Present and Future Applications of Artificial Intelligence in Kidney Transplantation
Abstract
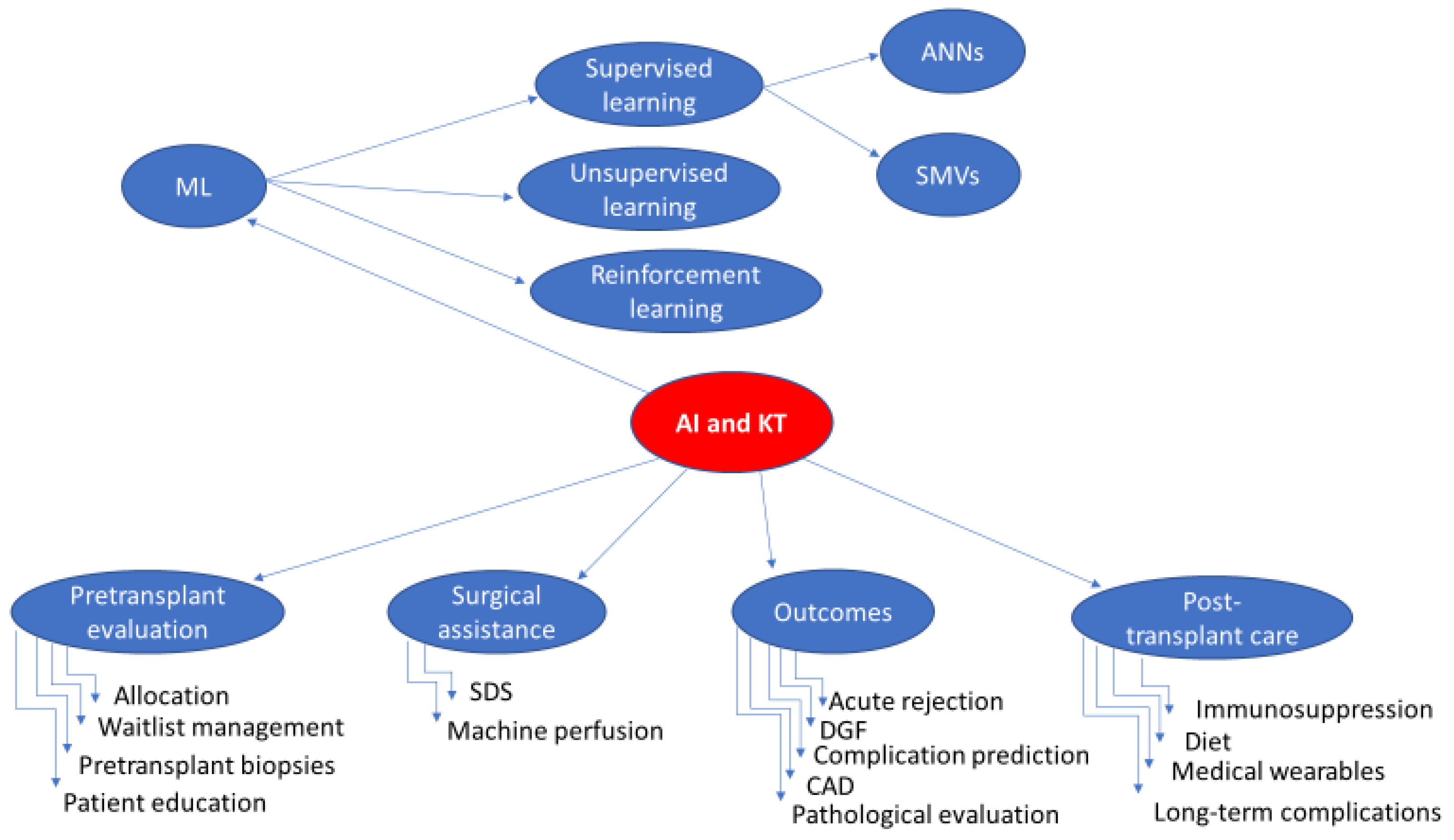
:1. Introduction
2. Current and Evolving Applications of AI in KT
2.1. Pretransplant Evaluation of Donors/Recipients
2.1.1. Allocation and Donor-Recipient Matching
2.1.2. Waitlist Management
2.1.3. Interpretation of Preoperative Grafts Biopsies
2.1.4. Patient Education
2.2. Surgical Assistance
2.2.1. AI-Powered Surgical Assistance Systems
2.2.2. Machine Perfusion
2.3. Outcomes
2.3.1. Earlier Acute Rejection Detection and Diagnosis
2.3.2. Delayed Graft Function Prediction
2.3.3. Post-Transplant Complication Prediction
2.3.4. Computer-Aided Diagnosis (CAD)
2.3.5. Pathological Evaluation of the Allograft
| Author, Year | Objective | AI method Used | Performance |
|---|---|---|---|
| Furness [83], 1999 | AR diagnosis | Neural network | 19/21 correct diagnoses |
| Hermsen [81], 2019 | Multiclass segmentation performance | CNNs/DL | DC 0.80/0.84 |
| Liu [84], 2019 | TCMR diagnosis | RNA-Seq-based ML | 55/67 TCMR 65/105 ABMR |
| Kim [85], 2019 | AR diagnosis | CNNs/DL | Sensitivity 0.7951 Specificity 0.9941 |
| Ligabue [86], 2020 | Kidney immunofluorescence reporting | CNNs/DL | Accuracy: 0.79–0.94 in different feature prediction |
| Wilbur [87], 2021 | Glomeruli identification | CNNs | Sensitivity 86% Specificity 92% |
| Kers [88], 2022 | Normal vs. rejection vs. other diseases | CNNs/DL | Rejection: AUROC (0.75 [0.73–0.76]) |
| Smith [82], 2023 | Non-glomerular inflammation quantification | CNNs | DC 0.858 |
2.4. Post-Transplant Care
2.4.1. Immunosuppressive Therapy
Tacrolimus
Cyclosporin
MMF
2.4.2. Dietary Issues
2.4.3. Medical Wearables
2.4.4. Prediction of Long-Term Outcomes
3. Future Applications of AI in KT
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badrouchi, S.; Bacha, M.M.; Hedri, H.; Ben Abdallah, T.; Abderrahim, E. Toward generalizing the use of artificial intelligence in nephrology and kidney transplantation. J. Nephrol. 2023, 36, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, S. Artificial Intelligence and Computer Vision. In Learn Computer Vision Using OpenCV: With Deep Learning CNNs and RNNs; Gollapudi, S., Ed.; Apress: Berkeley, CA, USA, 2019; pp. 1–29. [Google Scholar] [CrossRef]
- Rawashdeh, B.; Kim, J.; AlRyalat, S.A.; Prasad, R.; Cooper, M. ChatGPT and Artificial Intelligence in Transplantation Research: Is It Always Correct? Cureus 2023, 15, e42150. [Google Scholar] [CrossRef] [PubMed]
- Niel, O.; Bastard, P. Artificial Intelligence in Nephrology: Core Concepts, Clinical Applications, and Perspectives. Am. J. Kidney Dis. 2019, 74, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Seyahi, N.; Ozcan, S.G. Artificial intelligence and kidney transplantation. World J. Transplant. 2021, 11, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, A.; Iftene, A.; Jugrin, D.; Popa, I.V.; Lupu, P.M.; Vlad, C.; Covic, A. Using Artificial Intelligence Resources in Dialysis and Kidney Transplant Patients: A Literature Review. Biomed. Res. Int. 2020, 2020, 9867872. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sorstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Kim, S.H.; Jo, M.W.; Go, D.S.; Ryu, D.R.; Park, J. Economic burden of chronic kidney disease in Korea using national sample cohort. J. Nephrol. 2017, 30, 787–793. [Google Scholar] [CrossRef]
- Hueso, M.; Vellido, A.; Montero, N.; Barbieri, C.; Ramos, R.; Angoso, M.; Cruzado, J.M.; Jonsson, A. Artificial Intelligence for the Artificial Kidney: Pointers to the Future of a Personalized Hemodialysis Therapy. Kidney Dis. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.S.; Li, J.R.; Wang, S.S.; Chen, C.S.; Yang, C.K.; Lin, C.Y.; Hung, S.C.; Ho, H.C.; Ou, Y.C.; Chiu, K.Y.; et al. Influence of Surgical Complications on Outcomes in Kidney Transplantation Patients. In Vivo 2023, 37, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Voora, S.; Adey, D.B. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Schaubel, D.E.; Guidinger, M.K.; Andreoni, K.A.; Wolfe, R.A.; Merion, R.M.; Port, F.K.; Sung, R.S. A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 2009, 88, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Biggins, S.W.; Haselby, D.G.; Kim, W.R.; Wedd, J.; Lamb, K.; Thompson, B.; Segev, D.L.; Gustafson, S.; Kandaswamy, R.; et al. Kidney, pancreas and liver allocation and distribution in the United States. Am. J. Transplant. 2012, 12, 3191–3212. [Google Scholar] [CrossRef]
- Clayton, P.A.; McDonald, S.P.; Snyder, J.J.; Salkowski, N.; Chadban, S.J. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am. J. Transplant. 2014, 14, 1922–1926. [Google Scholar] [CrossRef]
- Stegall, M.D.; Stock, P.G.; Andreoni, K.; Friedewald, J.J.; Leichtman, A.B. Why do we have the kidney allocation system we have today? A history of the 2014 kidney allocation system. Hum. Immunol. 2017, 78, 4–8. [Google Scholar] [CrossRef]
- Schwantes, I.R.; Axelrod, D.A. Technology-Enabled Care and Artificial Intelligence in Kidney Transplantation. Curr. Transplant. Rep. 2021, 8, 235–240. [Google Scholar] [CrossRef]
- Bae, S.; Massie, A.B.; Luo, X.; Anjum, S.; Desai, N.M.; Segev, D.L. Changes in Discard Rate After the Introduction of the Kidney Donor Profile Index (KDPI). Am. J. Transplant. 2016, 16, 2202–2207. [Google Scholar] [CrossRef]
- Ali, H.; Mohamed, M.; Molnar, M.Z.; Fulop, T.; Burke, B.; Shroff, A.; Shroff, S.; Briggs, D.; Krishnan, N. Deceased-Donor Kidney Transplant Outcome Prediction Using Artificial Intelligence to Aid Decision-Making in Kidney Allocation. ASAIO J. 2024, 70, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Massie, A.B.; Thomas, A.G.; Bahn, G.; Luo, X.; Jackson, K.R.; Ottmann, S.E.; Brennan, D.C.; Desai, N.M.; Coresh, J.; et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am. J. Transplant. 2019, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Elster, E.A.; Stevens, K.; Graybill, J.C.; Gillern, S.; Phinney, S.; Salifu, M.O.; Jindal, R.M. Bayesian modeling of pretransplant variables accurately predicts kidney graft survival. Am. J. Nephrol. 2012, 36, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kilambi, V.; Bui, K.; Hazen, G.B.; Friedewald, J.J.; Ladner, D.P.; Kaplan, B.; Mehrotra, S. Evaluation of Accepting Kidneys of Varying Quality for Transplantation or Expedited Placement With Decision Trees. Transplantation 2019, 103, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Mark, E.; Goldsman, D.; Gurbaxani, B.; Keskinocak, P.; Sokol, J. Using machine learning and an ensemble of methods to predict kidney transplant survival. PLoS ONE 2019, 14, e0209068. [Google Scholar] [CrossRef]
- Paquette, F.X.; Ghassemi, A.; Bukhtiyarova, O.; Cisse, M.; Gagnon, N.; Della Vecchia, A.; Rabearivelo, H.A.; Loudiyi, Y. Machine Learning Support for Decision-Making in Kidney Transplantation: Step-by-step Development of a Technological Solution. JMIR Med. Inform. 2022, 10, e34554. [Google Scholar] [CrossRef]
- Sapiertein Silva, J.F.; Ferreira, G.F.; Perosa, M.; Nga, H.S.; de Andrade, L.G.M. A machine learning prediction model for waiting time to kidney transplant. PLoS ONE 2021, 16, e0252069. [Google Scholar] [CrossRef]
- Heston, T.F.; Norman, D.J.; Barry, J.M.; Bennett, W.M.; Wilson, R.A. Cardiac risk stratification in renal transplantation using a form of artificial intelligence. Am. J. Cardiol. 1997, 79, 415–417. [Google Scholar] [CrossRef]
- Moeckli, B.; Sun, P.; Lazeyras, F.; Morel, P.; Moll, S.; Pascual, M.; Buhler, L.H. Evaluation of donor kidneys prior to transplantation: An update of current and emerging methods. Transpl. Int. 2019, 32, 459–469. [Google Scholar] [CrossRef]
- Stewart, Z.A.; Shah, S.A.; Formica, R.N.; Kandaswamy, R.; Paramesh, A.S.; Friedman, J.; Squires, R.; Cooper, M.; Axelrod, D.A. A call to action: Feasible strategies to reduce the discard of transplantable kidneys in the United States. Clin. Transplant. 2020, 34, e13990. [Google Scholar] [CrossRef]
- Husain, S.A.; Chiles, M.C.; Lee, S.; Pastan, S.O.; Patzer, R.E.; Tanriover, B.; Ratner, L.E.; Mohan, S. Characteristics and Performance of Unilateral Kidney Transplants from Deceased Donors. Clin. J. Am. Soc. Nephrol. 2018, 13, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Sanchez, C.I.; Timofeeva, N.; Hermsen, M.; Nagtegaal, I.; Kovacs, I.; Hulsbergen-van de Kaa, C.; Bult, P.; van Ginneken, B.; van der Laak, J. Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Sci. Rep. 2016, 6, 26286. [Google Scholar] [CrossRef] [PubMed]
- Serag, A.; Ion-Margineanu, A.; Qureshi, H.; McMillan, R.; Saint Martin, M.J.; Diamond, J.; O’Reilly, P.; Hamilton, P. Translational AI and Deep Learning in Diagnostic Pathology. Front. Med. 2019, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.N.; Liu, T.C.; Wilson, P.C.; Swamidass, S.J.; Gaut, J.P. Development and Validation of a Deep Learning Model to Quantify Glomerulosclerosis in Kidney Biopsy Specimens. JAMA Netw. Open 2021, 4, e2030939. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Mogetta, A.; Meiburger, K.M.; Gambella, A.; Molinaro, L.; Barreca, A.; Papotti, M.; Molinari, F. Karpinski Score under Digital Investigation: A Fully Automated Segmentation Algorithm to Identify Vascular and Stromal Injury of Donors’ Kidneys. Electronics 2020, 9, 1644. [Google Scholar] [CrossRef]
- Salvi, M.; Mogetta, A.; Gambella, A.; Molinaro, L.; Barreca, A.; Papotti, M.; Molinari, F. Automated assessment of glomerulosclerosis and tubular atrophy using deep learning. Comput. Med. Imaging Graph. 2021, 90, 101930. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Marletta, S.; Hermsen, M.; van der Laak, J.; Munari, E.; Furian, L.; Vistoli, F.; Zaza, G.; Cardillo, M.; et al. Artificial intelligence applications for pre-implantation kidney biopsy pathology practice: A systematic review. J. Nephrol. 2022, 35, 1801–1808. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Kirchner, G.J.; Kim, R.Y.; Weddle, J.B.; Bible, J.E. Can Artificial Intelligence Improve the Readability of Patient Education Materials? Clin. Orthop. Relat. Res. 2023, 481, 2260–2267. [Google Scholar] [CrossRef]
- Chew, H.S.J. The Use of Artificial Intelligence-Based Conversational Agents (Chatbots) for Weight Loss: Scoping Review and Practical Recommendations. JMIR Med. Inform. 2022, 10, e32578. [Google Scholar] [CrossRef]
- Zhang, J.; Oh, Y.J.; Lange, P.; Yu, Z.; Fukuoka, Y. Artificial Intelligence Chatbot Behavior Change Model for Designing Artificial Intelligence Chatbots to Promote Physical Activity and a Healthy Diet: Viewpoint. J. Med. Internet Res. 2020, 22, e22845. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hein, L.; Vedula, S.S.; Speidel, S.; Navab, N.; Kikinis, R.; Park, A.; Eisenmann, M.; Feussner, H.; Forestier, G.; Giannarou, S.; et al. Surgical data science for next-generation interventions. Nat. Biomed. Eng. 2017, 1, 691–696. [Google Scholar] [CrossRef] [PubMed]
- De Backer, P.; Van Praet, C.; Simoens, J.; Peraire Lores, M.; Creemers, H.; Mestdagh, K.; Allaeys, C.; Vermijs, S.; Piazza, P.; Mottaran, A.; et al. Improving Augmented Reality Through Deep Learning: Real-time Instrument Delineation in Robotic Renal Surgery. Eur. Urol. 2023, 84, 86–91. [Google Scholar] [CrossRef]
- Piana, A.; Gallioli, A.; Amparore, D.; Diana, P.; Territo, A.; Campi, R.; Gaya, J.M.; Guirado, L.; Checcucci, E.; Bellin, A.; et al. Three-dimensional Augmented Reality-guided Robotic-assisted Kidney Transplantation: Breaking the Limit of Atheromatic Plaques. Eur. Urol. 2022, 82, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Vedula, S.S.; Ishii, M.; Hager, G.D. Objective Assessment of Surgical Technical Skill and Competency in the Operating Room. Annu. Rev. Biomed. Eng. 2017, 19, 301–325. [Google Scholar] [CrossRef]
- Sriwastwa, A.; Ravi, P.; Emmert, A.; Chokshi, S.; Kondor, S.; Dhal, K.; Patel, P.; Chepelev, L.L.; Rybicki, F.J.; Gupta, R. Generative AI for medical 3D printing: A comparison of ChatGPT outputs to reference standard education. 3D Print. Med. 2023, 9, 21. [Google Scholar] [CrossRef]
- Markgraf, W.; Malberg, H. Preoperative Function Assessment of Ex Vivo Kidneys with Supervised Machine Learning Based on Blood and Urine Markers Measured during Normothermic Machine Perfusion. Biomedicines 2022, 10, 3055. [Google Scholar] [CrossRef]
- Barah, M.; Mehrotra, S. Predicting Kidney Discard Using Machine Learning. Transplantation 2021, 105, 2054–2071. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar] [CrossRef]
- Sommer, F.; Sun, B.; Fischer, J.; Goldammer, M.; Thiele, C.; Malberg, H.; Markgraf, W. Hyperspectral Imaging during Normothermic Machine Perfusion-A Functional Classification of Ex Vivo Kidneys Based on Convolutional Neural Networks. Biomedicines 2022, 10, 397. [Google Scholar] [CrossRef]
- Zaza, G.; Neri, F.; Bruschi, M.; Granata, S.; Petretto, A.; Bartolucci, M.; di Bella, C.; Candiano, G.; Stallone, G.; Gesualdo, L.; et al. Proteomics reveals specific biological changes induced by the normothermic machine perfusion of donor kidneys with a significant up-regulation of Latexin. Sci. Rep. 2023, 13, 5920. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Cooper, J.E. Acute antibody-mediated rejection in kidney transplant recipients. Transplant. Rev. 2017, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.; Schlaefer, A.; Budde, K.; Schroeter, K.; Neumayer, H.H. Recognition of critical situations from time series of laboratory results by case-based reasoning. J. Am. Med. Inform. Assoc. 2002, 9, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.; Chatzikyrkou, C.; Broecker, V.; Schiffer, E.; Jaensch, L.; Iphoefer, A.; Mengel, M.; Mullen, W.; Mischak, H.; Haller, H.; et al. Diagnosis of subclinical and clinical acute T-cell-mediated rejection in renal transplant patients by urinary proteome analysis. Proteom. Clin. Appl. 2011, 5, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.; Sur, S.; Sigdel, T.; Nguyen, M.; Crespo, E.; Torija, A.; Meneghini, M.; Goma, M.; Sirota, M.; Bestard, O.; et al. Peripheral Blood RNA Sequencing Unravels a Differential Signature of Coding and Noncoding Genes by Types of Kidney Allograft Rejection. Kidney Int. Rep. 2020, 5, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Dong, J.; Rose, C.; Gill, J.S. The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int. 2016, 89, 1331–1336. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Ty, R.; Barba, L.; Sender, M. Prediction of early graft function in renal transplantation using a computer neural network. Transplant. Proc. 1998, 30, 1316–1317. [Google Scholar] [CrossRef]
- Santori, G.; Fontana, I.; Valente, U. Application of an artificial neural network model to predict delayed decrease of serum creatinine in pediatric patients after kidney transplantation. Transplant. Proc. 2007, 39, 1813–1819. [Google Scholar] [CrossRef]
- Kawakita, S.; Beaumont, J.L.; Jucaud, V.; Everly, M.J. Personalized prediction of delayed graft function for recipients of deceased donor kidney transplants with machine learning. Sci. Rep. 2020, 10, 18409. [Google Scholar] [CrossRef]
- Brier, M.E.; Ray, P.C.; Klein, J.B. Prediction of delayed renal allograft function using an artificial neural network. Nephrol. Dial. Transplant. 2003, 18, 2655–2659. [Google Scholar] [CrossRef]
- Decruyenaere, A.; Decruyenaere, P.; Peeters, P.; Vermassen, F.; Dhaene, T.; Couckuyt, I. Prediction of delayed graft function after kidney transplantation: Comparison between logistic regression and machine learning methods. BMC Med. Inform. Decis. Mak. 2015, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, A.; Stojanowski, J.; Rydzynska, K.; Kusztal, M.; Krajewska, M. Artificial Intelligence-A Tool for Risk Assessment of Delayed-Graft Function in Kidney Transplant. J. Clin. Med. 2021, 10, 5244. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.D.; de Andrade, L.G.M.; Barroso, F.V.C.; Oliveira, C.M.C.; Daher, E.F.; Fernandes, P.; Esmeraldo, R.M.; Sandes-Freitas, T.V. The impact of deceased donor maintenance on delayed kidney allograft function: A machine learning analysis. PLoS ONE 2020, 15, e0228597. [Google Scholar] [CrossRef] [PubMed]
- Quinino, R.M.; Agena, F.; Modelli de Andrade, L.G.; Furtado, M.; Chiavegatto Filho, A.D.P.; David-Neto, E. A Machine Learning Prediction Model for Immediate Graft Function After Deceased Donor Kidney Transplantation. Transplantation 2023, 107, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tang, Z.; Hu, X.; Lu, S.; Miao, B.; Hong, S.; Bai, H.; Sun, C.; Qiu, J.; Liang, H.; et al. Machine learning for the prediction of severe pneumonia during posttransplant hospitalization in recipients of a deceased-donor kidney transplant. Ann. Transl. Med. 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Gong, H.; Tian, H.; Zhuang, Q.; Li, J.; Cheng, K.; Ming, Y. The study of the association between immune monitoring and pneumonia in kidney transplant recipients through machine learning models. J. Transl. Med. 2020, 18, 370. [Google Scholar] [CrossRef]
- Sheppard, D.; McPhee, D.; Darke, C.; Shrethra, B.; Moore, R.; Jurewitz, A.; Gray, A. Predicting cytomegalovirus disease after renal transplantation: An artificial neural network approach. Int. J. Med. Inform. 1999, 54, 55–76. [Google Scholar] [CrossRef]
- Bae, S.; Massie, A.B.; Caffo, B.S.; Jackson, K.R.; Segev, D.L. Machine learning to predict transplant outcomes: Helpful or hype? A national cohort study. Transpl. Int. 2020, 33, 1472–1480. [Google Scholar] [CrossRef]
- Hamilton, D.; Miola, U.J.; Mousa, D. Interpretation of captopril transplant renography using a feed forward neural network. J. Nucl. Med. 1996, 37, 1649–1652. [Google Scholar]
- El-Baz, A.; Fahmi, R.; Yuksel, S.; Farag, A.A.; Miller, W.; El-Ghar, M.A.; Eldiasty, T. A new CAD system for the evaluation of kidney diseases using DCE-MRI. In Medical Image Computing and Computer-Assisted Intervention, Proceedings of the MICCAI 2006: 9th International Conference, Part II, Copenhagen, Denmark, 1–6 October 2006; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 446–453. [Google Scholar] [CrossRef]
- El-Baz, A.; Gimel’farb, G.; El-Ghar, M.A. New motion correction models for automatic identification of renal transplant rejection. In Medical Image Computing and Computer-Assisted Intervention, Proceedings of the MICCAI 2007: 10th International Conference, Part II, Brisbane, Australia, 29 October–2 November 2007; Springer: Berlin/Heidelberg, Germany, 2007; Volume 10, pp. 235–243. [Google Scholar] [CrossRef]
- Shehata, M.; Khalifa, F.; Soliman, A.; Ghazal, M.; Taher, F.; El-Ghar, M.A.; Dwyer, A.C.; Gimel’farb, G.; Keynton, R.S.; El-Baz, A. Computer-Aided Diagnostic System for Early Detection of Acute Renal Transplant Rejection Using Diffusion-Weighted MRI. IEEE Trans. Biomed. Eng. 2019, 66, 539–552. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Shehata, M.; Shalaby, A.; Khalifa, F.; Mahmoud, A.; El-Ghar, M.A.; Dwyer, A.C.; Ghazal, M.; Hajjdiab, H.; Keynton, R.; et al. A Novel CNN-Based CAD System for Early Assessment of Transplanted Kidney Dysfunction. Sci. Rep. 2019, 9, 5948. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.; Shalaby, A.; Switala, A.E.; El-Baz, M.; Ghazal, M.; Fraiwan, L.; Khalil, A.; El-Ghar, M.A.; Badawy, M.; Bakr, A.M.; et al. A multimodal computer-aided diagnostic system for precise identification of renal allograft rejection: Preliminary results. Med. Phys. 2020, 47, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Beetz, N.L.; Geisel, D.; Shnayien, S.; Auer, T.A.; Globke, B.; Ollinger, R.; Trippel, T.D.; Schachtner, T.; Fehrenbach, U. Effects of Artificial Intelligence-Derived Body Composition on Kidney Graft and Patient Survival in the Eurotransplant Senior Program. Biomedicines 2022, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.I.; Furness, P.N.; Nicholson, M. Diagnosis of early acute renal allograft rejection by evaluation of multiple histological features using a Bayesian belief network. J. Clin. Pathol. 1998, 51, 108–113. [Google Scholar] [CrossRef]
- Halloran, P.F.; Reeve, J.P.; Pereira, A.B.; Hidalgo, L.G.; Famulski, K.S. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: New insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2014, 85, 258–264. [Google Scholar] [CrossRef]
- Reeve, J.; Bohmig, G.A.; Eskandary, F.; Einecke, G.; Lefaucheur, C.; Loupy, A.; Halloran, P.F.; MMDx-Kidney Study Group. Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2017, 2, e94197. [Google Scholar] [CrossRef]
- Reeve, J.; Bohmig, G.A.; Eskandary, F.; Einecke, G.; Gupta, G.; Madill-Thomsen, K.; Mackova, M.; Halloran, P.F.; Group, I.M.-K.S. Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am. J. Transplant. 2019, 19, 2719–2731. [Google Scholar] [CrossRef]
- Hermsen, M.; Ciompi, F.; Adefidipe, A.; Denic, A.; Dendooven, A.; Smith, B.H.; van Midden, D.; Brasen, J.H.; Kers, J.; Stegall, M.D.; et al. Convolutional Neural Networks for the Evaluation of Chronic and Inflammatory Lesions in Kidney Transplant Biopsies. Am. J. Pathol. 2022, 192, 1418–1432. [Google Scholar] [CrossRef]
- Smith, B.; Grande, J.; Ryan, M.; Smith, M.; Denic, A.; Hermsen, M.; Park, W.; Kremers, W.; Stegall, M. Automated scoring of total inflammation in renal allograft biopsies. Clin. Transplant. 2023, 37, e14837. [Google Scholar] [CrossRef]
- Furness, P.N.; Levesley, J.; Luo, Z.; Taub, N.; Kazi, J.I.; Bates, W.D.; Nicholson, M.L. A neural network approach to the biopsy diagnosis of early acute renal transplant rejection. Histopathology 1999, 35, 461–467. [Google Scholar] [CrossRef]
- Liu, P.; Tseng, G.; Wang, Z.; Huang, Y.; Randhawa, P. Diagnosis of T-cell-mediated kidney rejection in formalin-fixed, paraffin-embedded tissues using RNA-Seq-based machine learning algorithms. Hum. Pathol. 2019, 84, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Choi, G.; Go, H.; Cho, Y.; Lee, H.; Lee, A.R.; Park, B.; Kim, N. A Fully Automated System Using A Convolutional Neural Network to Predict Renal Allograft Rejection: Extra-validation with Giga-pixel Immunostained Slides. Sci. Rep. 2019, 9, 5123. [Google Scholar] [CrossRef] [PubMed]
- Ligabue, G.; Pollastri, F.; Fontana, F.; Leonelli, M.; Furci, L.; Giovanella, S.; Alfano, G.; Cappelli, G.; Testa, F.; Bolelli, F.; et al. Evaluation of the Classification Accuracy of the Kidney Biopsy Direct Immunofluorescence through Convolutional Neural Networks. Clin. J. Am. Soc. Nephrol. 2020, 15, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, D.C.; Smith, M.L.; Cornell, L.D.; Andryushkin, A.; Pettus, J.R. Automated identification of glomeruli and synchronised review of special stains in renal biopsies by machine learning and slide registration: A cross-institutional study. Histopathology 2021, 79, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.; Bulow, R.D.; Klinkhammer, B.M.; Breimer, G.E.; Fontana, F.; Abiola, A.A.; Hofstraat, R.; Corthals, G.L.; Peters-Sengers, H.; Djudjaj, S.; et al. Deep learning-based classification of kidney transplant pathology: A retrospective, multicentre, proof-of-concept study. Lancet Digit. Health 2022, 4, e18–e26. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Wiseman, A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin. J. Am. Soc. Nephrol. 2021, 16, 1264–1271. [Google Scholar] [CrossRef]
- Basuli, D.; Roy, S. Beyond Human Limits: Harnessing Artificial Intelligence to Optimize Immunosuppression in Kidney Transplantation. J. Clin. Med. Res. 2023, 15, 391–398. [Google Scholar] [CrossRef]
- McMichael, J.; Irish, W.; McCauley, J.; Shapiro, R.; Gordon, R.; Van Thiel, D.H.; Lieberman, R.; Warty, V.S.; Fung, J.; Starzl, T.E. Evaluation of a novel “intelligent” dosing system for optimizing FK 506 therapy. Transplant. Proc. 1991, 23, 2780–2782. [Google Scholar]
- Seeling, W.; Plischke, M.; Schuh, C. Knowledge-based tacrolimus therapy for kidney transplant patients. Stud. Health Technol. Inform. 2012, 180, 310–314. [Google Scholar]
- Storset, E.; Asberg, A.; Skauby, M.; Neely, M.; Bergan, S.; Bremer, S.; Midtvedt, K. Improved Tacrolimus Target Concentration Achievement Using Computerized Dosing in Renal Transplant Recipients--A Prospective, Randomized Study. Transplantation 2015, 99, 2158–2166. [Google Scholar] [CrossRef]
- Tang, J.; Liu, R.; Zhang, Y.L.; Liu, M.Z.; Hu, Y.F.; Shao, M.J.; Zhu, L.J.; Xin, H.W.; Feng, G.W.; Shang, W.J.; et al. Application of Machine-Learning Models to Predict Tacrolimus Stable Dose in Renal Transplant Recipients. Sci. Rep. 2017, 7, 42192. [Google Scholar] [CrossRef] [PubMed]
- Niel, O.; Bastard, P. Artificial intelligence improves estimation of tacrolimus area under the concentration over time curve in renal transplant recipients. Transpl. Int. 2018, 31, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Thishya, K.; Vattam, K.K.; Naushad, S.M.; Raju, S.B.; Kutala, V.K. Artificial neural network model for predicting the bioavailability of tacrolimus in patients with renal transplantation. PLoS ONE 2018, 13, e0191921. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Zhang, X.; Zheng, C.; Zhu, L.; Zhu, M.; Cheng, Z.; Luo, X. A novel random forest integrative approach based on endogenous CYP3A4 phenotype for predicting tacrolimus concentrations and dosages in Chinese renal transplant patients. J. Clin. Pharm. Ther. 2020, 45, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Gaies, E.; Ben Sassi, M.; Charfi, R.; Salouage, I.; Jebabli, N.; ElJebari, H.; Klouz, A.; Daghfous, R.; Trabelsi, S. Therapeutic durg monitoring of cyclosporin using area under the curve in nephrotic syndrome. Tunis. Med. 2019, 97, 360–364. [Google Scholar]
- Jorga, A.; Holt, D.W.; Johnston, A. Therapeutic drug monitoring of cyclosporine. Transplant. Proc. 2004, 36 (Suppl. S2), 396S–403S. [Google Scholar] [CrossRef]
- Camps-Valls, G.; Porta-Oltra, B.; Soria-Olivas, E.; Martin-Guerrero, J.D.; Serrano-Lopez, A.J.; Perez-Ruixo, J.J.; Jimenez-Torres, N.V. Prediction of cyclosporine dosage in patients after kidney transplantation using neural networks. IEEE Trans. Biomed. Eng. 2003, 50, 442–448. [Google Scholar] [CrossRef]
- Goren, S.; Karahoca, A.; Onat, F.Y.; Goren, M.Z. Prediction of cyclosporine A blood levels: An application of the adaptive-network-based fuzzy inference system (ANFIS) in assisting drug therapy. Eur. J. Clin. Pharmacol. 2008, 64, 807–814. [Google Scholar] [CrossRef]
- Bergan, S.; Brunet, M.; Hesselink, D.A.; Johnson-Davis, K.L.; Kunicki, P.K.; Lemaitre, F.; Marquet, P.; Molinaro, M.; Noceti, O.; Pattanaik, S.; et al. Personalized Therapy for Mycophenolate: Consensus Report by the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2021, 43, 150–200. [Google Scholar] [CrossRef]
- Woillard, J.B.; Labriffe, M.; Debord, J.; Marquet, P. Mycophenolic Acid Exposure Prediction Using Machine Learning. Clin. Pharmacol. Ther. 2021, 110, 370–379. [Google Scholar] [CrossRef]
- CISTEM Immunosuppression Complication Risk Rejection Tool. Available online: https://cistem.wustl.edu (accessed on 19 October 2023).
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Zeimbekis, A.; Kastorini, C.M.; Stefanadis, C. Adherence to the Mediterranean diet is associated with renal function among healthy adults: The ATTICA study. J. Ren. Nutr. 2010, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Moon, Y.P.; Scarmeas, N.; Gu, Y.; Gardener, H.; Cheung, K.; Wright, C.B.; Sacco, R.L.; Nickolas, T.L.; Elkind, M.S. The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin. J. Am. Soc. Nephrol. 2014, 9, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Neto, A.W.; Oste, M.C.J.; Sotomayor, C.G.; van den Berg, E.; Geleijnse, J.M.; Berger, S.P.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Mediterranean Style Diet and Kidney Function Loss in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2020, 15, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Oste, M.C.; Corpeleijn, E.; Navis, G.J.; Keyzer, C.A.; Soedamah-Muthu, S.S.; van den Berg, E.; Postmus, D.; de Borst, M.H.; Kromhout, D.; Bakker, S.J. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care 2017, 5, e000283. [Google Scholar] [CrossRef]
- Stachowska, E.; Gutowska, I.; Strzelczak, A.; Wesolowska, T.; Safranow, K.; Ciechanowski, K.; Chlubek, D. The use of neural networks in evaluation of the direction and dynamics of changes in lipid parameters in kidney transplant patients on the Mediterranean diet. J. Ren. Nutr. 2006, 16, 150–159. [Google Scholar] [CrossRef]
- Aziz, S.; Ahmed, A.; Abd-Alrazaq, A.; Qidwai, U.; Farooq, F.; Sheikh, J. Estimating Blood Glucose Levels Using Machine Learning Models with Non-Invasive Wearable Device Data. Stud. Health Technol. Inform. 2023, 305, 283–286. [Google Scholar] [CrossRef]
- Mario, B.; Walpola, H.; Kisal, R.; De Silva, S. Kidney Transplant aftercare with IOT Medical Wearables. Preprint 2017. [Google Scholar] [CrossRef]
- Simic-Ogrizovic, S.; Furuncic, D.; Lezaic, V.; Radivojevic, D.; Blagojevic, R.; Djukanovic, L. Using ANN in selection of the most important variables in prediction of chronic renal allograft rejection progression. Transplant. Proc. 1999, 31, 368. [Google Scholar] [CrossRef]
- Lofaro, D.; Maestripieri, S.; Greco, R.; Papalia, T.; Mancuso, D.; Conforti, D.; Bonofiglio, R. Prediction of chronic allograft nephropathy using classification trees. Transplant. Proc. 2010, 42, 1130–1133. [Google Scholar] [CrossRef]
- Badrouchi, S.; Bacha, M.M.; Ahmed, A.; Ben Abdallah, T.; Abderrahim, E. Predicting long-term outcomes of kidney transplantation in the era of artificial intelligence. Sci. Rep. 2023, 13, 21273. [Google Scholar] [CrossRef]
- Improta, G.; Mazzella, V.; Vecchione, D.; Santini, S.; Triassi, M. Fuzzy logic-based clinical decision support system for the evaluation of renal function in post-Transplant Patients. J. Eval. Clin. Pract. 2020, 26, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, M.; Aubert, O.; Divard, G.; Reese, P.P.; Kamar, N.; Yoo, D.; Chin, C.S.; Bailly, E.; Buchler, M.; Ladriere, M.; et al. Dynamic prediction of renal survival among deeply phenotyped kidney transplant recipients using artificial intelligence: An observational, international, multicohort study. Lancet Digit. Health 2021, 3, e795–e805. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kong, G.; Wang, L.; Zhang, L.; Zhao, M.H. Big data in nephrology: Are we ready for the change? Nephrology 2019, 24, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Nosrati, M. Artificial Intelligence in Regenerative Medicine: Applications and Implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Shademan, A.; Decker, R.S.; Opfermann, J.D.; Leonard, S.; Krieger, A.; Kim, P.C. Supervised autonomous robotic soft tissue surgery. Sci. Transl. Med. 2016, 8, 337ra364. [Google Scholar] [CrossRef]
- Castelvecchi, D. Can we open the black box of AI? Nature 2016, 538, 20–23. [Google Scholar] [CrossRef]
- Char, D.S.; Shah, N.H.; Magnus, D. Implementing Machine Learning in Health Care—Addressing Ethical Challenges. N. Engl. J. Med. 2018, 378, 981–983. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Jingwa, K.A.; Okoye, O.K.; John Okah, M.; Ladele, J.A.; Farah, A.H.; Alimi, H.A. Ethical implications of AI and robotics in healthcare: A review. Medicine 2023, 102, e36671. [Google Scholar] [CrossRef]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsifa, E.; Mavroeidis, V.K. Present and Future Applications of Artificial Intelligence in Kidney Transplantation. J. Clin. Med. 2024, 13, 5939. https://doi.org/10.3390/jcm13195939
Kotsifa E, Mavroeidis VK. Present and Future Applications of Artificial Intelligence in Kidney Transplantation. Journal of Clinical Medicine. 2024; 13(19):5939. https://doi.org/10.3390/jcm13195939
Chicago/Turabian StyleKotsifa, Evgenia, and Vasileios K. Mavroeidis. 2024. "Present and Future Applications of Artificial Intelligence in Kidney Transplantation" Journal of Clinical Medicine 13, no. 19: 5939. https://doi.org/10.3390/jcm13195939





