Incidence and Predictors of Early and Late Radial Artery Occlusion after Percutaneous Coronary Intervention and Coronary Angiography: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
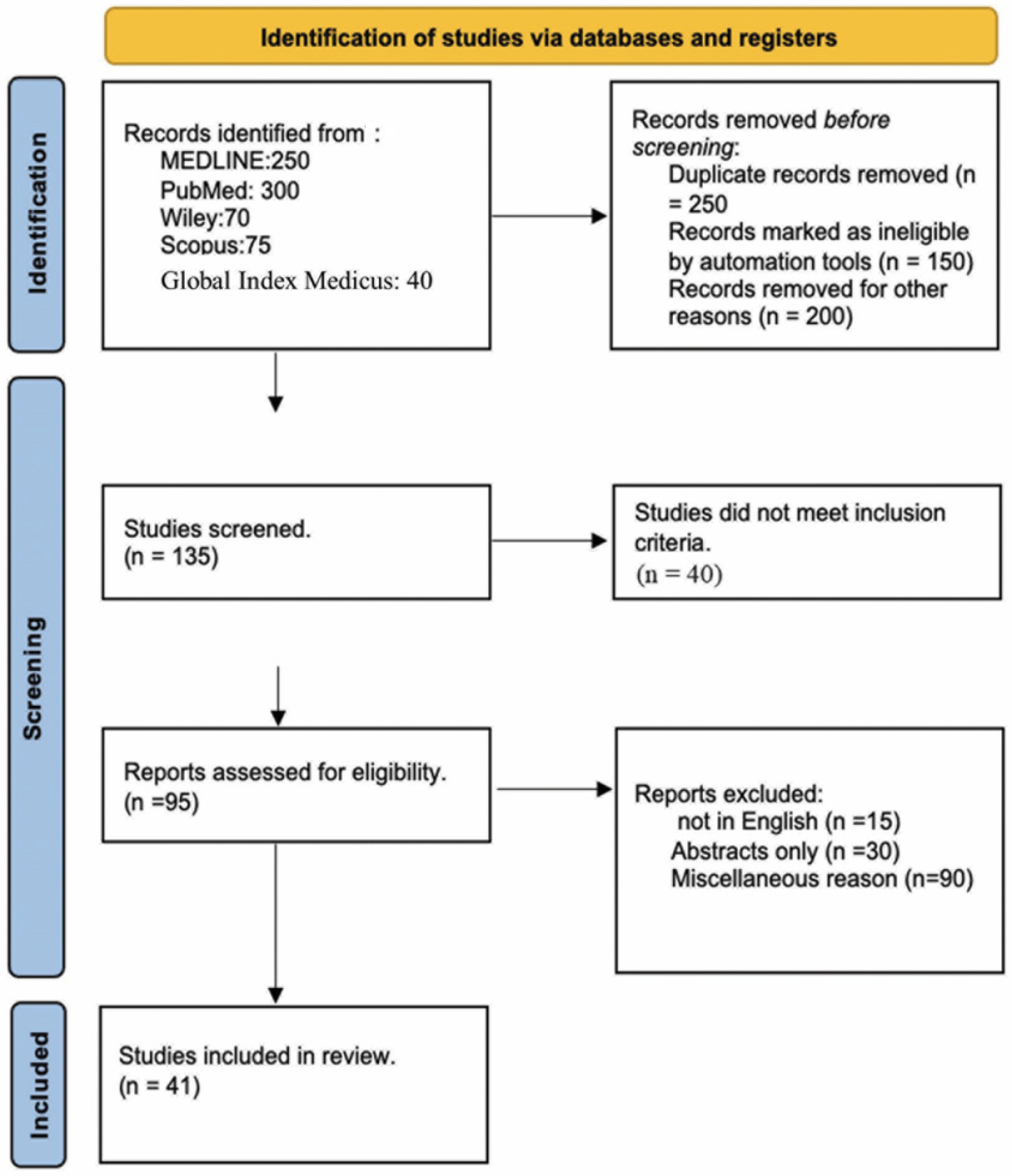
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Outcomes
2.6. Statistical Analysis
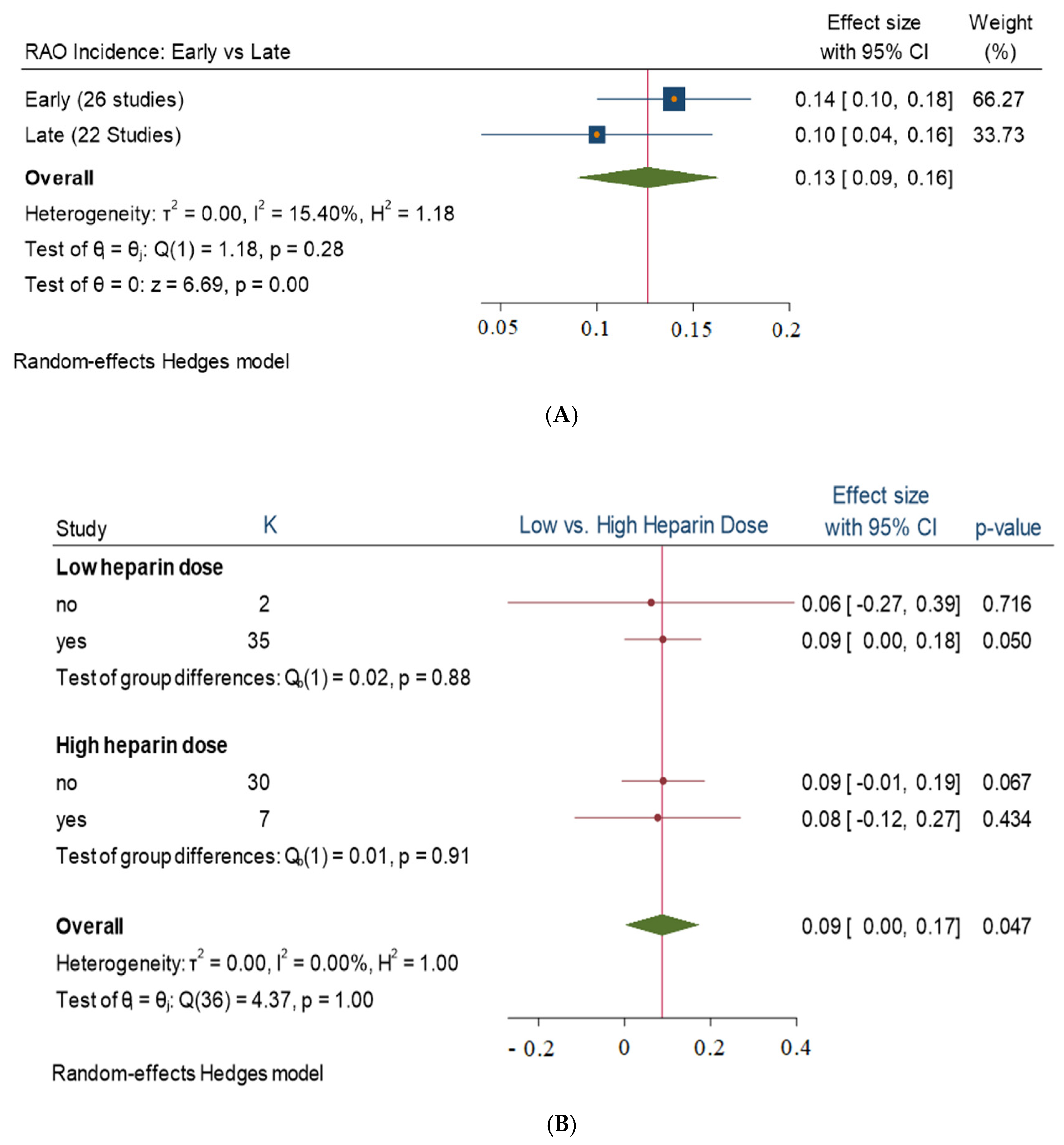
3. Results
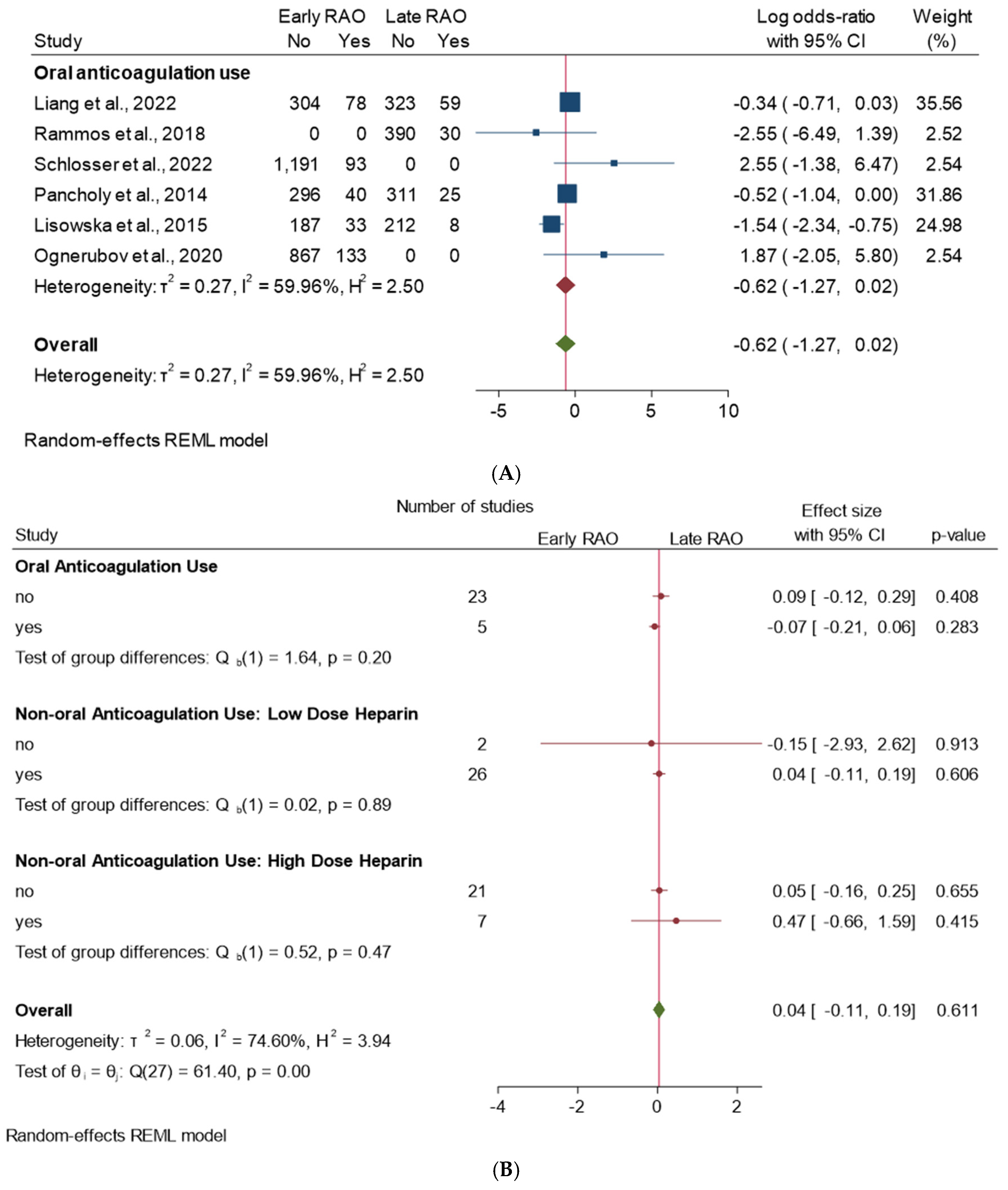
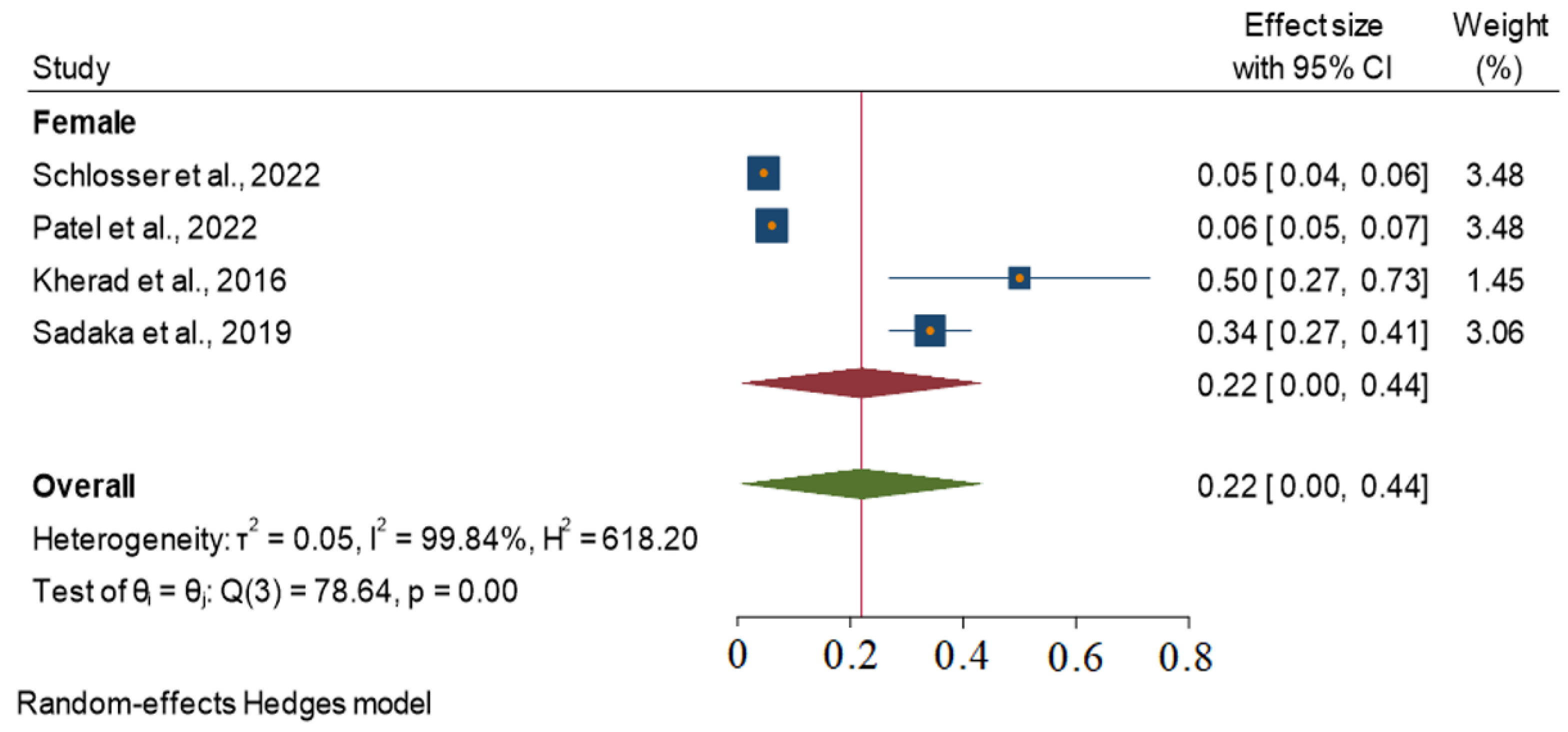
3.1. Stratified Analysis
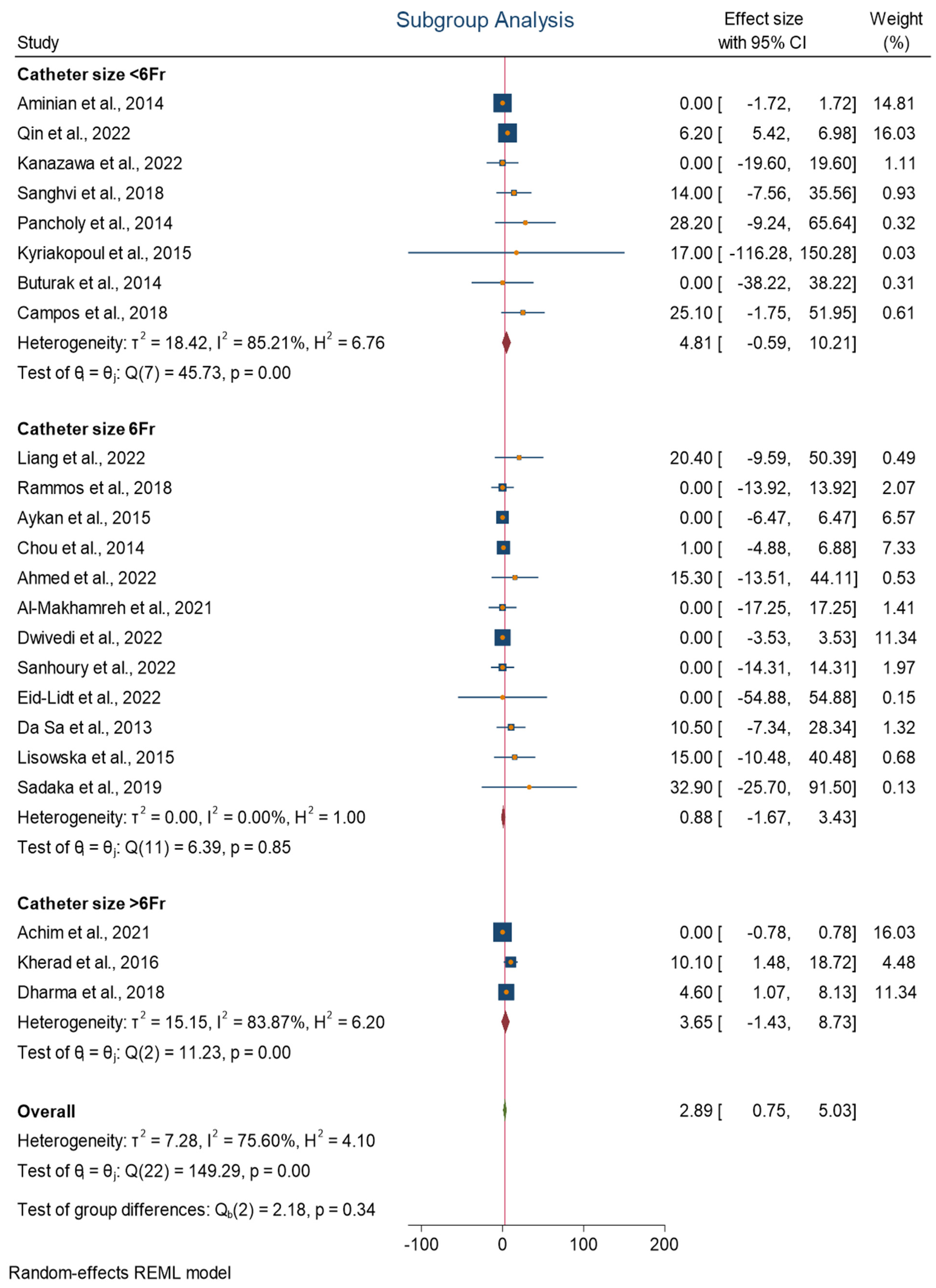
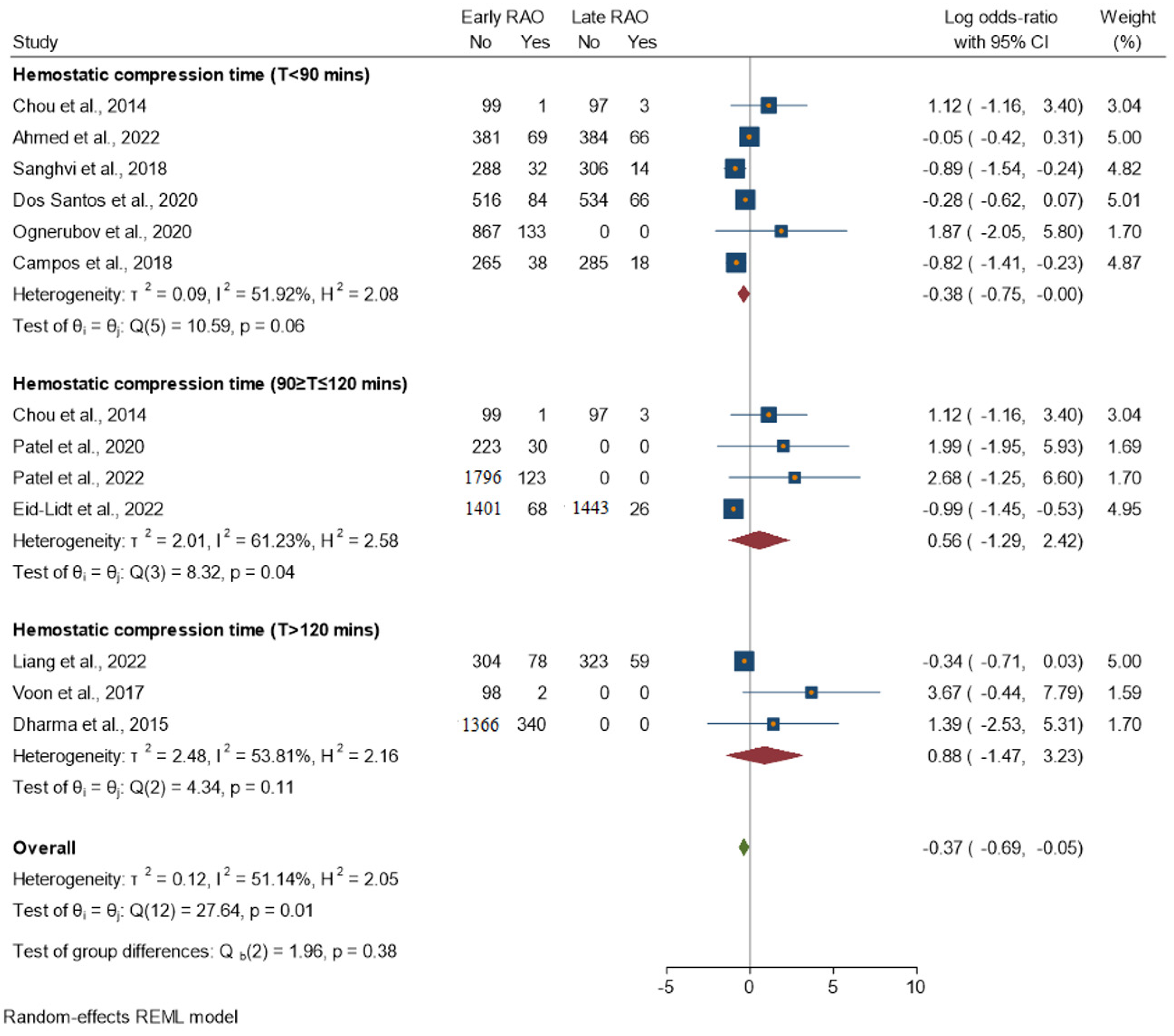
3.2. Subgroup Analysis
- T < 90 min: Moderate heterogeneity (I2 = 51.92%). The homogeneity study-specific effect-size test is not rejected, with a chi-squared test statistic (Q) of 10.59 and a p-value of 0.06.
- T 90–120 min: Moderate heterogeneity (I2 = 61.23%). The test of homogeneity study-specific effect size is rejected, with a chi-squared test statistic (Q) of 8.32 and a p-value of 0.04.
- T > 120 min: Moderate heterogeneity (I2 = 53.81%). The homogeneity study-specific effect-size test is not rejected, with a chi-squared test statistic (Q) of 8.32 and a p-value of 0.11.
3.3. Heterogeneity Assessment
3.4. Meta-Regression
3.5. Publication Bias
4. Discussion
4.1. Potential Application of These Results
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bertrand, O.F.; Rao, S.V.; Pancholy, S.; Jolly, S.S.; Rodés-Cabau, J.; Larose, É.; Costerousse, O.; Hamon, M.; Mann, T. Transradial approach for coronary angiography and interventions: Results of the first international transradial practice survey. JACC Cardiovasc. Interv. 2010, 3, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.M.; Galvin, S.D.; Javid, M.; Matalanis, G. Should the radial artery be used as a bypass graft following radial access coronary angiography. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Hirano, K.; Abe, T.; Imaeda, S.; Hashimoto, K.; Ryuzaki, T.; Yokokura, S.; Saito, T.; Yamazaki, H.; Tabei, R.; et al. Trans-radial percutaneous coronary intervention for patients with severe chronic renal insufficiency and/or on dialysis. Heart Vessels 2019, 34, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Stables, R.H.; Pauriah, M.; Hakeem, A.; Mills, J.D.; Palmer, N.D.; Perry, R.A.; Morris, J.L. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: A randomized study. JACC Cardiovasc. Interv. 2010, 3, 475–483. [Google Scholar] [CrossRef]
- Karrowni, W.; Vyas, A.; Giacomino, B.; Schweizer, M.; Blevins, A.; Girotra, S.; Horwitz, P.A. Radial versus femoral access for primary percutaneous interventions in ST-segment elevation myocardial infarction patients: A meta-analysis of randomized controlled trials. JACC Cardiovasc. Interv. 2013, 6, 814–823. [Google Scholar] [CrossRef]
- Rao, S.V.; Ou, F.-S.; Wang, T.Y.; Roe, M.T.; Brindis, R.; Rumsfeld, J.S.; Peterson, E.D. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: A report from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 2008, 1, 379–386. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf (accessed on 27 May 2024).
- Sinha, S.K.; Jha, M.J.; Mishra, V.; Thakur, R.; Goel, A.; Kumar, A.; Singh, A.K.; Sachan, M.; Varma, C.M.; Krishna, V. Radial Artery Occlusion—Incidence, Predictors and Long-term outcome after TRAnsradial Catheterization: Clinico-Doppler ultrasound-based study (RAIL-TRAC study). Acta Cardiol. 2017, 72, 318–327. [Google Scholar] [CrossRef]
- Maqsood, M.H.; Pancholy, S.; Tuozzo, K.A.; Moskowitz, N.; Rao, S.V.; Bangalore, S. Optimal Hemostatic Band Duration After Transradial Angiography or Intervention: Insights From a Mixed Treatment Comparison Meta-Analysis of Randomized Trials. Circ. Cardiovasc. Interv. 2023, 16, e012781. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; India, L.; Sharma, A.K.; Nayak, G.R.; Chaudhary, G.K.; Chandra, S.; Pradhan, A.; Vishwakarma, P.; Bhandari, M.; Sethi, R. Factors influencing radial artery occlusion after transradial coronary intervention in the Indian population. Anatol. J. Cardiol. 2022, 26, 105–111. [Google Scholar] [CrossRef]
- Mattea, V.; Salomon, C.; Menck, N.; Lauten, P.; Malur, F.M.; Schade, A.; Steinborn, F.; Costello-Boerrigter, L.; Neumeister, A.; Lapp, H. Low rate of access site complications after transradial coronary catheterization: A prospective ultrasound study. Int. J. Cardiol. Heart Vasc. 2017, 14, 46–52. [Google Scholar] [CrossRef]
- Pancholy, S.; Coppola, J.; Patel, T.; Roke-Thomas, M. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002686. [Google Scholar] [CrossRef]
- Horie, K.; Tada, N.; Isawa, T.; Matsumoto, T.; Taguri, M.; Kato, S.; Honda, T.; Ootomo, T.; Inoue, N. A randomised comparison of incidence of radial artery occlusion and symptomatic radial artery spasm associated with elective transradial coronary intervention using 6.5 Fr SheathLess Eaucath Guiding Catheter vs. 6.0 Fr Glidesheath Slender. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 13, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Saito, S.; Takahashi, A.; Bernat, I.; Jobe, R.L.; Kajiya, T.; Gilchrist, I.C.; Louvard, Y.; Kiemeneij, F.; van Royen, N.; et al. Impact of sheath size and hemostasis time on radial artery patency after transradial coronary angiography and intervention in Japanese and non-Japanese patients: A substudy from RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT) randomized multicenter trial. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 92, 844–851. [Google Scholar] [CrossRef]
- Bernat, I.; Aminian, A.; Pancholy, S.; Mamas, M.; Gaudino, M.; Nolan, J.; Gilchrist, I.C.; Saito, S.; Hahalis, G.N.; Ziakas, A.; et al. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention: An International Consensus Paper. JACC Cardiovasc. Interv. 2019, 12, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- The FUTURA/OASIS-8 Trial Group; Steg, P.G.; Jolly, S.S.; Mehta, S.R.; Afzal, R.; Xavier, D.; Rupprecht, H.-J.; Lopez-Sendon, J.L.; Budaj, A.; Diaz, R.; et al. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: The FUTURA/OASIS-8 randomized trial. JAMA 2010, 304, 1339–1349. [Google Scholar] [CrossRef]
- Hahalis, G.N.; Leopoulou, M.; Tsigkas, G.; Xanthopoulou, I.; Patsilinakos, S.; Patsourakos, N.G.; Ziakas, A.; Kafkas, N.; Koutouzis, M.; Tsiafoutis, I.; et al. Multicenter Randomized Evaluation of High Versus Standard Heparin Dose on Incident Radial Arterial Occlusion After Transradial Coronary Angiography: The SPIRIT OF ARTEMIS Study. JACC Cardiovasc. Interv. 2018, 11, 2241–2250. [Google Scholar] [CrossRef]
- Schlosser, J.; Herrmann, L.; Böhme, T.; Bürgelin, K.; Löffelhardt, N.; Nührenberg, T.; Mashayekhi, K.; Valina, C.M.; Neumann, F.-J.; Hochholzer, W. Incidence and predictors of radial artery occlusion following transradial coronary angiography: The proRadial trial. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2023, 112, 1175–1185. [Google Scholar] [CrossRef]
- Sadaka, M.A.; Etman, W.; Ahmed, W.; Kandil, S.; Eltahan, S. Incidence and predictors of radial artery occlusion after transradial coronary catheterization. Egypt. Heart J. EHJ Off. Bull. Egypt. Soc. Cardiol. 2019, 71, 12. [Google Scholar] [CrossRef]
- Pancholy, S.; Coppola, J.; Patel, T.; Roke-Thomas, M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): A randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2008, 72, 335–340. [Google Scholar] [CrossRef]
- Cubero, J.M.; Lombardo, J.; Pedrosa, C.; Diaz-Bejarano, D.; Sanchez, B.; Fernandez, V.; Gomez, C.; Vazquez, R.; Molano, F.J.; Pastor, L.F. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP). Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2009, 73, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.; Gordin, J.; Sallam, T.; Wachsner, R.; Meymandi, S.; Traina, M. Facilitated patent haemostasis after transradial catheterisation to reduce radial artery occlusion. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2015, 11, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, A.; Knapp, M.; Tycińska, A.; Sielatycki, P.; Sawicki, R.; Kralisz, P.; Musiał, W.J. Radial access during percutaneous interventions in patients with acute coronary syndromes: Should we routinely monitor radial artery patency by ultrasonography promptly after the procedure and in long-term observation? Int. J. Cardiovasc. Imaging 2015, 31, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Fujimoto, K.; Miyao, Y.; Koga, H.; Hirata, Y. Access site-related complications after transradial catheterization can be reduced with smaller sheath size and statins. Cardiovasc. Interv. Ther. 2012, 27, 174–180. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Doi, S.A.; Barendregt, J.J.; Khan, S.; Thalib, L.; Williams, G.M. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp. Clin. Trials 2015, 45 Pt A, 130–138. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.M.; Barendregt, J.J.M.; Doi, S.A.M. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lin, Q.; Zhu, Q.; Zhou, X.; Fang, Y.; Wang, L.; Xiang, G.; Zheng, K.I.; Huang, W.; Shan, P. Short-Term Postoperative Use of Rivaroxaban to Prevent Radial Artery Occlusion After Transradial Coronary Procedure: The RESTORE Randomized Trial. Circ. Cardiovasc. Interv. 2022, 15, e011555. [Google Scholar] [CrossRef]
- Roy, S.; Choxi, R.; Wasilewski, M.; Jovin, I.S. Novel oral anticoagulants in the treatment of radial artery occlusion. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, 1133–1137. [Google Scholar] [CrossRef]
- Rammos, C.; Burghardt, A.; Lortz, J.; Azizy, O.; Jánosi, R.A.; Steinmetz, M.; Rassaf, T. Impact of anticoagulation and vasoactive medication on regained radial artery patency after catheterization: A case-control study. Eur. J. Med. Res. 2018, 23, 25. [Google Scholar] [CrossRef]
- Aykan, A.; Gökdeniz, T.; Gül, I.; Kalaycıoğlu, E.; Çetin, M.; Hatem, E.; Çavuşoğlu, I.G.; Karabay, C.Y.; Güler, A.; Aykan, D.A.; et al. Comparison of low dose versus standard dose heparin for radial approach in elective coronary angiography? Int. J. Cardiol. 2015, 187, 389–392. [Google Scholar] [CrossRef]
- Chou, M.-T.; Chiang, C.-Y. PT200 Effect of Short-Time Compression with Kaolin-Filled Pad on Radial Artery Occlusion After Transradial Acess Catheterization. Glob. Heart 2014, 9, e207. [Google Scholar] [CrossRef]
- Sharma, A.K.; Razi, M.; Prakash, N.; Sharma, A.; Sarraf, S.; Sinha, S.; Pandey, U.; Thakur, R.; Verma, C.; Krishna, V. A comparative assessment of Dorsal radial artery access versus classical radial artery access for percutaneous coronary angiography-a randomized control trial (DORA trial). Indian Heart J. 2020, 72, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Abbas, E.; Bakr, A.H.; Demitry, S.R.; Algowhary, M.I. Prevention of radial artery occlusion by simultaneous ulnar and radial compression (PRO-SURC). A randomized duplex ultrasound follow-up study. Int. J. Cardiol. 2022, 363, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Shah, S.; Patel, B.A.; Patel, T.M. Randomized COmparison of Isolated Radial Artery ComPrEssioN Versus Radial and Ipsilateral Ulnar Artery Compression in Achieving Radial Artery Patency: The OPEN-Radial Trial. J. Invasive Cardiol. 2020, 32, 476–482. [Google Scholar] [PubMed]
- Patel, P.; Sethi, N.; Patel, G.A.; Kalisetti, D.; Patel, T.M. Evaluation of the incidence of radial artery occlusion using different introducer sheaths and hemostasis techniques. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 100, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yang, X.; Cheng, W.; Wang, J.; Jin, Z. Different Antiplatelet Strategies for Radial Artery Protection After Transradial Coronary Angiography-A Prospective Observational Cohort Study. Front. Cardiovasc. Med. 2022, 9, 913008. [Google Scholar] [CrossRef]
- Kanazawa, T.; Shimamura, K.; Nagao, K.; Yukawa, H.; Aida, K.; Kobayashi, Y.; Takahashi, N.; Nakagawa, E.; Itoh, H.; Hayashi, F.; et al. Angiographic evaluation of radial artery injury after transradial approach for percutaneous coronary intervention. Cardiovasc. Interv. Ther. 2022, 37, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Makhamreh, H.; Shaban, A.; Elfawair, M.; Noori, S.; Al-Khaleefa, F.; Rahahleh, L.; AlRamahi, B. Rate of late radial artery occlusion following cardiac catheterization at Jordan University Hospital. Future Cardiol. 2021, 17, 1225–1232. [Google Scholar] [CrossRef]
- Achim, A.; Kákonyi, K.; Jambrik, Z.; Nagy, F.; Tóth, J.; Sasi, V.; Hausinger, P.; Nemes, A.; Varga, A.; Bertrand, O.F.; et al. Distal Radial Artery Access for Coronary and Peripheral Procedures: A Multicenter Experience. J. Clin. Med. 2021, 10, 5974. [Google Scholar] [CrossRef]
- Xie, L.; Wei, X.; Xie, Z.; Jia, S.; Xu, S.; Wang, K. Feasibility of Distal Radial Access for Coronary Angiography and Percutaneous Coronary Intervention: A Single Center Experience. Cardiology 2021, 146, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; AyyazUlHaq, M.; Cahill, C.; Mannix, K.; Ahern, C.; Hennessy, T.; Arnous, S.; Kiernan, T. Randomized study comparing incidence of radial artery occlusion post-percutaneous coronary intervention between two conventional compression devices using a novel air-inflation technique. World J. Cardiol. 2017, 9, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Kherad, B.; Köhncke, C.; Spillmann, F.; Post, H.; Noutsias, M.; Pieske, B.; Krackhardt, F.; Tschöpe, C. Postprocedural radial artery occlusion rate using a sheathless guiding catheter for left ventricular endomyocardial biopsy performed by transradial approach. BMC Cardiovasc. Disord. 2016, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, K.A.; Montgomery, M.; Varghese, V. Effect of hemostatic device on radial artery occlusion: A randomized comparison of compression devices in the radial hemostasis study. Cardiovasc. Revascularization Med. Mol. Interv. 2018, 19, 934–938. [Google Scholar] [CrossRef]
- dos Santos, S.M.; Wainstein, R.V.; Valle, F.H.; Corrêa, C.L.; Aliti, G.B.; Ruschel, K.B.; Gonçalves, S.C.; Wainstein, M.V.; Rabelo-Silva, E.R. Two HEmostasis Methods After TransradIal Catheterization: THEMATIC Randomized Clinical Trial. J. Cardiovasc. Nurs. 2020, 35, 217–222. [Google Scholar] [CrossRef]
- Markovic, S.; Imhof, A.; Kunze, M.; Rottbauer, W.; Wöhrle, J. Standardized radial approach reduces access site complications: A prospective observational registry. Coron. Artery Dis. 2015, 26, 56. [Google Scholar] [CrossRef]
- Sanhoury, M.I.; Sobhy, M.A.; Saddaka, M.A.; Nassar, M.A.; Elwany, M.N. Distal radial approach between theory and clinical practice. Time to go distal! Egypt. Heart J. EHJ Off. Bull. Egypt. Soc. Cardiol. 2022, 74, 8. [Google Scholar] [CrossRef]
- Batchelor, W.; Dahya, V.; McGee, D.; Katopodis, J.; Dixon, W.; Campbell, J.; Meredith, A.; Knap, P.; Parkin, M.; Noel, T. Ultrahigh-resolution ultrasound characterization of access site trauma and intimal hyperplasia following use of a 7F sheathless guide versus 6F sheath/guide combination for transradial artery PCI: Results of the PRAGMATIC trial. Am. Heart J. 2018, 198, 75–83. [Google Scholar] [CrossRef]
- Dharma, S.; Kedev, S.; Patel, T.; Rao, S.V.; Gilchrist, I.C. Different Spasmolytic Regimens (Nitroglycerin vs Verapamil) and the Incidence of Radial Artery Occlusion After Transradial Catheterization. J. Invasive Cardiol. 2018, 30, 461–464. [Google Scholar]
- Eid-Lidt, G.; Reyes-Carrera, J.; Farjat-Pasos, J.I.; Saenz, A.L.; Bravo, C.A.; Rangel, S.N.; Salido, D.Z.; Servin, N.S.V.; Soto-López, M.E.; Gaspar, J. Prevention of Radial Artery Occlusion of 3 Hemostatic Methods in Transradial Intervention for Coronary Angiography. JACC Cardiovasc. Interv. 2022, 15, 1022–1029. [Google Scholar] [CrossRef]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- de Sá, B.J.L.; Barros, L.d.F.; Brandão, S.C.S.; Victor, E.G. Interference of Reprocessed Introducers in Radial Artery Occlusion after Cardiac Catheterization. Rev. Bras. Cardiol. Invasiva Engl. Ed. 2013, 21, 270–275. [Google Scholar] [CrossRef]
- Gokhroo, R.; Kishor, K.; Ranwa, B.; Bisht, D.; Gupta, S.; Padmanabhan, D.; Avinash, A. Ulnar Artery Interventions Non-Inferior to Radial Approach: AJmer Ulnar ARtery (AJULAR) Intervention Working Group Study Results. J. Invasive Cardiol. 2016, 28, 1–8. [Google Scholar]
- Pacchioni, A.; Mugnolo, A.; Sanchez, J.S.; Sgueglia, G.A.; Pesarini, G.; Bellamoli, M.; Saccà, S.; Ribichini, F.; Reimers, B.; Gasparini, G.L. Radial artery occlusion after conventional and distal radial access: Impact of preserved flow and time-to-hemostasis in a propensity-score matching analysis of 1163 patients. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 99, 827–835. [Google Scholar] [CrossRef]
- Pancholy, S.B.; Ahmed, I.; Bertrand, O.F.; Patel, T. Frequency of Radial Artery Occlusion After Transradial Access in Patients Receiving Warfarin Therapy and Undergoing Coronary Angiography. Am. J. Cardiol. 2014, 113, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, V.; Xanthopoulos, A.; Papamichalis, M.; Skoularigkis, S.; Tzavara, C.; Papadakis, E.; Patsilinakos, S.; Triposkiadis, F.; Skoularigis, J. Patent hemostasis of radial artery: Comparison of two methods. World J. Cardiol. 2021, 13, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Buturak, A.; Gorgulu, S.; Norgaz, T.; Voyvoda, N.; Sahingoz, Y.; Degirmencioglu, A.; Dagdelen, S. The long-term incidence and predictors of radial artery occlusion following a transradial coronary procedure. Cardiol. J. 2014, 21, 350–356. [Google Scholar] [CrossRef]
- Monsegu, J.; Hamon, M.; Diaz, J.F.; Briki, R.; Masotti, M.; Valdes, M.; Louvard, Y.; Hildick-Smith, D. TCT-418 Doppler Control of Radial Artery After Use of TR Band Following Coronarography and/or Angioplasty: DRABAND study results. J. Am. Coll. Cardiol. 2012, 60 (Suppl. S17), B119. [Google Scholar] [CrossRef]
- Dharma, S.; Kedev, S.; Patel, T.; Kiemeneij, F.; Gilchrist, I.C. A novel approach to reduce radial artery occlusion after transradial catheterization: Postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter. Cardiovasc. Interv. 2015, 85, 818–825. [Google Scholar] [CrossRef]
- Ognerubov, D.V.; Sedaghat, A.; Provatorov, S.I.; Tereshchenko, A.S.; Bertrand, O.F.; Bernat, I.; Arutyunyan, G.K.; Pogorelova, O.A.; Tripoten, M.I.; Balakhonova, T.V.; et al. A Randomized Trial Comparing Short versus Prolonged Hemostasis with Rescue Recanalization by Ipsilateral Ulnar Artery Compression: Impact on Radial Artery Occlusion—The RESCUE-RAO Trial. J. Intervent. Cardiol. 2020, 2020, e7928961. [Google Scholar] [CrossRef]
- Jirous, S.; Bernat, I.; Slezak, D.; Miklik, R.; Rokyta, R. Post-procedural radial artery occlusion and patency detection using duplex ultrasound vs. the reverse Barbeau test. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2020, 22, F23–F29. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.d.C.; Alves, C.M.R.; Tsunemi, M.H.; Peterlini, M.A.S.; Avelar, A.F.M. Randomized clinical study on radial artery compression time after elective coronary angiography. Rev. Lat. Am. Enfermagem 2018, 26, e3084. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, A.; Mautong, H.; Ahmed, K.; Aloul, Z.; Montero-Cabezas, J.; Marasco, S. Incidence and Predictors of Early and Late Radial Artery Occlusion after Percutaneous Coronary Intervention and Coronary Angiography: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5882. https://doi.org/10.3390/jcm13195882
Khalid A, Mautong H, Ahmed K, Aloul Z, Montero-Cabezas J, Marasco S. Incidence and Predictors of Early and Late Radial Artery Occlusion after Percutaneous Coronary Intervention and Coronary Angiography: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(19):5882. https://doi.org/10.3390/jcm13195882
Chicago/Turabian StyleKhalid, Aisha, Hans Mautong, Kayode Ahmed, Zaina Aloul, Jose Montero-Cabezas, and Silvana Marasco. 2024. "Incidence and Predictors of Early and Late Radial Artery Occlusion after Percutaneous Coronary Intervention and Coronary Angiography: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 19: 5882. https://doi.org/10.3390/jcm13195882





