Breath Analysis via Gas Chromatography–Mass Spectrometry (GC-MS) in Chronic Coronary Syndrome (CCS): A Proof-of-Concept Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- (a)
- Patients without an indication for myocardial revascularization;
- (b)
- Patients with an indication for myocardial revascularization.
- Group 1: patients without any lesion in the epicardial coronary arteries;
- Group 2: patients without significant lesions in the epicardial coronary arteries recommended for optimal medical therapy;
- Group 3: patients with lesions in small-caliber coronary arteries, recommended for optimal medical therapy;
- Group 4: patients with significant lesions in the epicardial coronary arteries, recommended for percutaneous coronary intervention (PCI);
- Group 5: patients with significant lesions in the epicardial coronary arteries, recommended for coronary artery bypass grafting (CABG).
2.2. Pulmonary and Cardiovascular Function Test
2.3. Exhaled Air Collection
2.4. Analysis of Exhaled Air
2.4.1. Analysis with Gas Sensor Array
2.4.2. Analysis with GC-MS
2.5. Ethical Consent
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar]
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990-2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 00, 1–101. [Google Scholar]
- Gerszten, R.E.; Wang, T.J. The search for new cardiovascular biomarkers. Nature 2008, 451, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Gerszten, R.E. Metabolomics and cardiovascular biomarker discovery. Clin. Chem. 2012, 58, 139–147. [Google Scholar] [CrossRef]
- Cikach, F.S.; Dweik, R.A. Cardiovascular biomarkers in exhaled breath. Prog. Cardiovasc. Dis. 2012, 55, 34–43. [Google Scholar] [CrossRef]
- van de Kant, K.D.G.; van der Sande, L.J.T.M.; Jöbsis, Q.; van Schayck, O.C.P.; Dompeling, E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: A systematic review. Respir. Res. 2012, 13, 117. [Google Scholar] [CrossRef]
- Scarlata, S.; Pennazza, G.; Santonico, M.; Pedone, C.; Antonelli Incalzi, R. Exhaled breath analysis by electronic nose in respiratory diseases. Expert. Rev. Mol. Diagn. 2015, 15, 933–956. [Google Scholar] [CrossRef]
- Marcondes-Braga, F.G.; Batista, G.L.; Bacal, F.; Gutz, I. Exhaled Breath Analysis in Heart Failure. Curr. Heart Fail. Rep. 2016, 13, 166–171. [Google Scholar] [CrossRef]
- De Vincentis, A.; Pennazza, G.; Santonico, M.; Vespasiani-Gentilucci, U.; Galati, G.; Gallo, P.; Vernile, C.; Pedone, C.; Antonelli Incalzi, R.; Picardi, A. Breath-print analysis by e-nose for classifying and monitoring chronic liver disease: A proof-of-concept study. Sci. Rep. 2016, 6, 25337. [Google Scholar] [CrossRef]
- Bajo-Fernández, M.; Souza-Silva, É.A.; Barbas, C.; Rey-Stolle, M.F.; García, A. GC-MS-based metabolomics of volatile organic compounds in exhaled breath: Applications in health and disease. A review. Front. Mol. Biosci. 2023, 10, 1295955. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Ruszkiewicz, D.M.; Meister, A.; Bartolomeu, C.; Atkar-Khattra, S.; Thomas, C.L.P.; Lam, S. Breath testing for SARS-CoV-2 infection. eBioMedicine 2023, 92, 104584. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath. Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.D.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath. Res. 2021, 15, 034001. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm (accessed on 27 September 2024).
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Kunos, L.; Bikov, A.; Lazar, Z.; Korosi, B.Z.; Benedek, P.; Losonczy, G.; Horvath, I. Evening and morning exhaled volatile compound patterns are different in obstructive sleep apnoea assessed with electronic nose. Sleep. Breath. 2015, 19, 247–253. [Google Scholar] [CrossRef]
- Santonico, M.; Pennazza, G.; Grasso, S.; D’Amico, A.; Bizzarri, M. Design and test of a biosensor-based multisensorial system: A proof of concept study. Sensors 2013, 13, 16625–16640. [Google Scholar] [CrossRef]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar® bags for breath-gas sampling and analysis. J. Breath. Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.chem.agilent.com (accessed on 25 September 2024).
- Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 25 September 2024).
- Vallejo, M.; García, A.; Tuñón, J.; García-Martínez, D.; Angulo, S.; Martin-Ventura, J.L.; Blanco-Colio, L.M.; Almeida, P.; Egido, J.; Barbas, C. Plasma fingerprinting with GC-MS in acute coronary syndrome. Anal. Bioanal. Chem. 2009, 394, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L.; Magaret, C.A.; Gaggin, H.K.; Rhyne, R.F.; Gandhi, P.U.; Kelly, N.; Simon, M.L.; Motiwala, S.R.; Belcher, A.M.; et al. A Clinical and Biomarker Scoring System to Predict the Presence of Obstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 1147–1156. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Di Giulio, C.; Pokorski, M. Pathologies currently identified by exhaled biomarkers. Respir. Physiol. Neurobiol. 2013, 187, 128–134. [Google Scholar] [CrossRef]
- Einoch Amor, R.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef]
- Christiansen, A.; Davidsen, J.R.; Titlestad, I.; Vestbo, J.; Baumbach, J. A systematic review of breath analysis and detection of volatile organic compounds in COPD. J. Breath. Res. 2016, 10, 034002. [Google Scholar] [CrossRef]
- Azim, A.; Barber, C.; Dennison, P.; Riley, J.; Howarth, P. Exhaled volatile organic compounds in adult asthma: A systematic review. Eur. Respir. J. 2019, 54, 1900056. [Google Scholar] [CrossRef]
- Finamore, P.; Scarlata, S.; Cardaci, V.; Incalzi, R.A. Exhaled Breath Analysis in Obstructive Sleep Apnea Syndrome: A Review of the Literature. Medicina 2019, 55, 538. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F.E. Diagnostic potential of breath analysis--focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S.; Sobotka, P.A.; Leja, F.L.; Euler, D.E. Breath pentane and plasma lipid peroxides in ischemic heart disease. Free Radic. Biol. Med. 1995, 19, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, E.; Allard, J.P. Breath alkanes as a marker of oxidative stress in different clinical conditions. Free Radic. Biol. Med. 2000, 28, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Greenberg, J.; Grodman, R.; Salazar, M. Breath markers of oxidative stress in patients with unstable angina. Heart Dis. 2003, 5, 95–99. [Google Scholar] [CrossRef]
- Kupari, M.; Lommi, J.; Ventilä, M.; Karjalainen, U. Breath acetone in congestive heart failure. Am. J. Cardiol. 1995, 76, 1076–1078. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Segreti, A.; Incalzi, R.A.; Lombardi, M.; Miglionico, M.; Nusca, A.; Pennazza, G.; Santonico, M.; Grasso, S.; Grigioni, F.; Di Sciascio, G. Characterization of inflammatory profile by breath analysis in chronic coronary syndromes. J. Cardiovasc Med. 2020, 21, 675–681. [Google Scholar] [CrossRef]


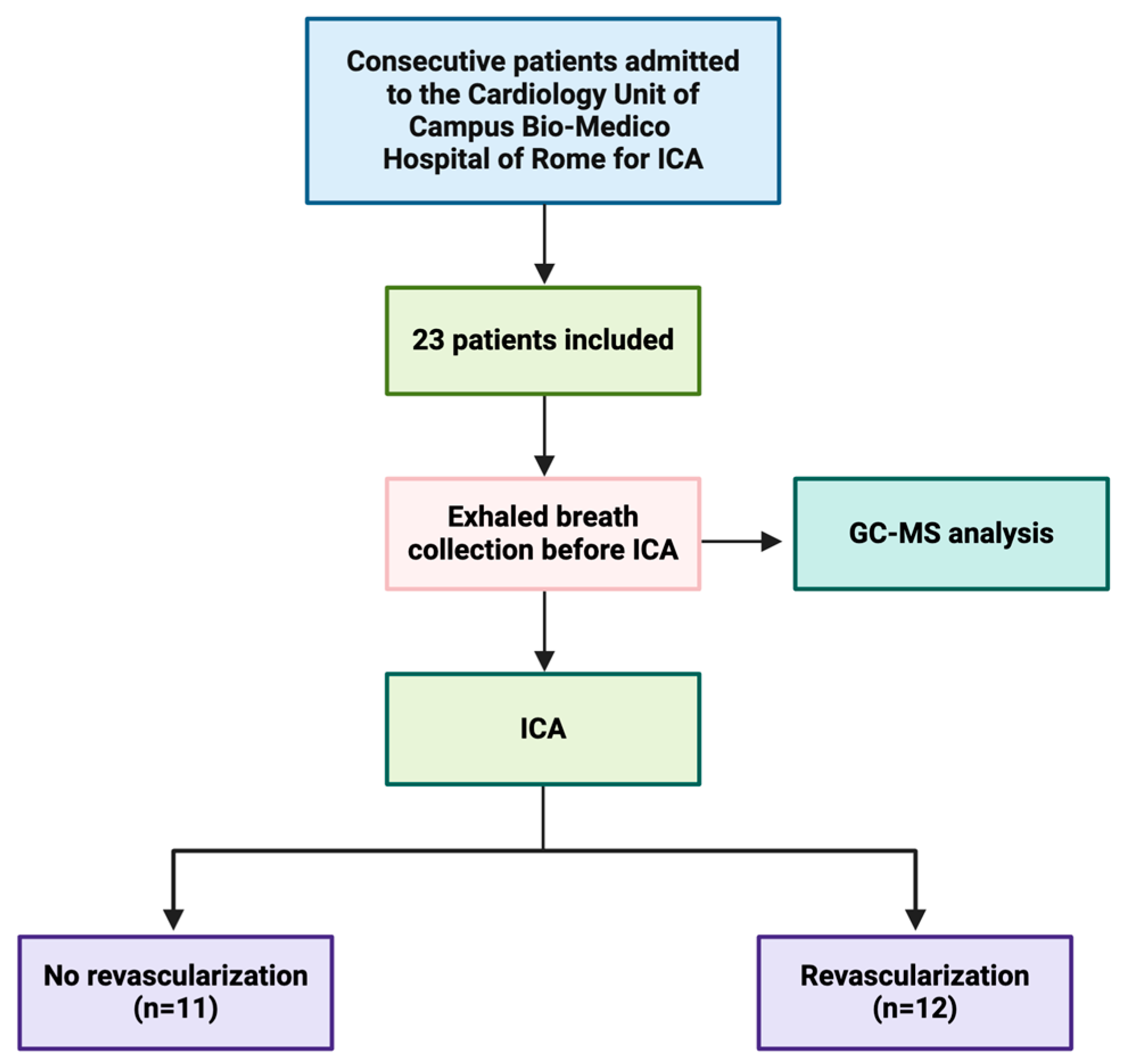
| No Revascularization (n = 11) | Revascularization (n = 12) | p Value | |
|---|---|---|---|
| Age | 67.09 ± 5.27 | 67.83 ± 4.5 | 0.843 |
| Male patients | 54.54% | 91.67% | 0.06 |
| BMI | 28.76 ± 1.7 | 29.4 ± 2.4 | 0.714 |
| Type 2 diabetes | 18.18% | 33.33% | 0.640 |
| Hypertension | 100.00% | 75% | 0.210 |
| Prior PCI/CABG | 18.18% | 25% | 0.545 |
| Smoking history | 63.64% | 75% | 0.660 |
| Dyslipidemia | 72.78% | 91.7% | 0.316 |
| Carotid atherosclerosis | 72.78% | 75% | 0.640 |
| EF | 0.6 ± 0.04 | 0.56 ± 0.07 | 0.360 |
| FEV1/FVC | 78.6 ± 5.76 | 77.4 ± 4.47 | 0.744 |
| SYNTAX score | 3.3 ± 1.9 | 14.8 ± 8.4 | <0.05 |
| Group 1, 2, 3 vs. 4, 5 GC-MS (n = 23) | ||
|---|---|---|
| Group 1, 2, 3 | Group 4, 5 | |
| 1, 2, 3 | 10 | 1 |
| 4, 5 | 5 | 7 |
| Group 1, 2, 3 vs. 4, 5 GC-MS (n = 23) | |||
|---|---|---|---|
| Group 1 | Group 2, 3 | Group 4, 5 | |
| 1 | 1 | 1 | 1 |
| 2, 3 | 0 | 4 | 4 |
| 4, 5 | 0 | 0 | 12 |
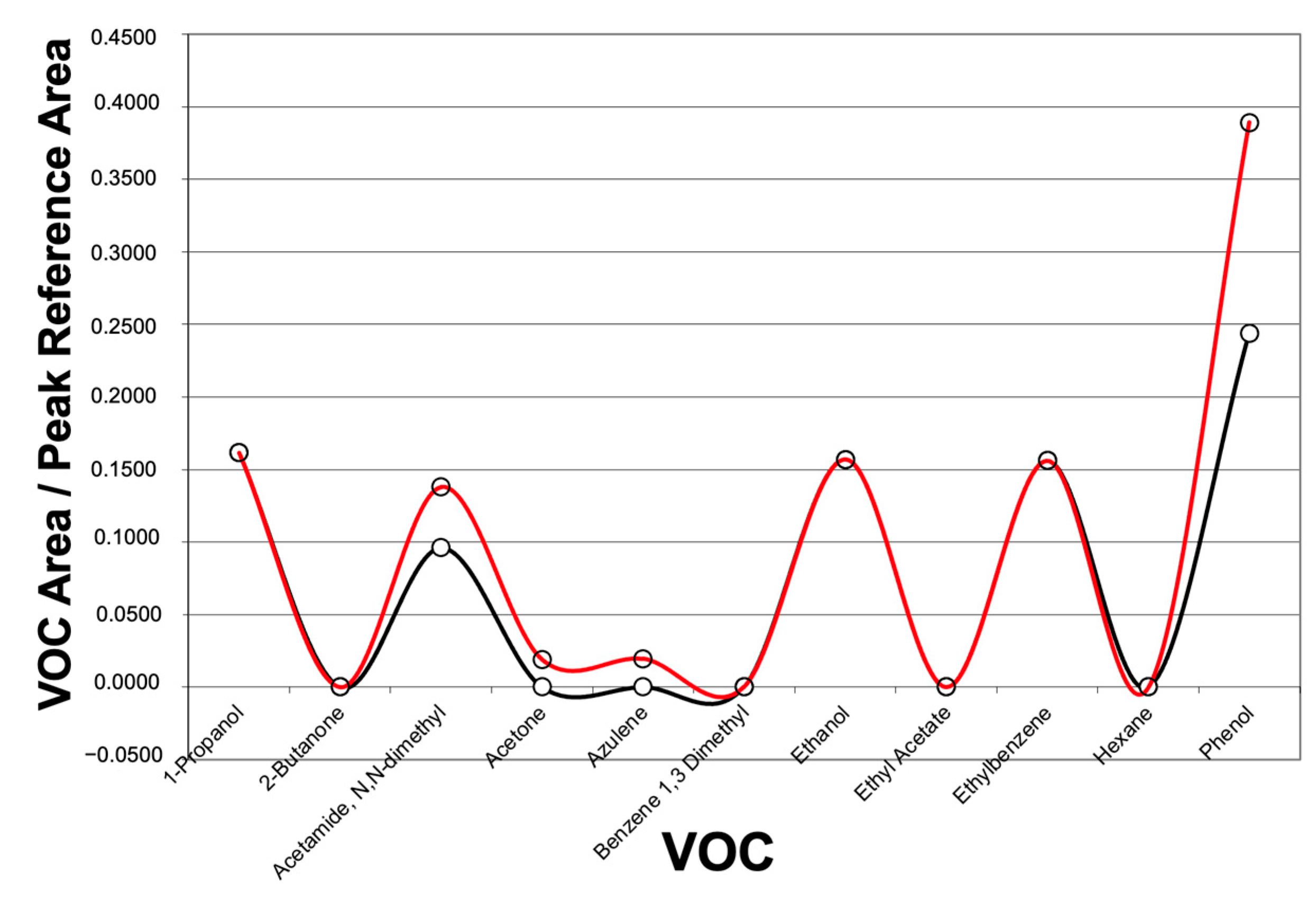
| 36 VOCs Detected by GC-MS | ||
|---|---|---|
| 1-Propanol | Dodecane | Isopropyl alcohol |
| 2-Butanone | Dodecane + Decane,2,3,5,8-tetramethyl | Octane, 4-methyl |
| Acetamide, N, N, -dimethyl | Dodecane, 2,6,11-trimethyl | Pentane |
| Acetic Acid | Ethanol | Pentane, 2,3-dimethyl |
| Acetone | Ethyl acetate | Pentane, 2-methyl |
| Azulene | Ethylbenzene | Pentane, 2-mehtyl + cyclopentane |
| Benzene 1,3 dimethyl | Formic acid, butyl ester | 1-Pentene |
| Butane | Hexane | Phenol |
| Butane, 2,3-dimethyl | Hexane, 2,3,5-trimethyl- | Styrene |
| Cyclopropane, ethyl | Hexane, 2-methyl | Trichloromethane |
| Decane, 2,4,6-trimethyl | Hexane, 3-methyl | Toluene |
| Decane, 3,7-dimethyl | Isobutane | Undecane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, M.; Segreti, A.; Miglionico, M.; Pennazza, G.; Tocca, L.; Amendola, L.; Vergallo, R.; Di Sciascio, G.; Porto, I.; Grigioni, F.; et al. Breath Analysis via Gas Chromatography–Mass Spectrometry (GC-MS) in Chronic Coronary Syndrome (CCS): A Proof-of-Concept Study. J. Clin. Med. 2024, 13, 5857. https://doi.org/10.3390/jcm13195857
Lombardi M, Segreti A, Miglionico M, Pennazza G, Tocca L, Amendola L, Vergallo R, Di Sciascio G, Porto I, Grigioni F, et al. Breath Analysis via Gas Chromatography–Mass Spectrometry (GC-MS) in Chronic Coronary Syndrome (CCS): A Proof-of-Concept Study. Journal of Clinical Medicine. 2024; 13(19):5857. https://doi.org/10.3390/jcm13195857
Chicago/Turabian StyleLombardi, Marco, Andrea Segreti, Marco Miglionico, Giorgio Pennazza, Lorenzo Tocca, Luca Amendola, Rocco Vergallo, Germano Di Sciascio, Italo Porto, Francesco Grigioni, and et al. 2024. "Breath Analysis via Gas Chromatography–Mass Spectrometry (GC-MS) in Chronic Coronary Syndrome (CCS): A Proof-of-Concept Study" Journal of Clinical Medicine 13, no. 19: 5857. https://doi.org/10.3390/jcm13195857
APA StyleLombardi, M., Segreti, A., Miglionico, M., Pennazza, G., Tocca, L., Amendola, L., Vergallo, R., Di Sciascio, G., Porto, I., Grigioni, F., & Antonelli Incalzi, R. (2024). Breath Analysis via Gas Chromatography–Mass Spectrometry (GC-MS) in Chronic Coronary Syndrome (CCS): A Proof-of-Concept Study. Journal of Clinical Medicine, 13(19), 5857. https://doi.org/10.3390/jcm13195857











