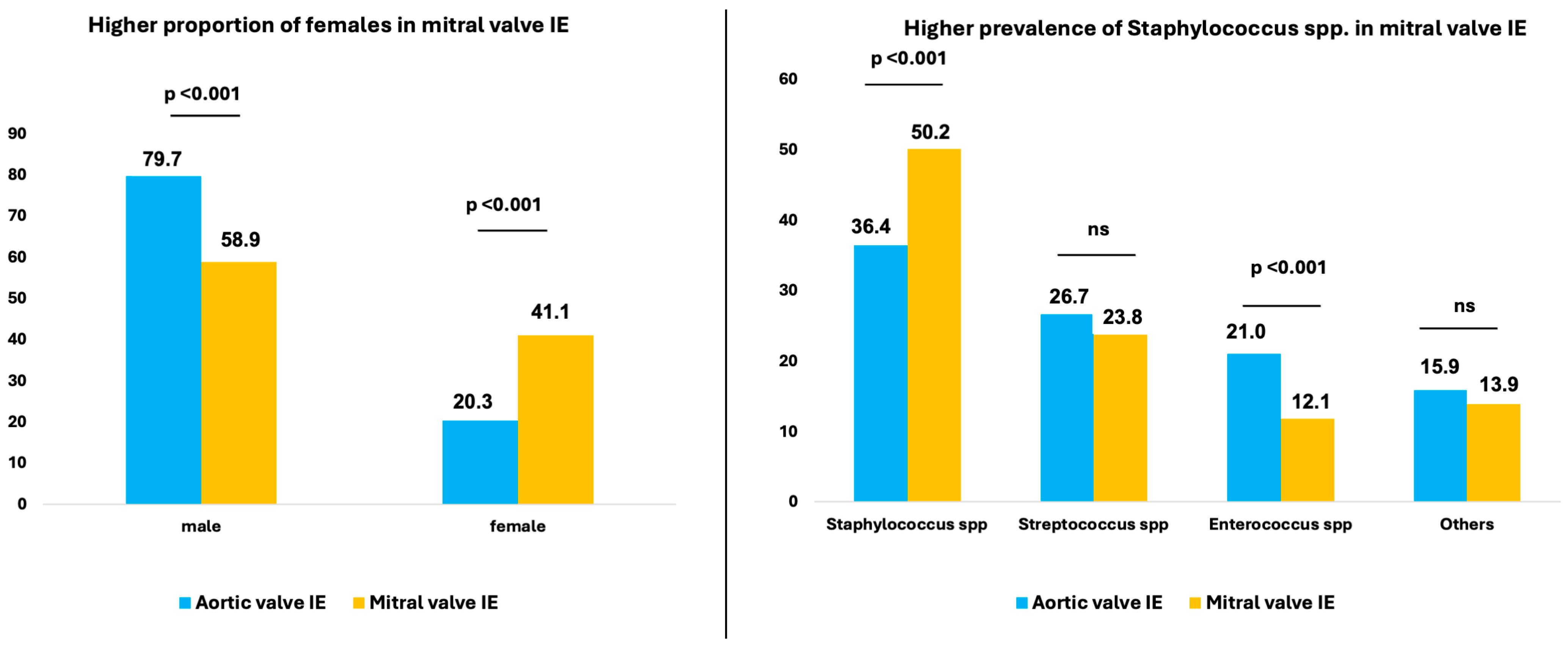
| male | 79.7% (1930/2423) | 58.9% (869/1476) | <0.001 † |
| female | 20.3% (493/2423) | 41.1% (607/1476) | <0.001 † |
| BMI (kg/m2) | 26.0 [23.7–29.3] | 25.5 [23.2–29.1] | 0.004 # |
| Underlying conditions/comorbidities | |||
| Hypertension | 50.5% (1223/2423) | 49.2% (726/1476) | 0.435 † |
| Diabetes | 24.5% (593/2423) | 29.1% (430/1476) | 0.001 † |
| Hyperlipidemia | 21.2% (489/2308) | 18.9% (270/1427) | 0.094 † |
| Smoking | 19.4% (459/2364) | 13.9% (201/1448) | <0.001 † |
| COPD | 10.3% (247/2404) | 10.0% (144/1438) | 0.796 † |
| Peripheral artery disease | 7.8% (189/2423) | 7.5% (111/1476) | 0.750 † |
| Pulmonary hypertension | 14.5% (351/2423) | 21.3% (314/1476) | <0.001 † |
| Preoperative CKD | 37.4% (906/2423) | 38.5% (568/1476) | 0.496 † |
| Preoperative hemodialysis | 7.0% (169/2423) | 8.6% (127/1476) | 0.062 † |
| Preoperative stroke | 19.3% (464/2402) | 28.2% (412/1463) | <0.001 † |
| Coronary artery disease | 27.4% (605/2208) | 29.8% (400/1342) | 0.123 † |
| Myocardial infarction | 7.4% (178/2398) | 6.8% (98/1442) | 0.467 † |
| Septic cerebral embolism | 17.7% (430/2423) | 25.4% (375/1476) | <0.001 † |
| LVEF | |||
| ≥50% | 70.3% (1639/2332) | 78.6% (1122/1428) | <0.001 † |
| ≥30% to 50% | 25.7% (599/2332) | 18.7% (267/1428) | <0.001 † |
| <30% | 4.0% (94/2332) | 2.7% (39/1428) | 0.036 † |
| Alcohol abusus | 7.3% (156/2124) | 6.4% (83/1294) | 0.301 † |
| Preoperative ventilation | 7.5% (182/2423) | 11.3% (167/1476) | <0.001 † |
| EuroSCORE | 11.0 [6.0–17.1] | 10.0 [5.0–18.0] | 0.062 # |
| Microbiology | |||
| Positive blood culture | 61.4% (1487/2423) | 67.8% (1000/1476) | <0.001 † |
| Staphylococcus spp. | 36.4% (497/1364) | 50.2% (456/908) | <0.001 † |
| Streptococcus spp. | 26.7% (364/1364) | 23.8% (216/908) | 0.121 † |
| Enterococcus spp. | 21.0% (286/1364) | 12.1% (110/908) | <0.001 † |
| Other microorganisms | 15.9% (217/1364) | 13.9% (126/908) | 0.185 † |
| Echocardiography | |||
| Presence of vegetation | 57.1% (1384/2423) | 66.6% (983/1475) | <0.001 † |
| Prosthetic valve endocarditis | 33.4% (789/2362) | 16.6% (239/1441) | <0.001 † |
| Pacemaker associated IE | 0.6% (15/2423) | 0.7% (11/1476) | 0.639 † |
| Operative data | |||
| Ascending aortic/root/arch surgery | 24.3% (558/2423) | 2.0% (29/1476) | <0.001 † |
| Concomitant CABG | 12.1% (292/2423) | 13.6% (200/1476) | 0.172 † |
| CPB time | 104.0 [76.0–152.0] | 112.0 [85.0–144.0] | 0.014 # |
| Crossclamp time | 71.0 [51.0–103.0] | 71.0 [54.0–95.0] | 0.469 # |
| Redo operation | 35.0% (848/2423) | 21.2% (313/1476) | <0.001 † |
| Aortic Valve IE (n = 2423) | Mitral Valve IE (n = 1476) | p Value | |
|---|---|---|---|
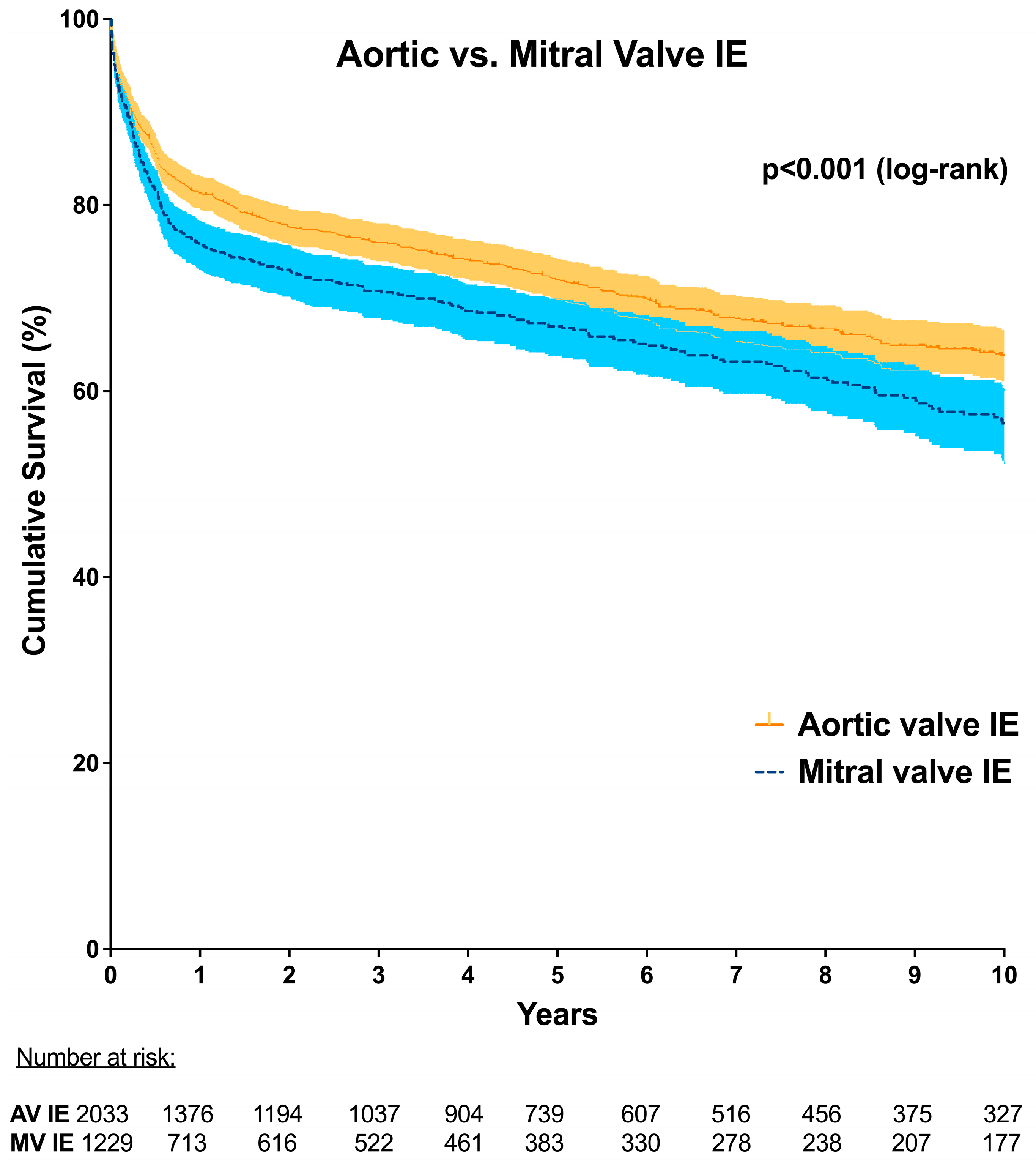
| 30 day mortality (day 0–30) | 14.6% (314/2153) | 16.7% (206/1231) | 0.095 † |
| 1 year mortality (day 0–365) | 29.0% (549/1893) | 35.3% (384/1088) | <0.001 † |
| Re-exploration | 21.7% (527/2423) | 20.7% (306/1476) | 0.452 † |
| Postoperative stroke | 14.4% (309/2143) | 17.7% (233/1319) | 0.011 † |
| Postoperative hemodialysis | 13.9% (320/2299) | 19.3% (273/1414) | <0.001 † |
| Tracheostomy | 7.0% (169/2423) | 10.2% (150/1476) | <0.001 † |
| ICU stay (days) | 3.0 [1.0–7.0] | 3.0 [1.0–5.0] | <0.001 # |
| Hospital stay (days) | 14.0 [9.0–21.0] | 13.0 [8.0–21.0] | 0.362 # |
| 30 Day Mortality | 1 Year Mortality | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age > 65 years | 1.554 | 1.121–2.154 | 0.008 | 2.046 | 1.564–2.676 | <0.001 |
| Male sex | 0.718 | 0.523–0.985 | 0.040 | 0.718 | 0.558–0.923 | 0.010 |
| LVEF < 30% | 2.537 | 1.310–4.916 | 0.006 | |||
| Coronary artery disease | 1.548 | 1.219–1.967 | <0.001 | |||
| Diabetes | 1.524 | 1.109–2.093 | 0.009 | 1.354 | 1.063–1.724 | 0.014 |
| Pulmonary hypertension | 1.424 | 1.078–1.882 | 0.013 | |||
| Alcohol | 1.830 | 1.259–2.660 | 0.002 | |||
| Preoperative CKD | 1.975 | 1.448–2.695 | <0.001 | 1.708 | 1.320–2.211 | <0.001 |
| Preoperative hemodialysis | 2.099 | 1.460–3.016 | <0.001 | |||
| Preoperative stroke | 1.414 | 1.109–1.803 | 0.005 | |||
| Preoperative ventilation | 2.104 | 1.398–3.169 | <0.001 | 1.537 | 1.095–2.156 | 0.013 |
| Urological focus | 1.410 | 1.024–1.941 | 0.035 | |||
| Wound focus | 1.461 | 1.034–2.065 | 0.032 | |||
| Septic embolism spleen | 1.360 | 1.071–1.728 | 0.012 | |||
| Staphylococcus spp. IE | 1.734 | 1.273–2.362 | <0.001 | |||
| Vegetation | 1.817 | 1.071–3.082 | 0.027 | |||
| Prosthetic valve IE | 1.536 | 1.115–2.117 | 0.009 | |||
| Redo | 1.569 | 1.236–1.991 | <0.001 | |||
| Mitral valve IE | 1.329 | 1.043–1.693 | 0.021 | |||
| Aortic Valve IE | Mitral Valve IE | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Male sex | 0.642 | 0.416–0.991 | 0.045 | |||
| LVEF < 30% | 4.452 | 2.182–9.084 | <0.001 | |||
| Myocardial infarction | 2.134 | 1.031–4.415 | 0.041 | |||
| Diabetes mellitus | 1.711 | 1.136–2.575 | 0.010 | |||
| Smoking | 0.436 | 0.220–0.864 | 0.017 | |||
| Preoperative CKD | 1.901 | 1.283–2.818 | 0.001 | 3.592 | 2.287–5.641 | <0.001 |
| Preoperative ventilation | 2.883 | 1.696–4.901 | <0.001 | |||
| Staphylococcus spp. IE | 1.810 | 1.225–2.673 | 0.004 | |||
| Prosthetic valve IE | 1.782 | 1.206–2.631 | 0.004 | |||
| Aortic Valve IE | Mitral Valve IE | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age >65 years | 1.824 | 1.259–2.643 | 0.001 | 2.302 | 1.557–3.404 | <0.001 |
| Male sex | 0.568 | 0.401–0.804 | 0.001 | |||
| LVEF >50% | 0.670 | 0.450–0.998 | 0.049 | |||
| Hypertension | 0.629 | 0.435–0.912 | 0.014 | 1.947 | 1.251–3.030 | 0.003 |
| Coronary artery disease | 1.610 | 1.165–2.224 | 0.004 | |||
| Diabetes mellitus | 1.951 | 1.344–2.832 | <0.001 | |||
| COPD | 1.614 | 1.098–2.372 | 0.015 | |||
| Preoperative CKD | 1.645 | 1.157–2.339 | 0.006 | 1.528 | 1.026–2.274 | 0.037 |
| Preoperative hemodialysis | 2.028 | 1.233–3.337 | 0.005 | 2.000 | 1.148–3.482 | 0.014 |
| Peripheral vascular disease | 1.964 | 1.330–2.900 | <0.001 | |||
| PAH | 1.495 | 1.021–2.189 | 0.039 | |||
| Alcohol abuse | 1.693 | 1.056–2.716 | 0.029 | |||
| Preoperative ventilation | 1.864 | 1.172–2.965 | 0.009 | |||
| Urological focus | 2.000 | 1.324–3.020 | <0.001 | |||
| Septic embolism kidney | 1.712 | 1.139–2.574 | 0.010 | |||
| Septic embolism spleen | 1.468 | 1.029–2.093 | 0.034 | |||
| Staphylococcus spp. IE | 1.735 | 1.198–2.513 | 0.004 | |||
| Redo | 1.699 | 1.230–2.347 | 0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, C.; Marin-Cuartas, M.; Tugtekin, S.-M.; Diab, M.; Saha, S.; Akhyari, P.; Elderia, A.; Muench, F.; Petrov, A.; Aubin, H.; et al. Aortic and Mitral Valve Endocarditis—Simply Left-Sided Endocarditis or Different Entities Requiring Individual Consideration?—Insights from the CAMPAIGN Database. J. Clin. Med. 2024, 13, 5841. https://doi.org/10.3390/jcm13195841
Weber C, Marin-Cuartas M, Tugtekin S-M, Diab M, Saha S, Akhyari P, Elderia A, Muench F, Petrov A, Aubin H, et al. Aortic and Mitral Valve Endocarditis—Simply Left-Sided Endocarditis or Different Entities Requiring Individual Consideration?—Insights from the CAMPAIGN Database. Journal of Clinical Medicine. 2024; 13(19):5841. https://doi.org/10.3390/jcm13195841
Chicago/Turabian StyleWeber, Carolyn, Mateo Marin-Cuartas, Sems-Malte Tugtekin, Mahmoud Diab, Shekhar Saha, Payam Akhyari, Ahmed Elderia, Florian Muench, Asen Petrov, Hug Aubin, and et al. 2024. "Aortic and Mitral Valve Endocarditis—Simply Left-Sided Endocarditis or Different Entities Requiring Individual Consideration?—Insights from the CAMPAIGN Database" Journal of Clinical Medicine 13, no. 19: 5841. https://doi.org/10.3390/jcm13195841
APA StyleWeber, C., Marin-Cuartas, M., Tugtekin, S.-M., Diab, M., Saha, S., Akhyari, P., Elderia, A., Muench, F., Petrov, A., Aubin, H., Misfeld, M., Lichtenberg, A., Hagl, C., Doenst, T., Matschke, K., Borger, M. A., Wahlers, T., & Luehr, M., on behalf of the Study Group “Clinical, Multicenter Project of Analysis of Infective Endocarditis in Germany” (CAMPAIGN). (2024). Aortic and Mitral Valve Endocarditis—Simply Left-Sided Endocarditis or Different Entities Requiring Individual Consideration?—Insights from the CAMPAIGN Database. Journal of Clinical Medicine, 13(19), 5841. https://doi.org/10.3390/jcm13195841







