Combined Use of GDF-15 and NT-Pro BNP for Outcome Prediction in Patients with Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Biochemical Analysis
2.3. Statistical Analysis
2.4. Follow-Up
3. Results
3.1. Study Population
3.2. Association of GDF-15 and NT-Pro BNP Levels with Study Endpoints
- (1)
- GDF-15 and NT-pro BNP levels below the cut-off value;
- (2)
- Only GDF-15 levels above the cut-off value;
- (3)
- Only NT-pro BNP levels above the cut-off value;
- (4)
- Both GDF-15 and NT-pro BNP levels above the cut-off value.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al.; ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Lelonek, M.; Grabowski, M.; Kasprzak, J.; Leszek, P.; Nessler, J.; Pawlak, A.; Rozentryt, P.; Straburzynska-Migaj, E.; Rubiś, P. An expert opinion of the Heart Failure Association of the Polish Cardiac Society on the 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure: Heart failure guidelines from a national perspective. Kardiol. Pol. 2022, 80, 239–246. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.; Coats, A.J. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef]
- Wussler, D.; Michou, E.; Belkin, M.; Kozhuharov, N.; Diebold, M.; Gualandro, D.M.; Breidthardt, T.; Mueller, C. Mortality prediction in acute heart failure: Scores or biomarkers? Swiss. Med. Wkly. 2020, 150, w20320. [Google Scholar] [CrossRef]
- Javaheri, A.; Ozcan, M.; Moubarak, L.; Smoyer, K.E.; Rossulek, M.I.; Revkin, J.H.; Groarke, J.D.; Tarasenko, L.C.; Kosiborod, M.N. Association between growth differentiation factor-15 and adverse outcomes among patients with heart failure: A systematic literature review. Heliyon 2024, 10, e35916. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Cheang, I.; Zhang, L.; Wang, K.; Wang, H.-M.; Wu, Q.-Y.; Zhou, Y.-L.; Zhou, F.; Xu, D.-J.; Zhang, H.-F.; et al. Growth differentiation factor-15 combined with N-terminal prohormone of brain natriuretic peptide increase 1-year prognosis prediction value for patients with acute heart failure. Chin. Med. J. 2019, 132, 2278–2285. [Google Scholar] [CrossRef]
- Hromas, R.; Hufford, M.; Sutton, J.; Xu, D.; Li, Y.; Lu, L. PLAB, a novel placental bone morphogenetic protein. Biochim. Biophys. Acta 1997, 1354, 40–44. [Google Scholar] [CrossRef]
- Moore, A.G.; Brown, D.A.; Fairlie, W.D.; Bauskin, A.R.; Brown, P.K.; Munier, M.L.C.; Russell, P.K.; Salamonsen, L.A.; Wallace, E.M.; Breit, S.N. The transforming growth factor-β superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J. Clin. Endocrinol. Metab. 2000, 85, 4781–4788. [Google Scholar]
- Fuchs, T.; Trollor, J.N.; Crawford, J.; Brown, D.A.; Baune, B.T.; Samaras, K.; Campbell, L.; Breit, S.N.; Brodaty, H.; Sachdev, P.; et al. Macrophage inhibitory cytokine-1 is associated with cognitive impairment and predicts cognitive decline—The Sydney Memory and Aging Study. Aging Cell 2013, 12, 882–889. [Google Scholar] [CrossRef]
- Desmedt, S.; Desmedt, V.; De Vos, L.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Growth differentiation factor 15, A novel biomarker with high clinical potential. Crit. Rev. Clin. Lab. Sci. 2019, 56, 333–350. [Google Scholar] [CrossRef]
- Piechota, W.; Krzesiński, P. Growth differentiation factor 15 as a biomarker in heart failure. Folia Cardiol. 2018, 13, 174–180. (In Polish) [Google Scholar] [CrossRef]
- Wollert, K.; Kempf, T.; Wallentin, L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Skowrońska, M.; Skrzyńska, M.; Machowski, M.; Bartoszewicz, Z.; Paczyńska, M.; Ou-Pokrzewińska, A.; Kurnicka, K.; Ciurzyński, M.; Roik, M.; Wiśniewska, M.; et al. Plasma growth differentiation factor 15 levels for predicting serious adverse events and bleeding in acute pulmonary embolism: A prospective observational study. Pol. Arch. Intern. Med. 2020, 130, 757–765. [Google Scholar] [PubMed]
- Wollert, K.C.; Kempf, T.; Peter, T.; Olofsson, S.; James, S.; Johnston, N.; Lindahl, B.; Horn-Wichmann, R.; Brabant, G.; Simoons, M.L.; et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation 2007, 115, 962–971. [Google Scholar] [CrossRef]
- Hagström, E.; Held, C.; Stewart, R.A.H.; Aylward, P.E.; Budaj, A.; Cannon, C.P.; Koenig, W.; Krug-Gourley, S.; Mohler, E.R.; Steg, P.G.; et al. STABILITY Investigators. Growth differentiation factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin. Chem. 2017, 63, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef]
- Liang, W.; Wei, F.; Yang, C.; Xie, F.; Shuai, X.-X.; Wang, M.; Yu, M. GDF-15 is associated with thrombus burden in patients with deep venous thrombosis. Thromb. Res. 2020, 187, 148–153. [Google Scholar] [CrossRef]
- Hijazi, Z.; Oldgren, J.; Andersson, U.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Reilly, P.A.; Yusuf, S.; Siegbahn, A.; Wallentin, L. Growth-differentiation factor 15 and risk of major bleeding in atrial fibrillation: Insights from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Am. Heart J. 2017, 190, 94–103. [Google Scholar] [CrossRef]
- Lindholm, D.; Hagström, E.; James, S.K.; Becker, R.C.; Cannon, C.P.; Himmelmann, A.; Katus, H.A.; Maurer, G.; López-Sendón, J.L.; Steg, P.G.; et al. Growth differentiation factor 15 at 1 month after an acute coronary syndrome is associated with increased risk of major bleeding. J. Am. Heart Assoc. 2017, 6, e005580. [Google Scholar] [CrossRef]
- Sharma, A.; Stevens, S.; Lucas, J.; Fiuzat, M.; Adams, K.F.; Whellan, D.J.; Donahue, M.P.; Kitzman, D.W.; Piña, I.L.; Zannad, F.; et al. Utility of growth differentiation factor-15, a marker of oxidative stress and inflammation, in chronic heart failure: Insights from the HF-ACTION study. JACC Heart Fail. 2017, 5, 724–734. [Google Scholar] [CrossRef]
- Chan, M.M.; Santhanakrishnan, R.; Chong, J.P.; Chen, Z.; Tai, B.C.; Liew, O.W.; Ng, T.P.; Ling, L.H.; Sim, D.; Leong, K.T.G.; et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 2016, 18, 81–88. [Google Scholar] [CrossRef]
- Takaoka, M.; Tadross, J.A.; Al-Hadithi, A.B.A.K.; Zhao, X.; Villena-Gutiérrez, R.; Tromp, J.; Absar, S.; Au, M.; Harrison, J.; Harrison, A.P.; et al. GDF15 antagonism limits severe heart failure and prevents cardiac cachexia. Cardiovasc. Res. 2024, cvae214. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Jarolim, P.; Palazzolo, M.G.; Bellavia, A.; Antman, E.M.; Eikelboom, J.; Granger, G.B.; Harrington, J.; Healey, J.; Hijazi, Z.; et al. Biomarkers for Heart failure in Atrial Fibrillation. J. Am. Coll. Cardiol. 2024, in press. [Google Scholar]
- McDowell, K.; Campbell, R.; Simpson, J.; Cunningham, J.W.; Desai, A.S.; Jhund, P.S.; Lefkowitz, M.P.; Rouleau, J.L.; Swedberg, K.; Zile, M.R.; et al. Incremental prognostic value of biomarkers in PARADIGM-HF. Eur. J. Heart Fail. 2023, 25, 1406–1414. [Google Scholar] [CrossRef]
- Noveanu, M.; Breidthardt, T.; Potocki, M.; Reichlin, T.; Twerenbold, R.; Uthoff, H.; Socrates, T.; Arenja, N.; Reiter, M.; Meissner, J. Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and long-term outcome in acute decompensated heart failure. Crit. Care 2011, 15, R1. [Google Scholar] [CrossRef]
- Demissei, B.G.; Cotter, G.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Severin, T.M.; Wang, Y.; et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: Results from the RELAX-AHF trial. Eur. J. Heart Fail. 2017, 19, 1001–1010. [Google Scholar] [CrossRef]
- Gürgöze, M.T.; van Vark, L.C.; Baart, S.J.; Kardys, I.; Akkerhuis, K.M.; Manintveld, O.C.; Postmus, D.; Hillege, H.L.; Lesman-Leegte, I.; Asselbergs, F.W.; et al. Multimarker analysis of serially measured GDF-15, NT-proBNP, ST2, GAL-3, cTnI, creatinine, and prognosis in acute heart failure. Circ. Heart Fail. 2023, 16, e009526. [Google Scholar] [CrossRef]
- Bettencourt, P.; Ferreira-Coimbra, J.; Rodrigues, P.; Marques, P.; Moreira, H.; Pinto, M.J.; Guimarães, J.T.; Lourenço, P. Towards a multi-marker prognostic strategy in acute heart failure: A role for GDF-15. ESC Heart Fail. 2018, 5, 1017–1022. [Google Scholar] [CrossRef]
| Variable | Event-Free Patients (n = 73) | Death or HF Rehospitalization (n = 31) | p-Value |
|---|---|---|---|
| Demographic data | |||
| Age, years, mean (SD) | 63 (±17) | 71 (±7) | 0.04 |
| Male, n (%) | 50 (69) | 27 (81) | 0.05 |
| New onset AHF, n (%) | 40 (55) | 11 (36) | 0.07 |
| BMI, kg/m2, mean (SD) | 31 (±6) | 27 (±5) | 0.02 |
| Medical history | |||
| Hypertension, n (%) | 48 (66) | 24 (77) | 0.24 |
| CAD, n (%) | 24 (33) | 21 (70) | 0.11 |
| Diabetes, n (%) | 22 (30) | 17 (55) | 0.02 |
| CKD, n (%) | 15 (21) | 14 (45) | 0.01 |
| Atrial fibrillation, n (%) | 32 (44) | 16 (52) | 0.47 |
| ICD/CRTD/CRT, n (%) | 12 (16) | 12 (39) | 0.35 |
| Etiology of AHF | |||
| Ischemic, n (%) | 25 (34) | 22 (71) | <0.001 |
| Valve disease, n (%) | 25 (34) | 17 (55) | 0.05 |
| Inflammatory, n (%) | 14 (19) | 1 (3) | 0.15 |
| DCM, n (%) | 6 (8) | 2 (7) | 0.76 |
| Arrhythmic, n (%) | 9 (12) | 5 (16) | 0.60 |
| Hospitalization data | |||
| HR, bpm, mean (SD) | 110 (±28) | 94 (±33) | 0.03 |
| SBP, mmHg, mean (SD) | 135 (±25) | 123 (±25) | 0.04 |
| NYHA class IV, n (%) | 47 (64) | 24 (77) | 0.25 |
| Hospital stay, days median (IQR) | 15 (12–23) | 15 (8–28) | 0.35 |
| Norepinephrine, n (%) | 8 (11) | 8 (26) | 0.06 |
| Dobutamine, n (%) | 6 (8) | 7 623) | 0.04 |
| Diuretics iv, n (%) | 68 (93) | 30 (97) | 0.47 |
| MCS–IABP, n (%) | 4 (6) | 2 (7) | 0.85 |
| β-blocker, n (%) | 67 (92) | 24 (77) | 0.04 |
| ARB, n (%) | 7 (10) | 2 (7) | 0.60 |
| ACEI, n (%) | 51 (70) | 15 (48) | 0.04 |
| MRA, n (%) | 54 (73) | 9 (29) | 0.05 |
| ARNI, n (%) | 8 (11) | 8 (26) | 0.06 |
| SGLT2 inhibitor, n (%) | 6 (8) | 1 (3) | 0.35 |
| Laboratory and echocardiographic parameters | |||
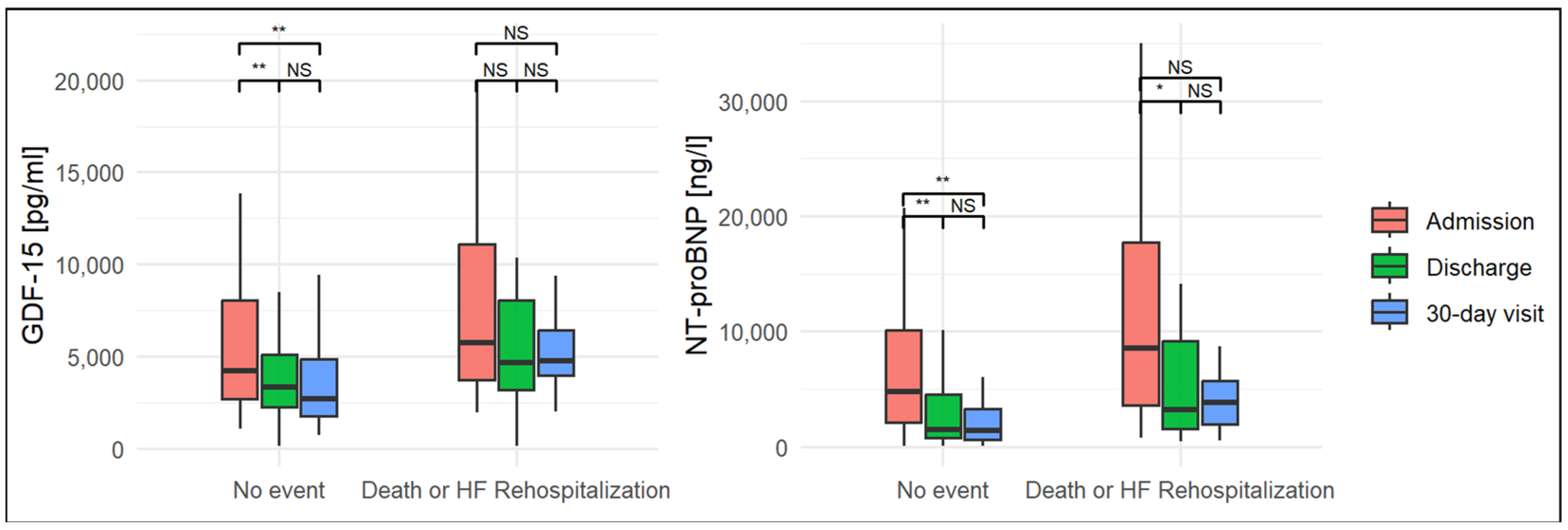
| GDF-15, pg/mL, median (IQR) | |||
| admission | 4216 (2717–8036) | 5735 (3704–11,103) | 0.03 |
| discharge | 3351 (2267–5108) | 4667 (3195–8056) | 0.10 |
| 30-day visit | 2718 (1741–4854) | 4736 (3988–6438) | 0.01 |
| NT-pro BNP, ng/L, median (IQR) | |||
| admission | 4805 (2124–10,108) | 8522 (3628–17,762) | 0.02 |
| discharge | 1452 (801–4538) | 3222 (1583–9193) | 0.03 |
| 30-day visit | 1412 (648–3249) | 3843 (1920–5744) | 0.01 |
| hsTnT, ng/L, median (IQR) admission | 41 (27–133) | 80 (31–533) | 0.09 |
| Creatinine, mg/dL, median (IQR) admission | 1.2 (0.97–1.36) | 1.3 (1.1–2.2) | 0.04 |
| Bilirubin, mg/dL, median (IQR) admission | 0.7 (0.53–1.42) | 1.5 (0.72–1.79) | 0.05 |
| Hemoglobin, g/dL, median (IQR) admission | 14 (11–15) | 12 (10.7–13.6) | 0.02 |
| LVEF, %, mean (SD) | 32 (±15) | 28 (±10) | 0.31 |
| LVEDd, mm, mean (SD) admission | 60 (±9) | 61 (±10) | 0.45 |
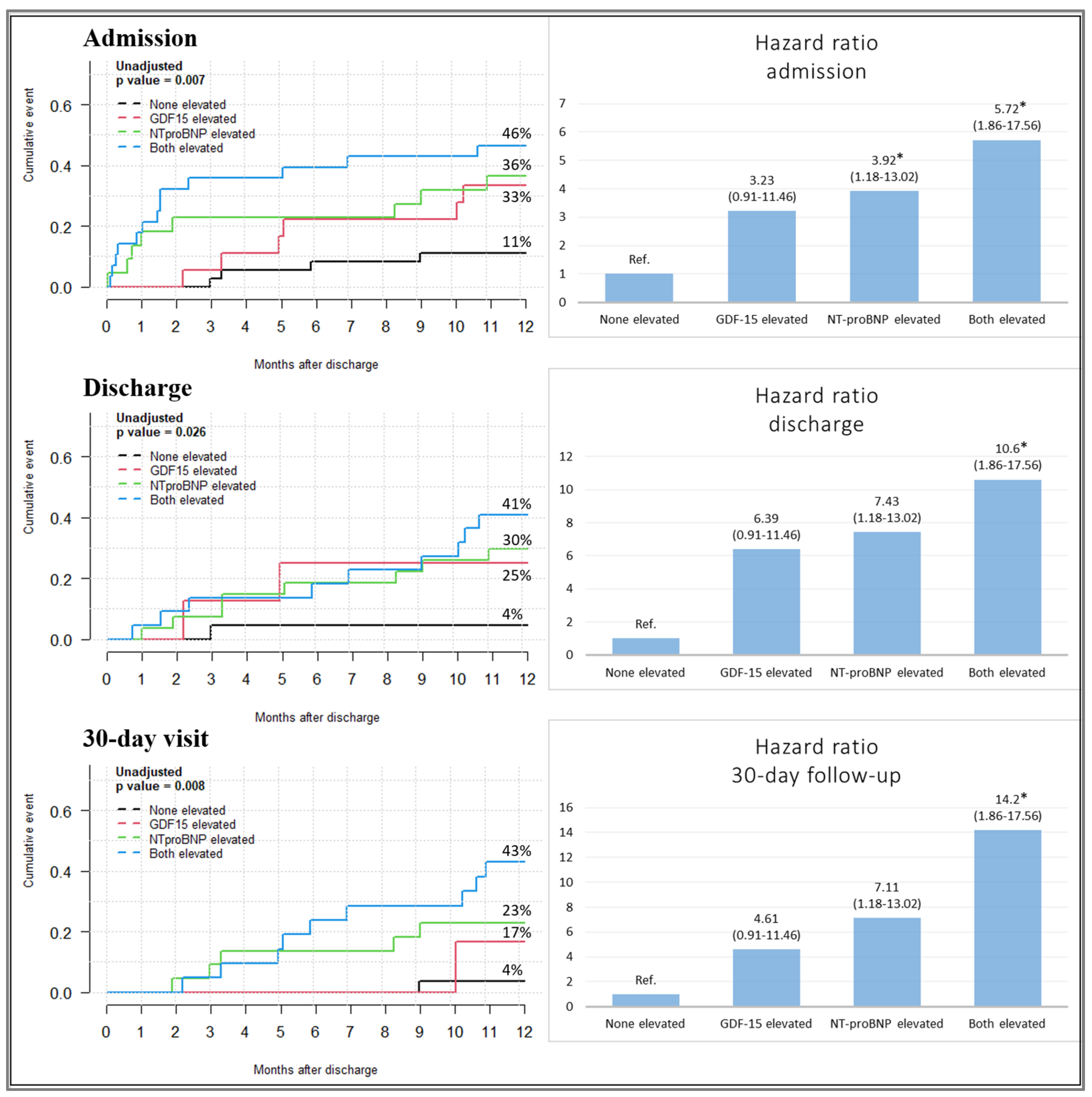
| Univariable Model | ||
|---|---|---|
| Variable | HR (95% CI) | p-value |
| NT-pro BNP and GDF-15 elevated admission | 5.56 (1.82–17.18) | 0.01 |
| NT-pro BNP and GDF-15 elevated discharge | 10.81 (1.37–85.41) | 0.02 |
| NT-pro BNP and GDF-15 elevated 30-day visit | 14.57 (1.84–115.23) | 0.01 |
| Age | 1.03 (1.01–1.06) | 0.02 |
| Ischemic etiology | 3.63 (1.67–7.89) | 0.001 |
| Diabetes | 2.31 (1.14–4.69) | 0.02 |
| Multivariable model | ||
| Variable | HR (95% CI) | p-value |
| Admission | ||
| NT-pro BNP and GDF-15 elevated | 4.38 (1.24–15.5) | 0.02 |
| Age | 1.03 (0.98–1.08) | 0.25 |
| Ischemic etiology | 2.35 (0.69–8.02) | 0.17 |
| Diabetes | 0.88 (0.24–3.17) | 0.88 |
| Discharge | ||
| NT-pro BNP and GDF-15 elevated | 5.62 (0.55–50.10) | 0.15 |
| Age | 1.05 (0.97–1.12) | 0.24 |
| Ischemic etiology | 0.43 (0.11–1.72) | 0.23 |
| Diabetes | 4.86 (0.77–30.67) | 0.09 |
| 30-day visit | ||
| NT-pro BNP and GDF-15 elevated | 10.15 (0.91–112.72) | 0.06 |
| Age | 1.00 (0.93–1.09) | 0.89 |
| Ischemic etiology | 1.42 (0.27–7.45) | 0.62 |
| Diabetes | 1.34 (0.28–6.46) | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płonka, J.; Klus, A.; Wężyk, N.; Dąbrowska, K.; Rzepiela, L.; Gawrylak-Dryja, E.; Nalewajko, K.; Feusette, P.; Gierlotka, M. Combined Use of GDF-15 and NT-Pro BNP for Outcome Prediction in Patients with Acute Heart Failure. J. Clin. Med. 2024, 13, 5936. https://doi.org/10.3390/jcm13195936
Płonka J, Klus A, Wężyk N, Dąbrowska K, Rzepiela L, Gawrylak-Dryja E, Nalewajko K, Feusette P, Gierlotka M. Combined Use of GDF-15 and NT-Pro BNP for Outcome Prediction in Patients with Acute Heart Failure. Journal of Clinical Medicine. 2024; 13(19):5936. https://doi.org/10.3390/jcm13195936
Chicago/Turabian StylePłonka, Joanna, Anna Klus, Natalia Wężyk, Klaudia Dąbrowska, Lidia Rzepiela, Ewa Gawrylak-Dryja, Krzysztof Nalewajko, Piotr Feusette, and Marek Gierlotka. 2024. "Combined Use of GDF-15 and NT-Pro BNP for Outcome Prediction in Patients with Acute Heart Failure" Journal of Clinical Medicine 13, no. 19: 5936. https://doi.org/10.3390/jcm13195936







