Modified Peritoneal Fenestration as a Preventive Method for Lymphocele after Kidney Transplantation: A Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Preoperative Data
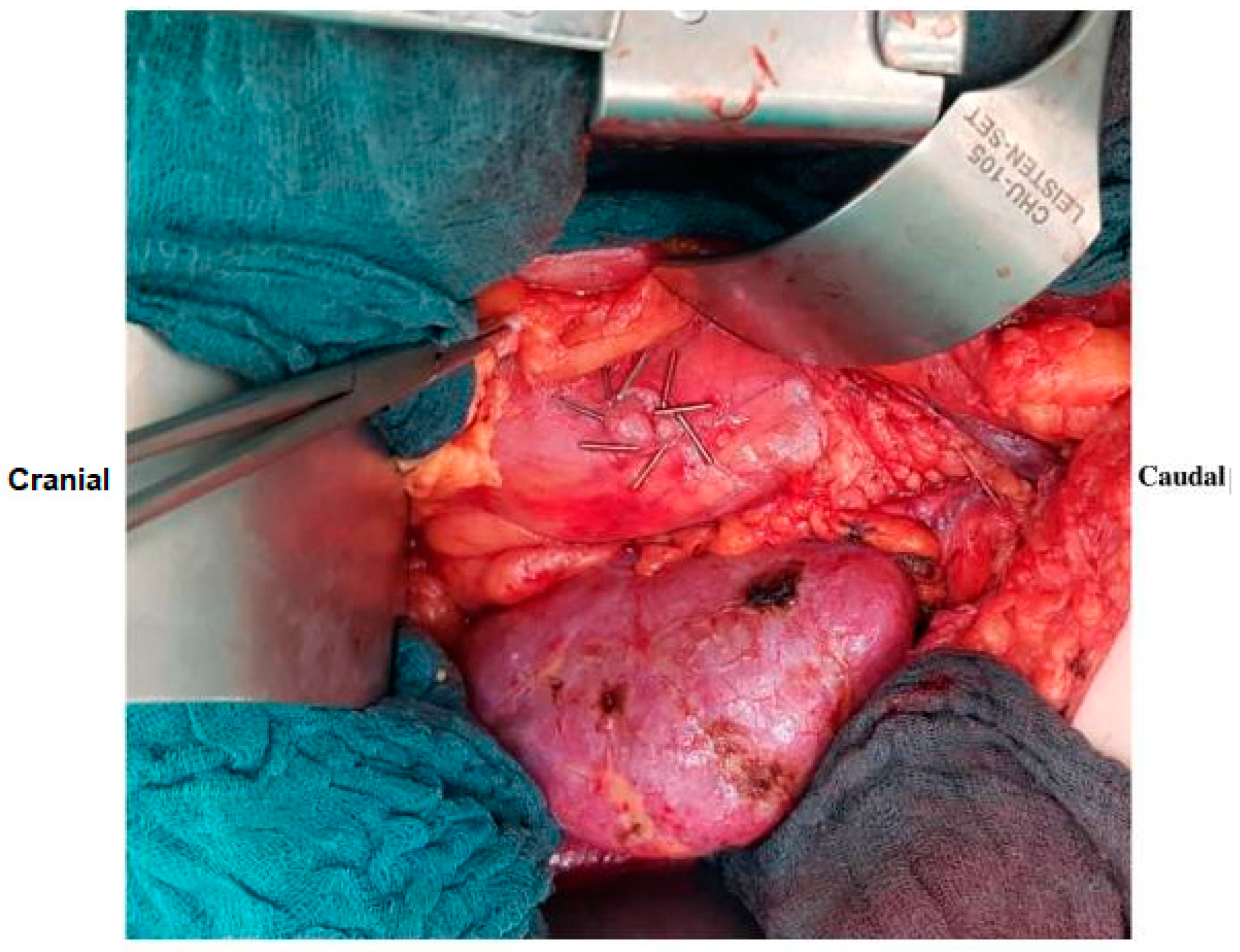
2.3. Surgical Procedure
2.4. Intraoperative and Postoperative Data
2.5. Propensity-Matching of Case and Control Groups
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atray, N.K.; Moore, F.; Zaman, F.; Caldito, G.; Abreo, K.; Maley, W.; Zibari, G.B. Post transplant lymphocele: A single centre experience. Clin. Transplant. 2004, 18 (Suppl. S12), 46–49. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Flechner, S.M.; Zhou, L.; Mastroianni, B.; Savas, K.; Derweesh, I.; Patel, P.; Modlin, C.; Goldfarb, D.; Novick, A.C. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation. J. Urol. 2004, 171, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Lucewicz, A.; Wong, G.; Lam, V.W.; Hawthorne, W.J.; Allen, R.; Craig, J.C.; Pleass, H.C. Management of Primary Symptomatic Lymphocele After Kidney Transplantation: A Systematic Review. Transplantation 2011, 92, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, A.; Kulu, Y.; Sabagh, M.; Khajeh, E.; Mohammadi, S.; Ghamarnejad, O.; Golriz, M.; Morath, C.; Bechstein, W.O.; Berlakovich, G.A.; et al. Consensus on definition and severity grading of lymphatic complications after kidney transplantation. Br. J. Surg. 2020, 107, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, A.L.; Heger, P.; Abbasi Dezfouli, S.; Golriz, M.; Mehrabi, A. Prophylaxis of lymphocele formation after kidney transplantation via peritoneal fenestration: A systematic review. Transpl. Int. 2017, 30, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Ranghino, A.; Segoloni, G.P.; Lasaponara, F.; Biancone, L. Lymphatic disorders after renal transplantation: New insights for an old complication. Clin. Kidney J. 2015, 8, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Derweesh, I.H.; Ismail, H.R.; Goldfarb, D.A.; Araki, M.; Zhou, L.; Modlin, C.; Krishnamurthi, V.; Flechner, S.M.; Novick, A.C. Intraoperative placing of drains decreases the incidence of lymphocele and deep vein thrombosis after renal transplantation. BJU Int. 2008, 101, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, V.; Vernillo, A.; Dumani, X.; Florio, I.; Pelosio, L.; Jamshidi, A.; Romagnuolo, R.; Calogero, A.; Carlomagno, N.; Santangeloa, M.; et al. Prevention of fluid effusion in kidney transplantation with the use of hemostatic biomaterials. Transplant. Proc. 2014, 46, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Berardinelli, L.; Raiteri, M.; Pasciucco, A.; Carini, M. The use of a polymeric sealant for prevention of posttransplantation lymphocele. Transplant. Proc. 2011, 43, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Sabagh, M.; Sabetkish, N.; Fakour, S.; Ramouz, A.; Weber, S.; Mieth, M.; Lurje, G.; Golriz, M.; Zeier, M.; Mehrabi, A.; et al. Methods to prevent lymphocele after kidney transplantation: Seeking the optimal technique for avoiding a preventable complication. Transplant. Rev. 2024, 38, 100877. [Google Scholar] [CrossRef] [PubMed]
- Golriz, M.; Sabagh, M.; Mohammadi, S.; Ghamarnejad, O.; Khajeh, E.; Mieth, M.; Al-Saeedi, M.; Diener, M.K.; Mihaljevic, A.L.; Morath, C.; et al. PREventive effect of FENestration with and without clipping on post-kidney transplantation lymphatic complications (PREFEN): Study protocol for a randomised controlled trial. BMJ Open 2020, 10, e032286. [Google Scholar] [CrossRef] [PubMed]
- Syversveen, T.; Midtvedt, K.; Brabrand, K.; Oyen, O.; Foss, A.; Scholz, T. Prophylactic peritoneal fenestration to prevent morbidity after kidney transplantation: A randomized study. Transplantation 2011, 92, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Golriz, M.; Sabagh, M.; Emami, G.; Mohammadi, S.; Ramouz, A.; Khajeh, E.; Ghamarnejad, O.; Morath, C.; Mieth, M.; Kulu, Y.; et al. Prophylactic Peritoneal Fenestration during Kidney Transplantation Can Reduce the Type C Lymphocele Formation. J. Clin. Med. 2021, 10, 5651. [Google Scholar] [CrossRef] [PubMed]
- Taweemonkongsap, T.; Srinualnad, S.; Nualyong, C.; Tantiwong, A.; Soontrapa, S. Novel technique to prevent lymphocele recurrence after laparoscopic lymphocele fenestration in renal transplant patients. J. Endourol. 2006, 20, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Beimler, J.; Schmidt, J.; Büchler, M.; Zeier, M.J. Heidelberger Manual der Nieren-und Pankreastransplantation; Heidelberg University Hospital: Heidelberg, Germany, 2007. [Google Scholar]
- Layman, R.E.; McNally, M.; Kilian, C.; Linn, J.; Roza, A.; Johnson, C.P.; Adams, M.B.; Shames, B.D. Does opening the peritoneum at the time of renal transplanation prevent lymphocele formation? Transplant. Proc. 2006, 38, 3524–3526. [Google Scholar] [CrossRef] [PubMed]
- Zaontz, M.R.; Firlit, C.F. Pelvic lymphocele after pediatric renal transplantation: A successful technique for prevention. J. Urol. 1988, 139, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, F.; Niedzwiecki, S.; Fikatas, P.; Nebrig, M.; Schmidt, S.C.; Kohler, S.; Weiss, S.; Schumacher, G.; Pascher, A.; Reinke, P.; et al. Symptomatic lymphoceles after kidney transplantation—Multivariate analysis of risk factors and outcome after laparoscopic fenestration. Clin. Transplant. 2010, 24, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Rath, T. Current Issues and Future Direction in Kidney Transplantation; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Lehner, L.J.; Hohberger, A.; Marschke, L.; Lachmann, N.; Peters, R.; Friedersdorff, F.; Khadzhynov, D.; Halleck, F.; Budde, K.; Staeck, O.; et al. Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles. J. Clin. Med. 2020, 9, 2841. [Google Scholar] [CrossRef] [PubMed]
- Zagdoun, E.; Ficheux, M.; Lobbedez, T.; Chatelet, V.; Thuillier-Lecouf, A.; Bensadoun, H.; Ryckelynck, J.-P.; de Ligny, B.H. Complicated lymphoceles after kidney transplantation. Transplant. Proc. 2010, 42, 4322–4325. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.; d’Ancona, F.C.; van der Meijden, W.A.; Poyck, P.P. Predictors of symptomatic lymphocele after kidney transplantation. Int. Urol. Nephrol. 2019, 51, 2161–2167. [Google Scholar] [CrossRef] [PubMed]

| All Peritoneal Fenestrations before Matching (n = 405) | Peritoneal Fenestration + Clipping (n = 25) | p | Peritoneal Fenestration without Clipping after Matching (n = 75) | p | ||
|---|---|---|---|---|---|---|
| Age (years), median (range) | 56 (6–87) | 41 (21–69) | 0.042 | 45 (18–71) | 0.723 | |
| Gender, (female), n (%) | 149 (26.8) | 7 (28.0) | 0.375 | 29 (38.7) | 0.471 | |
| BMI (kg/m2), median (range) | 25 (16.2–41.7) | 23.9 (17.4–36.3) | 0.094 | 24.6 (17.2–35.9) | 0.650 | |
| Previous abdominal surgery, n (%) | 143 (35.3) | 9 (36.0) | 0.944 | 19 (32.0) | 0.303 | |
| ASA, n (%) | 0.441 | 0.285 | ||||
| II | 42 (10.4) | 1 (4.0) | 9 (12.0) | |||
| III | 355 (87.6) | 24 (96.0) | 63 (84.0) | |||
| IV | 8 (2.0) | 0 (0.0) | 3 (4.0) | |||
| Donor age (years), median (range) | 56 (6–87) | 55 (37–81) | 0.325 | 57 (12–81) | 0.682 | |
| Donor type (deceased), n (%) | 301 (74.2) | 9 (36.0) | <0.001 | 43 (57.3) | 0.105 | |
| Implantation side (right), n (%) | 185 (45.7) | 15 (60.0) | 0.163 | 44 (58.7) | 1.000 | |
| Blood loss (mL), median (range) | 300 (500–1700) | 300 (100–1100) | 0.061 | 300 (100–2000) | 0.048 | |
| Operation time (min), median (range) | 208 (51–800) | 205 (145–402) | 0.828 | 205 (145–402) | 0.708 | |
| Total lymphocele, n (%) | 55 (13.6) | 3 (12.0) | 0.857 | 10 (13.3) | 0.863 | |
| Clinically relevant lymphocele, n (%) | 43 (10.6) | 2 (8.0) | 0.678 | 8 (10.7) | 0.829 | |
| Clinically Relevant (Grade B and C) Lymphocele (n = 10) | Grade A Lymphocele (n = 3) | p | |
|---|---|---|---|
| Recipient age (years), median (range) | 56.5 (45–71) | 39 (27–56) | 0.077 |
| Donor age (years), median (range) | 61 (34–81) | 63 (53–77) | 1.000 |
| BMI (kg/m2), median (range) | 24.9 (21.6–35.9) | 21 (17.7–22.6) | 0.298 |
| BMI (≥25 kg/m2), n (%) | 5 (50.0) | 0 (0.0) | 0.028 |
| Gender (female),n (%) | 4 (40.0) | 1 (33.3) | 1.000 |
| ASA, (>III), n (%) | 7 (70.0) | 3 (100.0) | 0.528 |
| Donor type (deceased), n (%) | 9 (90.0) | 2 (66.7) | 0.423 |
| Side of graft (right), n (%) | 6 (60.0) | 1 (33.3) | 0.559 |
| Side of kidney implantation (right), n (%) | 4 (40.0) | 1 (33.3) | 1.000 |
| Previous abdominal surgery,n (%) | 5 (50.0) | 3 (100.0) | 0.231 |
| Blood loss (mL), median (range) | 325 (230–1000) | 500 (150–500) | 0.559 |
| Operation time (min), median (range) | 220 (163–290) | 210 (205–345) | 0.917 |
| Univariate Analysis | |||
|---|---|---|---|
| OR | 95% CI | p | |
| Recipient age (>55 years) | 7.560 | 2.374–24.073 | <0.001 |
| Recipient gender (female) | 1.038 | 0.349–3.089 | 1.000 |
| BMI (≥25 kg/m2) | 1.432 | 0.387–5.302 | 0.738 |
| Previous abdominal surgery | 4.507 | 1.497–13.568 | 0.010 |
| Donor type (deceased) | 0.181 | 0.048–0.677 | 0.007 |
| Donor age (>60 years) | 3.239 | 1.088–9.642 | 0.056 |
| Operation time | 1.008 | 0.997–1.018 | 0.160 |
| Blood loss (>1000 mL) | 1.521 | 0.371–6.243 | 0.693 |
| Fenestration with clipping | 0.837 | 0.166–4.221 | 0.826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabagh, M.; Weber, S.; Sabetkish, N.; Ramouz, A.; Fakour, S.; Morath, C.; Mieth, M.; Zeier, M.; Khajeh, E.; Mehrabi, A.; et al. Modified Peritoneal Fenestration as a Preventive Method for Lymphocele after Kidney Transplantation: A Preliminary Report. J. Clin. Med. 2024, 13, 5878. https://doi.org/10.3390/jcm13195878
Sabagh M, Weber S, Sabetkish N, Ramouz A, Fakour S, Morath C, Mieth M, Zeier M, Khajeh E, Mehrabi A, et al. Modified Peritoneal Fenestration as a Preventive Method for Lymphocele after Kidney Transplantation: A Preliminary Report. Journal of Clinical Medicine. 2024; 13(19):5878. https://doi.org/10.3390/jcm13195878
Chicago/Turabian StyleSabagh, Mohammadsadegh, Sanaz Weber, Nastaran Sabetkish, Ali Ramouz, Sanam Fakour, Christian Morath, Markus Mieth, Martin Zeier, Elias Khajeh, Arianeb Mehrabi, and et al. 2024. "Modified Peritoneal Fenestration as a Preventive Method for Lymphocele after Kidney Transplantation: A Preliminary Report" Journal of Clinical Medicine 13, no. 19: 5878. https://doi.org/10.3390/jcm13195878
APA StyleSabagh, M., Weber, S., Sabetkish, N., Ramouz, A., Fakour, S., Morath, C., Mieth, M., Zeier, M., Khajeh, E., Mehrabi, A., & Golriz, M. (2024). Modified Peritoneal Fenestration as a Preventive Method for Lymphocele after Kidney Transplantation: A Preliminary Report. Journal of Clinical Medicine, 13(19), 5878. https://doi.org/10.3390/jcm13195878





