Temperature Simulation of an Ablation Needle for the Prediction of Tissue Necrosis during Liver Ablation
Abstract
1. Introduction
2. Materials and Methods
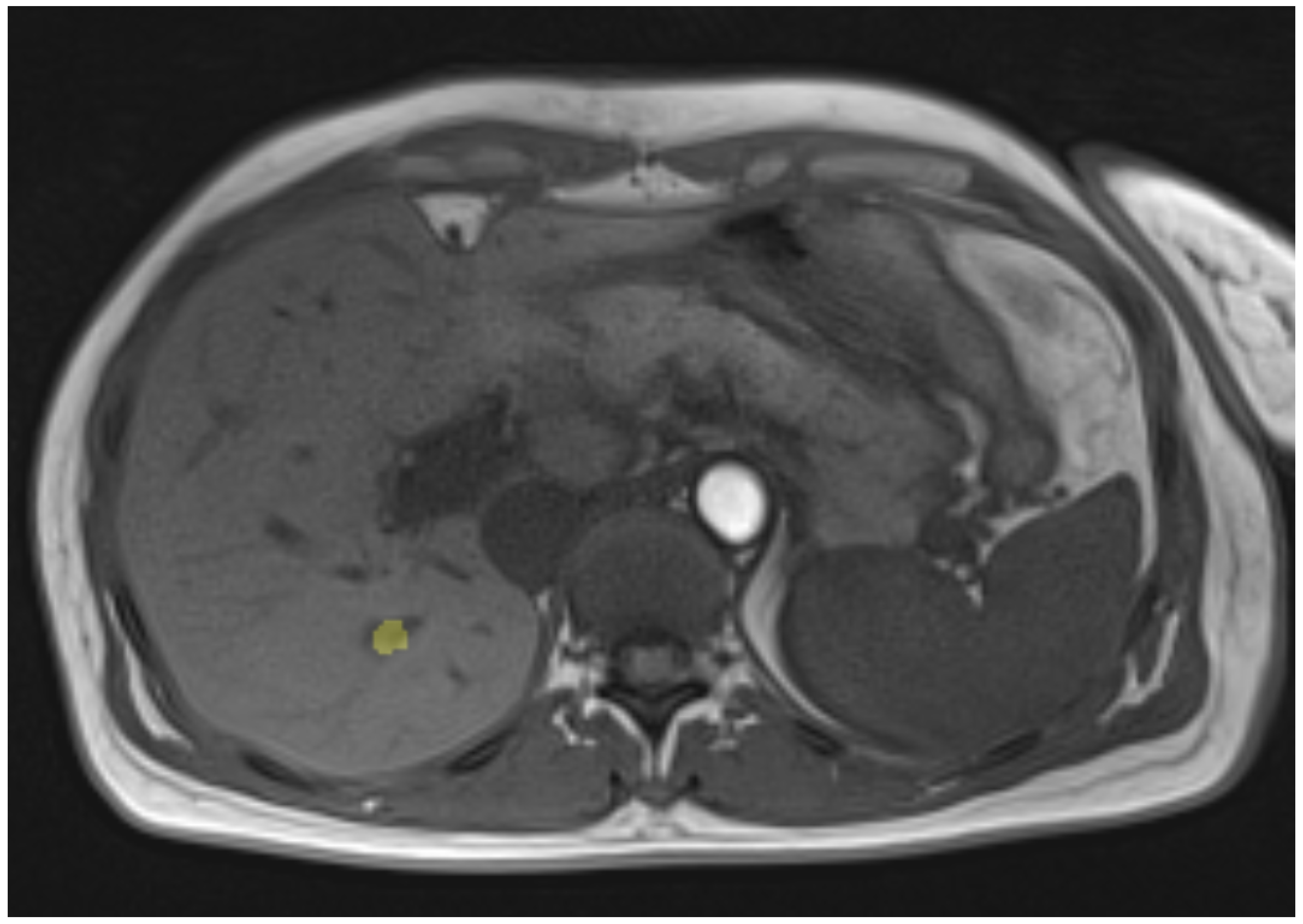
2.1. Magnetic Resonance Image-Guided Microwave Ablation Experimental Setup
2.2. Polyacrylamide Liver Phantoms
2.3. MR Measurements
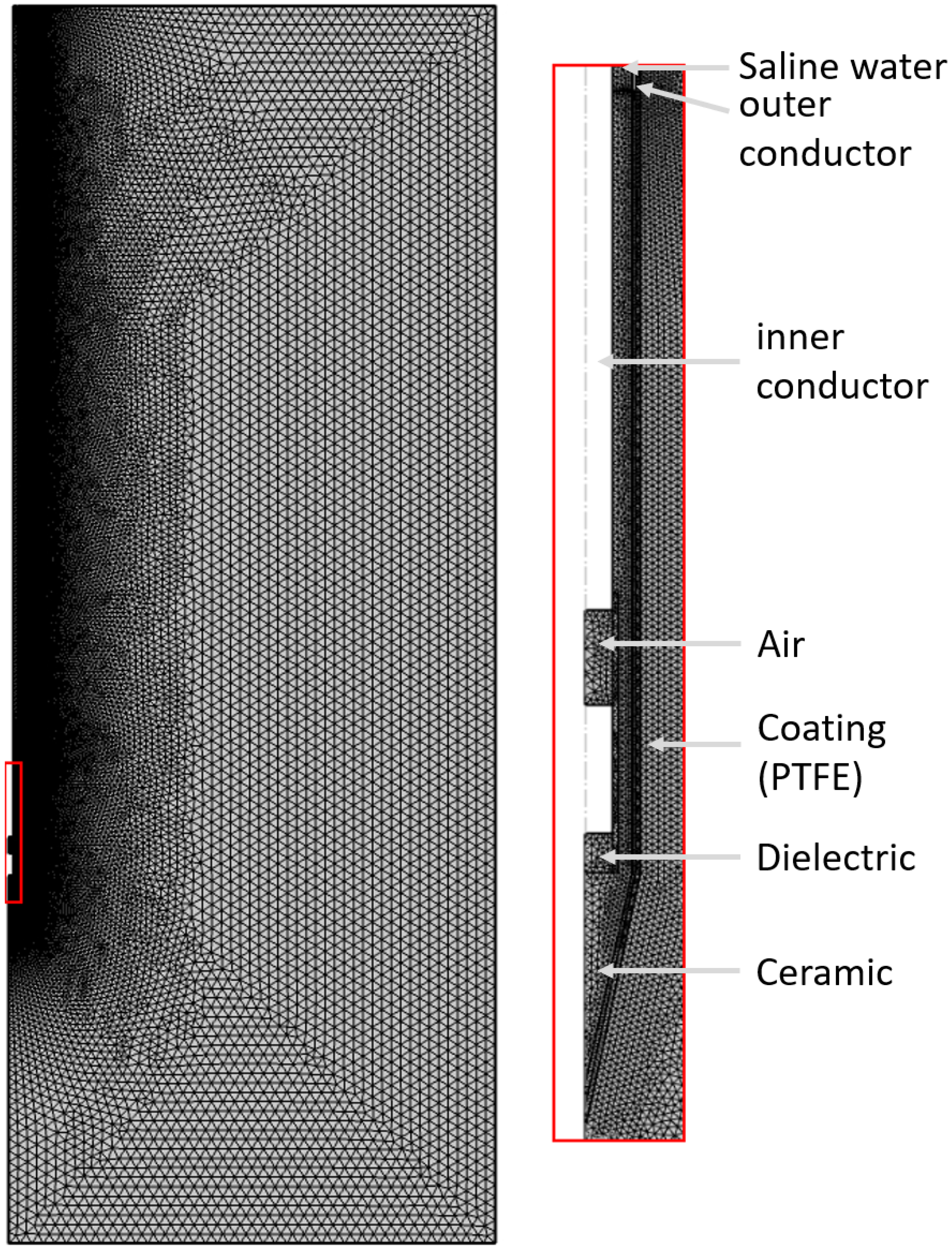
2.4. Computational Modeling
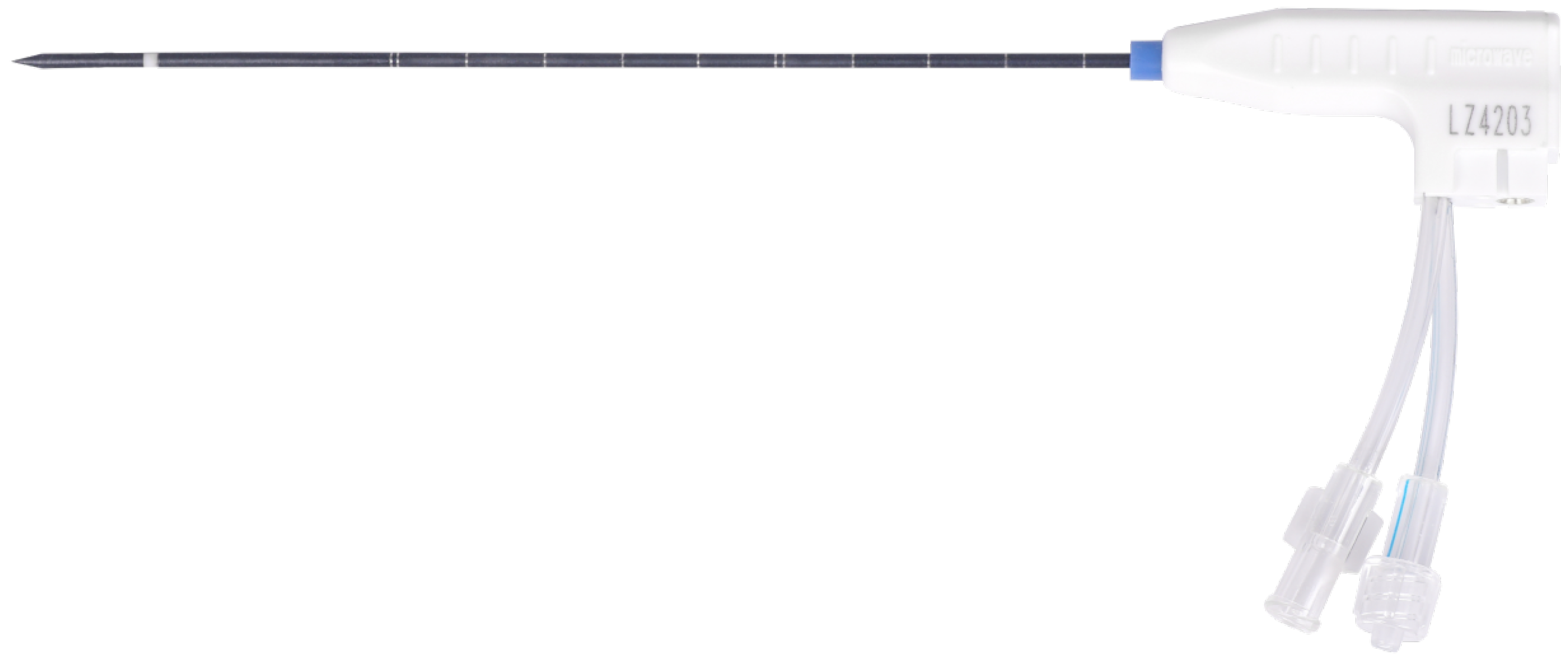
2.5. Modeling the MWA Needle
| Name | |||
|---|---|---|---|
| Variables | Value | Unit | Description |
| 1000 | kg/m3 | Density, blood | |
| 3639 | J/(kg·K) | Specific heat, blood | |
| 0.00361 | 1/s | Perfusion rate, blood | |
| 310.15 | K | Temperature, blood | |
| 290.15 | K | Temperature, phantom | |
| 43.03 | 1 | Relative permittivity, liver | |
| 54.8 | 1 | Relative permittivity, tumor | |
| 1.69 | S/m | Electric conductivity, liver | |
| 2 | S/m | Electric conductivity, tumor | |
| 2.03 | 1 | Relative permittivity, dielectric | |
| 2.6 | 1 | Relative permittivity, catheter | |
| f | 2.45 | GHz | Microwave frequency |
| 35 | W | Input microwave power phantom | |
| 42 | W | Input microwave power clinical case | |
| 3600 | J/(kg·K) | Specific heat capacity, liver | |
| 3760 | J/(kg·K) | Specific heat capacity, tumor | |
| 1069 | kg/m3 | Density, liver | |
| 1040 | kg/m3 | Density, tumor | |
| 0.55 | W/(m·K) | Thermal conductivity, liver | |
| 0.57 | W/(m·K) | Thermal conductivity, tumor | |
| 1000 | W/m3 | Metabolic heat, liver | |
| 1.3 × 1000 | W/m3 | Metabolic heat, tumor | |
2.6. Discretization
2.7. Analytic Approach
3. Results
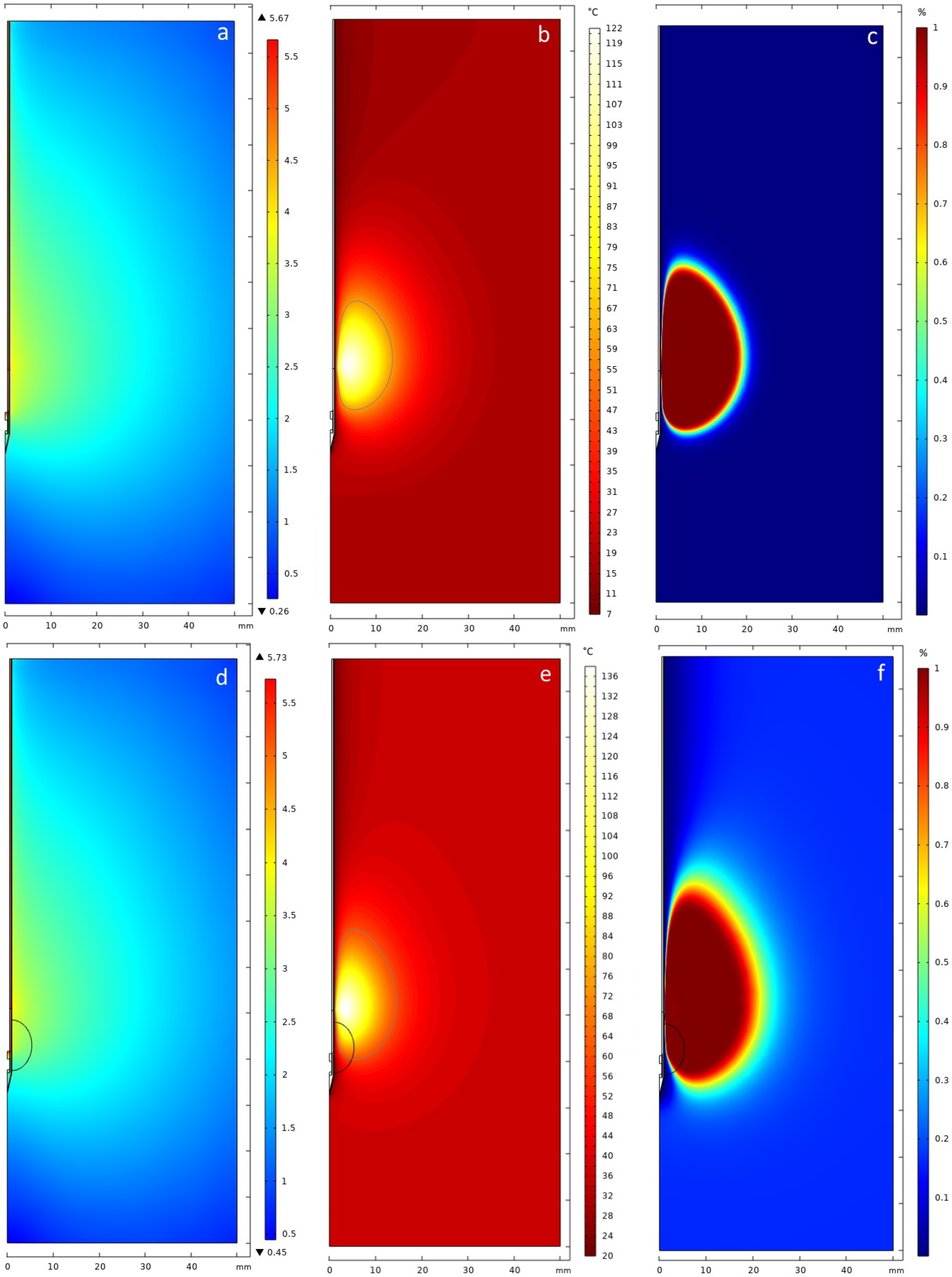
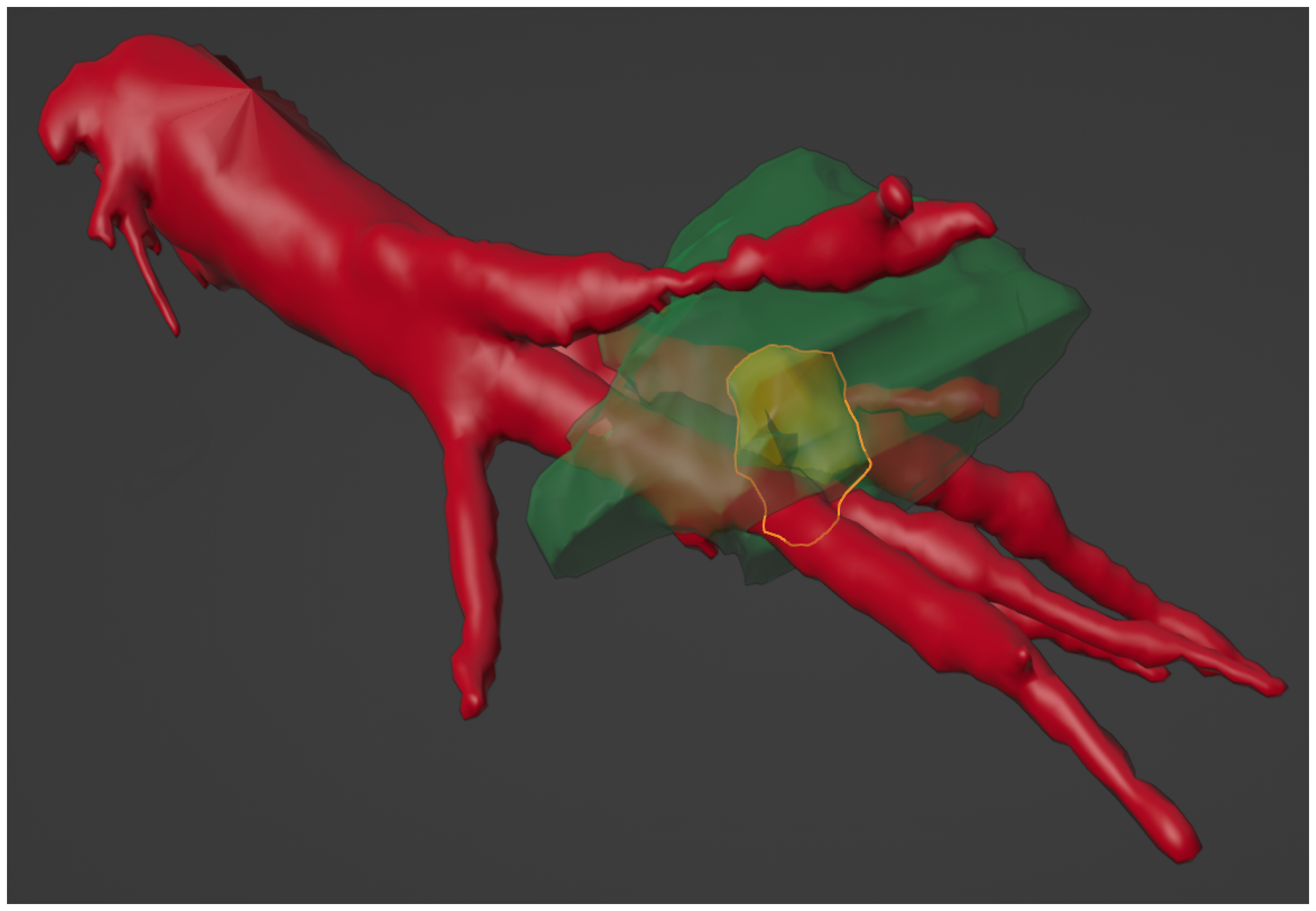
3.1. In Vitro Phantom
3.2. Clinical Case
4. Discussion
4.1. Implementation of the Simulation
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MWA | Microwave ablation |
| MRI | Magnetic resonance imaging |
References
- Wong, M.C.; Jiang, J.Y.; Goggins, W.B.; Liang, M.; Fang, Y.; Fung, F.D.; Leung, C.; Wang, H.H.; Wong, G.L.; Wong, V.W.; et al. International incidence and mortality trends of liver cancer: A global profile. Sci. Rep. 2017, 7, 45846. [Google Scholar] [CrossRef] [PubMed]
- Sarveazad, A.; Agah, S.; Babahajian, A.; Amini, N.; Bahardoust, M. Predictors of 5 year survival rate in hepatocellular carcinoma patients. J. Res. Med. Sci. 2019, 24, 86. [Google Scholar]
- Chong, C.C.; Lee, K.F.; Chu, C.M.; Chan, A.W.; Wong, J.; Chan, S.L.; Lok, H.T.; Fung, A.K.; Fong, A.K.; Cheung, Y.S.; et al. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: Application of ALBI score to patient selection. Health Promot. Board 2018, 20, 546–554. [Google Scholar] [CrossRef]
- Hui, T.C.; Kwan, J.; Pua, U. Advanced techniques in the percutaneous ablation of liver tumours. Diagnostics 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, H.; Prakash, P. Antenna designs for microwave tissue ablation. Crit. Rev. Biomed. Eng. 2018, 46, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Lyons, G.R.; Pua, B.B. Ablation planning software for optimizing treatment: Challenges, techniques, and applications. Tech. Vasc. Interv. Radiol. 2019, 22, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Melnik, R. Coupled thermo-electro-mechanical models for thermal ablation of biological tissues and heat relaxation time effects. Phys. Med. Biol. 2019, 64, 245008. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Wu, S.; Zhou, Z.; Bai, Y.; Ai, H. Characterization of 2450-MHz microwave thermal coagulation zone based on characteristic length growth model and shape variation factor. Int. J. Microw. Comput.-Aided Eng. 2019, 29, e21705. [Google Scholar] [CrossRef]
- Amabile, C.; Farina, L.; Lopresto, V.; Pinto, R.; Cassarino, S.; Tosoratti, N.; Goldberg, S.N.; Cavagnaro, M. Tissue shrinkage in microwave ablation of liver: An ex vivo predictive model. Int. J. Hyperth. 2017, 33, 101–109. [Google Scholar] [CrossRef]
- De Vita, E.; De Tommasi, F.; Massaroni, C.; Iadicicco, A.; Faiella, E.; Carassiti, M.; Grasso, R.F.; Schena, E.; Campopiano, S. Investigation of the Heat Sink Effect During Microwave Ablation in Hepatic Tissue: Experimental and Numerical Analysis. IEEE Sens. J. 2021, 21, 22743–22751. [Google Scholar] [CrossRef]
- Radjenović, B.; Sabo, M.; Šoltes, L.; Prnova, M.; Čičak, P.; Radmilović-Radjenović, M. On efficacy of microwave ablation in the thermal treatment of an early-stage hepatocellular carcinoma. Cancers 2021, 13, 5784. [Google Scholar] [CrossRef]
- Faridi, P.; Keselman, P.; Fallahi, H.; Prakash, P. Experimental assessment of microwave ablation computational modeling with MR thermometry. Med. Phys. 2020, 47, 3777–3788. [Google Scholar] [CrossRef]
- Pearce, J.A. Comparative analysis of mathematical models of cell death and thermal damage processes. Int. J. Hyperth. 2013, 29, 262–280. [Google Scholar] [CrossRef]
- Gorman, J.; Tan, W.; Abraham, J. Numerical Simulation of Microwave Ablation in the Human Liver. Processes 2022, 10, 361. [Google Scholar] [CrossRef]
- Horstmann, D.; zu Hartlage, K.M.; Reimert, D.; Caballero, J.J.L.; Belker, O.; Wacker, F.; Hensen, B.; Gutberlet, M. Implementation of a Real-time 3D-thermometry Pipeline in Gadgetron for Easy Clinical Integration. In Proceedings of the BVM Workshop, Braunschweig, Germany, 2–4 July 2023; p. 299. [Google Scholar]
- Bu-Lin, Z.; Bing, H.; Sheng-Li, K.; Huang, Y.; Rong, W.; Jia, L. A polyacrylamide gel phantom for radiofrequency ablation. Int. J. Hyperth. 2008, 24, 568–576. [Google Scholar] [CrossRef]
- Alpers, J.; Rötzer, M.; Gutberlet, M.; Wacker, F.; Hensen, B.; Hansen, C. Adaptive simulation of 3D thermometry maps for interventional MR-guided tumor ablation using Pennes’ bioheat equation and isotherms. Sci. Rep. 2022, 12, 20356. [Google Scholar] [CrossRef]
- Caballero, J.J.L.; zu Hartlage, K.M.; Gerlach, T.; Speck, O.; Wacker, F.; Hensen, B.; Gutberlet, M. Reduction of Electromagnetic Interferences of a commercially available MR approved microwave generator. iMRI Symp. Leipz. 2021, 125. [Google Scholar]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Du, Y.X.; Chen, Z.B.; Lang, L.; Ye, Z.; Yang, Q.; Shen, S.Q.; Lei, Z.Y.; Zhang, S.Q. Computer modeling and in vitro experimental study of water-cooled microwave ablation array. Minim. Invasive Ther. Allied Technol. 2021, 30, 12–20. [Google Scholar] [CrossRef]
- Afaghi, P.; Lapolla, M.A.; Ghandi, K. Percutaneous microwave ablation applications for liver tumors: Recommendations for COVID-19 patients. Heliyon 2021, 7, e06454. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.; Gabriel, C. The dielectric properties of biological tissues: Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251. [Google Scholar] [CrossRef] [PubMed]
- Radmilović-Radjenović, M.; Bošković, N.; Sabo, M.; Radjenović, B. An Analysis of Microwave Ablation Parameters for Treatment of Liver Tumors from the 3D-IRCADb-01 Database. Biomedicines 2022, 10, 1569. [Google Scholar] [CrossRef]
- Kim, C. Understanding the nuances of microwave ablation for more accurate post-treatment assessment. Future Oncol. 2018, 14, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- GmbH, M.M. Microwave. Available online: https://www.mml-medical.de/en/products/microwave/ (accessed on 5 August 2024).
- Selmi, M.; Bajahzar, A.; Belmabrouk, H. Effects of target temperature on thermal damage during temperature-controlled MWA of liver tumor. Case Stud. Therm. Eng. 2022, 31, 101821. [Google Scholar] [CrossRef]
- Kim, B.M.; Jacques, S.L.; Rastegar, S.; Thomsen, S.; Motamedi, M. Nonlinear finite-element analysis of the role of dynamic changes in blood perfusion and optical properties in laser coagulation of tissue. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 922–933. [Google Scholar]
- Zhang, S.; Wu, S.; Shang, S.; Qin, X.; Jia, X.; Li, D.; Cui, Z.; Xu, T.; Niu, G.; Bouakaz, A.; et al. Detection and monitoring of thermal lesions induced by microwave ablation using ultrasound imaging and convolutional neural networks. IEEE J. Biomed. Health Inform. 2019, 24, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, H.; Oguro, S.; Tuncali, K.; Morrison, P.R.; Tatli, S.; Shyn, P.B.; Silverman, S.G.; Hata, N. Multimodality non-rigid image registration for planning, targeting and monitoring during CT-guided percutaneous liver tumor cryoablation. Acad. Radiol. 2010, 17, 1334–1344. [Google Scholar] [CrossRef]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiong, Y.; Sun, J.; Wang, G.; Li, W.; Tang, T.; Li, J. The efficacy of microwave ablation versus liver resection in the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. Int. J. Surg. 2020, 77, 85–93. [Google Scholar] [CrossRef]
- Correa-Gallego, C.; Fong, Y.; Gonen, M.; D’Angelica, M.I.; Allen, P.J.; DeMatteo, R.P.; Jarnagin, W.R.; Kingham, T.P. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann. Surg. Oncol. 2014, 21, 4278–4283. [Google Scholar] [CrossRef]
- Prakash, P. Theoretical modeling for hepatic microwave ablation. Open Biomed. Eng. J. 2010, 4, 27. [Google Scholar] [CrossRef]
- Heshmat, A.; O’Connor, C.S.; Albuquerque Marques Silva, J.; Paolucci, I.; Jones, A.K.; Odisio, B.C.; Brock, K.K. Using Patient-Specific 3D Modeling and Simulations to Optimize Microwave Ablation Therapy for Liver Cancer. Cancers 2024, 16, 2095. [Google Scholar] [CrossRef]
- Ge, M.; Jiang, H.; Huang, X.; Zhou, Y.; Zhi, D.; Zhao, G.; Chen, Y.; Wang, L.; Qiu, B. A multi-slot coaxial microwave antenna for liver tumor ablation. Phys. Med. Biol. 2018, 63, 175011. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, S.J.; Heerink, W.J.; de Jong, K.P. Liver microwave ablation: A systematic review of various FDA-approved systems. Eur. Radiol. 2019, 29, 4026–4035. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, L.; Moser, M.A.; Zhang, W.; Zhang, B. A review of antenna designs for percutaneous microwave ablation. Phys. Medica 2021, 84, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.H.; Soltani, M.; Kashkooli, F.M.; Raahemifar, K. Use of microwave ablation for thermal treatment of solid tumors with different shapes and sizes—A computational approach. PLoS ONE 2020, 15, e0233219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, W.; Chen, Z.; Chen, D.; Du, Y.; Lang, L.; Ye, Z.; Shen, S.; Lei, Z.; Zhang, S. Water-cooled microwave ablation array for bloodless rapid transection of the liver. Int. J. Hyperth. 2021, 38, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Valori, M.; Rebaioli, L.; Marrocco, V.; Modica, F.; Bonelli, F.; Pascazio, G.; Portosi, V.; Prudenzano, F.; Fasano, A.; Lampignano, V.; et al. Manufacturing challenges and technological solutions for microwave ablation (MWA) probe prototyping. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2023, 237, 481–491. [Google Scholar] [CrossRef]
- Pfannenstiel, A.; Iannuccilli, J.; Cornelis, F.H.; Dupuy, D.E.; Beard, W.L.; Prakash, P. Shaping the future of microwave tumor ablation: A new direction in precision and control of device performance. Int. J. Hyperth. 2022, 39, 664–674. [Google Scholar] [CrossRef]
- Ringe, K.I.; Lutat, C.; Rieder, C.; Schenk, A.; Wacker, F.; Raatschen, H.J. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS ONE 2015, 10, e0134301. [Google Scholar] [CrossRef]





| Metric | Arrhenius | Critical Temp. 60 °C |
|---|---|---|
| Hausdorff D. | 12.021 mm | 15.017 mm |
| Euclidean D. | 2.504 mm | 3.754 mm |
| Dice score | 0.772 | 0.601 |
| Metric | Arrhenius | Critical Temp. 60 °C |
|---|---|---|
| Hausdorff D. | 9.862 mm | 11.045 mm |
| Euclidean D. | 3.061 mm | 2.502 mm |
| Dice score | 0.526 | 0.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Will, M.; Gerlach, T.; Saalfeld, S.; Gutberlet, M.; Düx, D.; Schröer, S.; Hille, G.; Wacker, F.; Hensen, B.; Berg, P. Temperature Simulation of an Ablation Needle for the Prediction of Tissue Necrosis during Liver Ablation. J. Clin. Med. 2024, 13, 5853. https://doi.org/10.3390/jcm13195853
Will M, Gerlach T, Saalfeld S, Gutberlet M, Düx D, Schröer S, Hille G, Wacker F, Hensen B, Berg P. Temperature Simulation of an Ablation Needle for the Prediction of Tissue Necrosis during Liver Ablation. Journal of Clinical Medicine. 2024; 13(19):5853. https://doi.org/10.3390/jcm13195853
Chicago/Turabian StyleWill, Maximilian, Thomas Gerlach, Sylvia Saalfeld, Marcel Gutberlet, Daniel Düx, Simon Schröer, Georg Hille, Frank Wacker, Bennet Hensen, and Philipp Berg. 2024. "Temperature Simulation of an Ablation Needle for the Prediction of Tissue Necrosis during Liver Ablation" Journal of Clinical Medicine 13, no. 19: 5853. https://doi.org/10.3390/jcm13195853
APA StyleWill, M., Gerlach, T., Saalfeld, S., Gutberlet, M., Düx, D., Schröer, S., Hille, G., Wacker, F., Hensen, B., & Berg, P. (2024). Temperature Simulation of an Ablation Needle for the Prediction of Tissue Necrosis during Liver Ablation. Journal of Clinical Medicine, 13(19), 5853. https://doi.org/10.3390/jcm13195853






