A Prospective, Randomized Trial of Bioresorbable Polymer Drug-Eluting Stents versus Fully Bioresorbable Scaffolds in Patients Undergoing Coronary Stenting
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants and Interventions
2.3. Outcomes and Definitions
2.4. Sample Size, Randomization, and Outcomes Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
- (1)
- BP-EES have superior angiographic performance as compared to BRS, with a significantly lower in-device percentage diameter stenosis at 6- to 8-month invasive surveillance.
- (2)
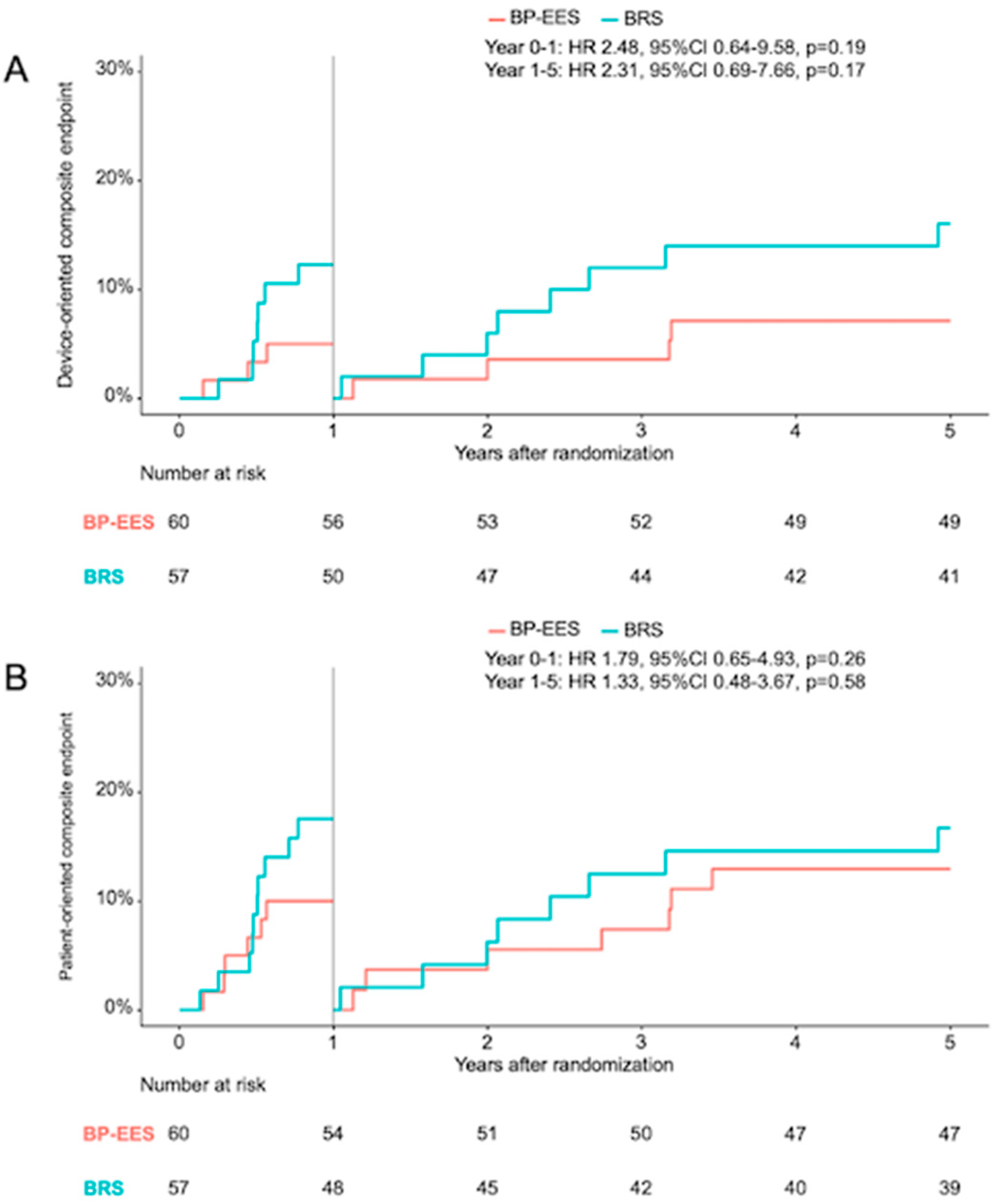
- Consistent with the angiographic data, the risk of DOCE after 12 months in the BRS group was doubled compared to BP-EES, although no statistically significant differences were found between either groups. This observation was also consistent throughout the 5-year clinical follow-up and the landmark analysis between 1 and 5 years.
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.L.; Kastrati, A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014, 100, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Kolodgie, F.D.; Harnek, J.; Guerrero, L.J.; Acampado, E.; Tefera, K.; Skorija, K.; Weber, D.K.; Gold, H.K.; Virmani, R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005, 112, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakazawa, G.; Kolodgie, F.D.; Virmani, R. Temporal course of neointimal formation after drug-eluting stent placement: Is our understanding of restenosis changing? JACC Cardiovasc. Interv. 2009, 2, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Joner, M.; Kastrati, A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009, 57, 567–584. [Google Scholar] [PubMed]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current Status of Bioresorbable Scaffolds in the Treatment of Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef]
- Meredith, I.T.; Verheye, S.; Dubois, C.L.; Dens, J.; Fajadet, J.; Carrié, D.; Walsh, S.; Oldroyd, K.G.; Varenne, O.; El-Jack, S.; et al. Primary endpoint results of the EVOLVE trial: A randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J. Am. Coll. Cardiol. 2012, 59, 1362–1370. [Google Scholar] [CrossRef]
- Meredith, I.T.; Verheye, S.; Weissman, N.J.; Barragan, P.; Scott, D.; Chávarri, M.V.; West, N.E.J.; Kelbæk, H.; Whitbourn, R.; Walters, D.L.; et al. Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: A randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention 2013, 9, 308–315. [Google Scholar] [CrossRef]
- Ali, Z.A.; Gao, R.; Kimura, T.; Onuma, Y.; Kereiakes, D.J.; Ellis, S.G.; Chevalier, B.; Vu, M.T.; Zhang, Z.; Simonton, C.A.; et al. Three-Year Outcomes with the Absorb Bioresorbable Scaffold: Individual-Patient-Data Meta-Analysis from the ABSORB Randomized Trials. Circulation 2018, 137, 464–479. [Google Scholar] [CrossRef]
- Cassese, S.; Byrne, R.A.; Ndrepepa, G.; Kufner, S.; Wiebe, J.; Repp, J.; Schunkert, H.; Fusaro, M.; Kimura, T.; Kastrati, A. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: A meta-analysis of randomised controlled trials. Lancet 2016, 387, 537–544. [Google Scholar] [CrossRef]
- Stone, G.W.; Biensock, S.W.; Neumann, F.J. Bioresorbable coronary scaffolds are ready for a comeback: Pros and cons. EuroIntervention 2023, 19, 199–202. [Google Scholar] [PubMed]
- Song, L.; Xu, B.; Chen, Y.; Zhou, Y.; Jia, S.; Zhong, Z.; Su, X.; Ma, Y.; Zhang, Q.; Liu, J.; et al. Thinner Strut Sirolimus-Eluting BRS Versus EES in Patients with Coronary Artery Disease: FUTURE-II Trial. JACC Cardiovasc. Interv. 2021, 14, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Gabriel Steg, P.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; Thygesen, K.; Alpert, J.S.; White, H.D.; et al. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef]
- Serruys, P.W.; Onuma, Y.; Ormiston, J.A.; de Bruyne, B.; Regar, E.; Dudek, D.; Thuesen, L.; Smits, P.C.; Chevalier, B.; McClean, D.; et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: Six-month clinical and imaging outcomes. Circulation 2010, 122, 2301–2312. [Google Scholar] [CrossRef]
- Puricel, S.; Arroyo, D.; Corpataux, N.; Baeriswyl, G.; Lehmann, S.; Kallinikou, Z.; Muller, O.; Allard, L.; Stauffer, J.C.; Togni, M.; et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J. Am. Coll. Cardiol. 2015, 65, 791–801. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Taniwaki, M.; Windecker, S. Coronary stents: Novel developments. Heart 2014, 100, 1051–1061. [Google Scholar] [CrossRef]
- Ellis, S.G.; Kereiakes, D.J.; Metzger, D.C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N. Engl. J. Med. 2015, 373, 1905–1915. [Google Scholar] [CrossRef]
- Ortega-Paz, L.; Capodanno, D.; Gori, T.; Nef, H.; Latib, A.; Caramanno, G.; Di Mario, C.; Naber, C.; Lesiak, M.; Capranzano, P.; et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: Development and internal validation of the PSP score. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 12, 2110–2117. [Google Scholar] [CrossRef]
- Puricel, S.; Cuculi, F.; Weissner, M.; Schmermund, A.; Jamshidi, P.; Nyffenegger, T.; Binder, H.; Eggebrecht, H.; Münzel, T.; Cook, S.; et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J. Am. Coll. Cardiol. 2016, 67, 921–931. [Google Scholar] [CrossRef]
- Nef, H.M.; Wiebe, J.; Kastner, J.; Mehilli, J.; Münzel, T.; Naber, C.; Neumann, T.; Richardt, G.; Schmermund, A.; Wöhrle, J.; et al. Everolimus-eluting bioresorbable scaffolds in patients with coronary artery disease: Results from the German-Austrian ABSORB RegIstRy (GABI-R). EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Kimura, T.; Gao, R.; Kereiakes, D.J.; Ellis, S.G.; Onuma, Y.; Chevalier, B.; Simonton, C.; Dressler, O.; Crowley, A.; et al. Time-Varying Outcomes with the Absorb Bioresorbable Vascular Scaffold During 5-Year Follow-up: A Systematic Meta-analysis and Individual Patient Data Pooled Study. JAMA Cardiol. 2019, 4, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of Very Late Bioresorbable Scaffold Thrombosis: The INVEST Registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Schukraft, S.; Arroyo, D.; Togni, M.; Goy, J.-J.; Wenaweser, P.; Stadelmann, M.; Baeriswyl, G.; Muller, O.; Stauffer, J.-C.; Puricel, S.; et al. Five-year angiographic, OCT and clinical outcomes of a randomized comparison of everolimus and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. Catheter. Cardiovasc. Interv. 2022, 99, 523–532. [Google Scholar] [CrossRef] [PubMed]
| BP-EES | BRS | p Value | |
|---|---|---|---|
| Patients | 60 | 57 | |
| Age | 66.7 ± 11.4 | 68.5 ± 10.5 | 0.38 |
| Female gender | 13 (21.7) | 16 (28.1) | 0.56 |
| Diabetes mellitus | 22 (36.7) | 16 (28.1) | 0.43 |
| Insulin dependent | 6 (27.3) | 3 (18.8) | 0.89 |
| Hypertension | 55 (91.7) | 49 (86.0) | 0.49 |
| Hyperlipidemia | 51 (85.0) | 45 (78.9) | 0.54 |
| Smoking | 27 (45.0) | 26 (45.6) | 0.63 |
| Family history of CAD | 21 (35.0) | 20 (35.1) | 1.00 |
| Prior myocardial infarction | 9 (15.0) | 12 (21.1) | 0.40 |
| Prior bypass surgery | 3 (5.0) | 3 (5.3) | 1.00 |
| Prior percutaneous coronary intervention | 30 (50.0) | 28 (49.1) | 0.84 |
| Number of vessels diseased | 0.72 | ||
| 1 vessel disease | 15 (25.0) | 18 (31.6) | |
| 2 vessel disease | 21 (35.0) | 19 (33.3) | |
| 3 vessel disease | 24 (40.0) | 20 (35.1) | |
| Clinical presentation | 0.61 | ||
| ST elevation myocardial infarction ≤ 48 h | 0 (0.0) | 1 (1.8) | |
| Non ST elevation myocardial infarction | 6 (10.0) | 4 (7.1) | |
| Unstable angina | 14 (23.3) | 11 (19.6) | |
| Stable angina | 21 (35.0) | 16 (28.6) | |
| Asymptomatic | 19 (31.7) | 24 (42.9) |
| BP-EES | BRS | p Value | |
|---|---|---|---|
| Culprit lesions | 63 | 59 | |
| Target vessel | 0.43 | ||
| Left anterior descending | 29 (46.0) | 25 (42.4) | |
| Left circumflex | 16 (25.4) | 11 (18.6) | |
| Right coronary artery | 18 (28.6) | 23 (39.0) | |
| ACC/AHA classification | 0.80 | ||
| A | 8 (12.7) | 6 (10.2) | |
| B1 | 22 (34.9) | 26 (44.1) | |
| B2 | 29 (46.0) | 24 (40.7) | |
| C | 4 (6.4) | 3 (5.1) | |
| Pre-dilation | 54 (85.7) | 58 (98.3) | 0.02 |
| Stent diameter, max (mm) | 3.2 ± 0.4 | 3.2 ± 0.3 | 0.71 |
| Number of primary devices used | 0.34 | ||
| 1 device | 51 (81.0) | 44 (74.6) | |
| 2 devices | 12 (19.0) | 13 (22.0) | |
| 3 devices | 0 | 2 (3.4) | |
| Total stented length (mm) | 26.8 ± 10.9 | 26.7 ± 12.1 | 0.98 |
| Overlap | 15 (23.8) | 16 (27.1) | 0.76 |
| Post-dilation | 41 (65.1) | 52 (88.1) | <0.01 |
| Nominal diameter balloon, max (mm) | 3.4 ± 0.7 | 3.5 ± 0.3 | 0.63 |
| Balloon pressure, max (atm) | 16.5 ± 4.8 | 18.2 ± 4.5 | 0.10 |
| Quantitative coronary angiography analysis | |||
| Pre-intervention | |||
| Reference diameter (mm) | 2.92 ± 0.41 | 2.91 ± 0.43 | 0.94 |
| Minimal lumen diameter (mm) | 1.18 ± 0.34 | 1.17 ± 0.40 | 0.81 |
| Diameter stenosis (%) | 59.5 ± 12.3 | 60.3 ± 12.8 | 0.73 |
| Lesion length (mm) | 12.3 ± 5.5 | 13.1 ± 5.7 | 0.41 |
| Post-intervention | |||
| Minimal lumen diameter (mm) | 2.75 ± 0.36 | 2.65 ± 0.42 | 0.15 |
| Diameter stenosis (%) (in-stent) | 10.2 ± 4.5 | 13.7 ± 6.0 | <0.001 |
| BP-EES | BRS | p Value | |
|---|---|---|---|
| Lesions/patients assessed | 44 | 46 | |
| Days to angiographic follow-up | 192 [180, 218] | 199 [186, 221] | 0.19 |
| In-segment analysis | |||
| late lumen loss (mm) | 0.15 ± 0.33 | 0.32 ± 0.52 | 0.07 |
| minimal lumen diameter (mm) | 2.32 ± 0.44 | 2.18 ± 0.64 | 0.23 |
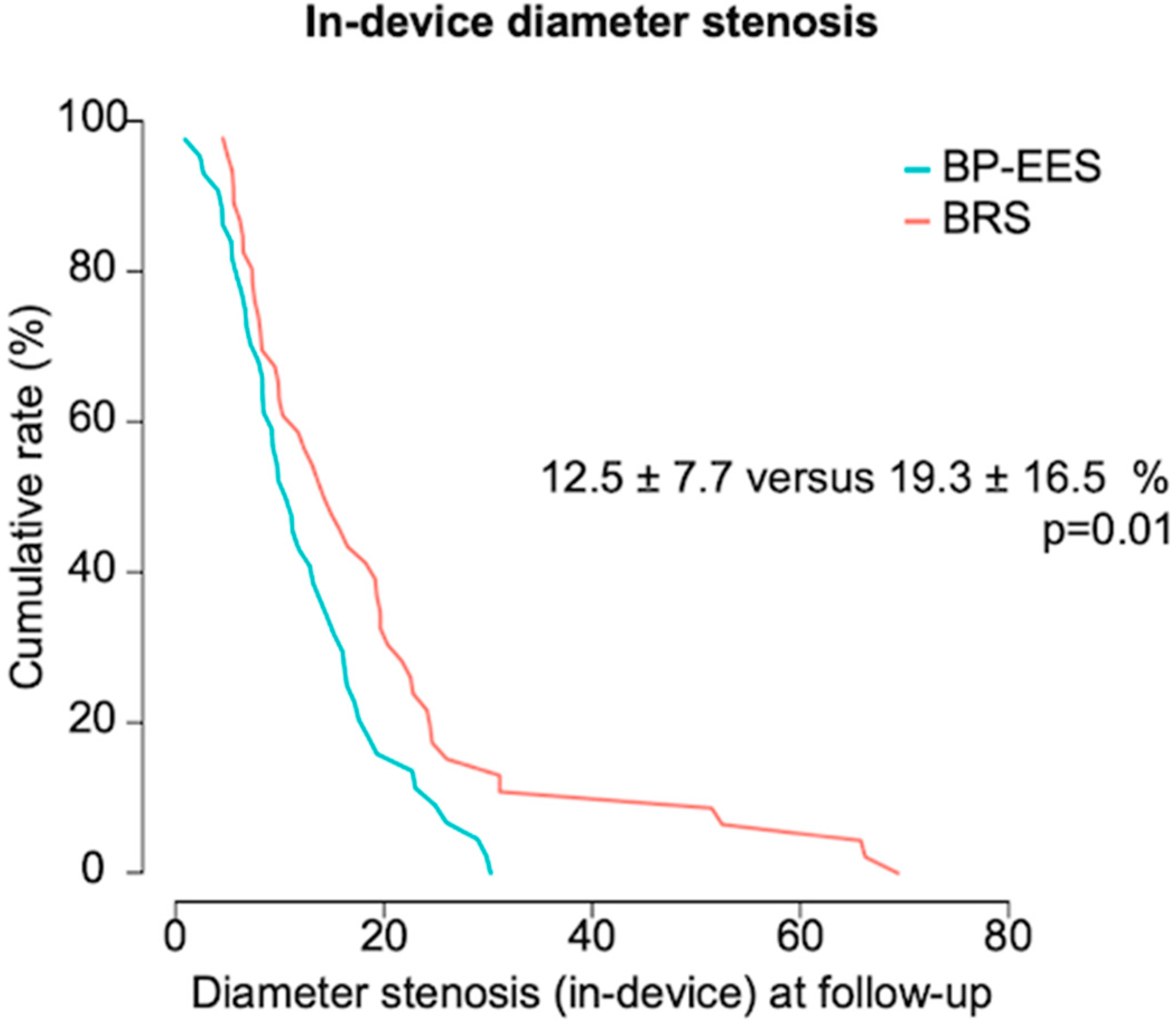
| diameter stenosis (%) | 23.6 ± 10.2 | 27.6 ± 16.6 | 0.17 |
| binary restenosis | 1 (2.3) | 6 (13.0) | 0.12 |
| In-stent analysis | |||
| late lumen loss (mm) | 0.13 ± 0.26 | 0.21 ± 0.47 | 0.31 |
| minimal lumen diameter (mm) | 2.64 ± 0.39 | 2.44 ± 0.65 | 0.08 |
| diameter stenosis (%) | 12.5 ± 7.7 | 19.3 ± 16.5 | 0.01 |
| binary restenosis | 0 (0.0) | 5 (10.9) | 0.07 |
| 12-Month Follow-Up | 5-Year Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| BP-EES | BRS | Hazard Ratio (95% CI) | p Value | BP-EES | BRS | Hazard Ratio (95% CI) | p Value | |
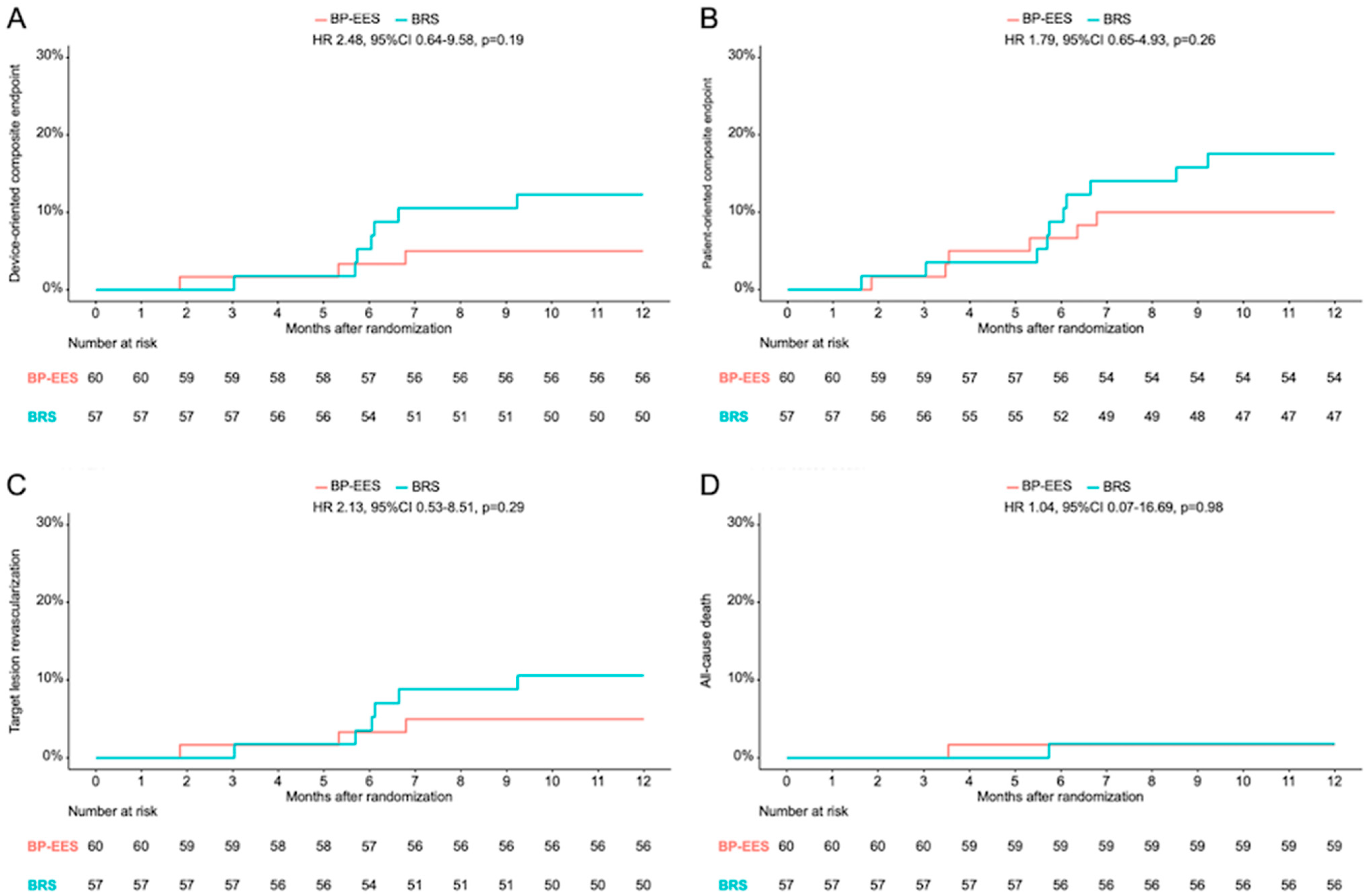
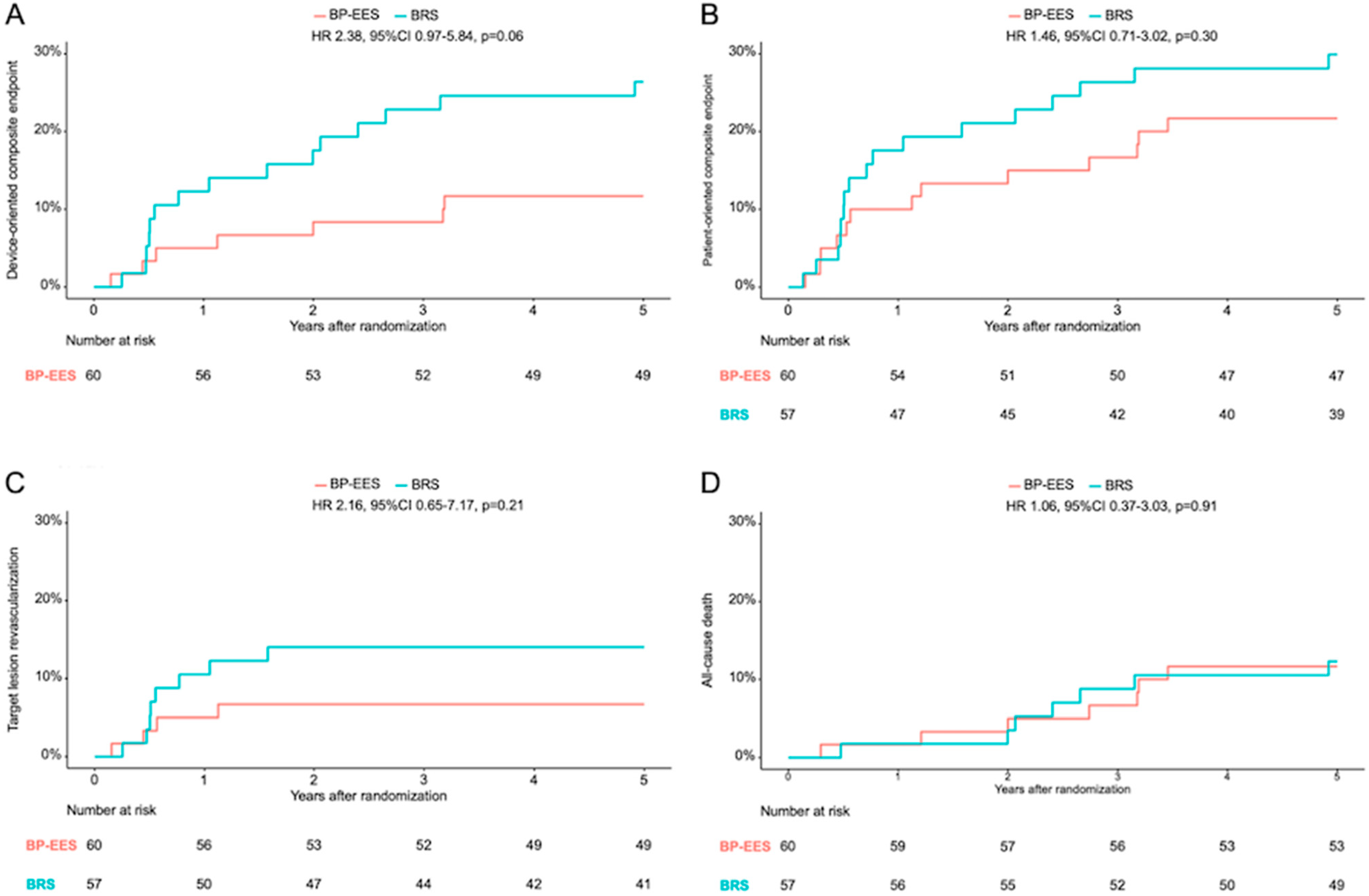
| Death | 1 (1.7) | 1 (1.8) | 1.04 (0.07–16.69) | 0.98 | 7 (11.7) | 7 (12.3) | 1.06 (0.37–3.03) | 0.91 |
| Cardiac death | 0 | 1 (1.8) | NA | 0.99 | 3 (5.0) | 7 (12.3) | 2.47 (0.64.9.57) | 0.19 |
| Device-oriented outcomes | ||||||||
| definite device thrombosis | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| probable device thrombosis | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| target vessel myocardial infarction | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| target lesion revascularization | 3 (5.0) | 6 (10.5) | 2.13 (0.53–8.51) | 0.29 | 4 (6.7) | 8 (14.0) | 2.16 (0.65–7.17) | 0.21 |
| device-oriented composite endpoint | 3 (5.0) | 7 (12.3) | 2.48 (0.64–9.58) | 0.19 | 7 (11.7) | 15 (26.4) | 2.38 (0.97–5.84) | 0.06 |
| Patient-oriented outcomes | ||||||||
| myocardial infarction | 0 | 1 (1.8) | NA | 0.99 | 0 | 1 (1.8) | NA | 0.99 |
| patient-oriented composite endpoint | 6 (10.0) | 10 (17.5) | 1.79 (0.65–4.93) | 0.26 | 13 (21.7) | 17 (29.9) | 1.46 (0.71–3.02) | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiebe, J.; Byrne, R.A.; Bradaric, C.; Kuna, C.; Kessler, T.; Pfleiderer, M.; Kufner, S.; Xhepa, E.; Hoppmann, P.; Joner, M.; et al. A Prospective, Randomized Trial of Bioresorbable Polymer Drug-Eluting Stents versus Fully Bioresorbable Scaffolds in Patients Undergoing Coronary Stenting. J. Clin. Med. 2024, 13, 5949. https://doi.org/10.3390/jcm13195949
Wiebe J, Byrne RA, Bradaric C, Kuna C, Kessler T, Pfleiderer M, Kufner S, Xhepa E, Hoppmann P, Joner M, et al. A Prospective, Randomized Trial of Bioresorbable Polymer Drug-Eluting Stents versus Fully Bioresorbable Scaffolds in Patients Undergoing Coronary Stenting. Journal of Clinical Medicine. 2024; 13(19):5949. https://doi.org/10.3390/jcm13195949
Chicago/Turabian StyleWiebe, Jens, Robert A. Byrne, Christian Bradaric, Constantin Kuna, Thorsten Kessler, Mathieu Pfleiderer, Sebastian Kufner, Erion Xhepa, Petra Hoppmann, Michael Joner, and et al. 2024. "A Prospective, Randomized Trial of Bioresorbable Polymer Drug-Eluting Stents versus Fully Bioresorbable Scaffolds in Patients Undergoing Coronary Stenting" Journal of Clinical Medicine 13, no. 19: 5949. https://doi.org/10.3390/jcm13195949
APA StyleWiebe, J., Byrne, R. A., Bradaric, C., Kuna, C., Kessler, T., Pfleiderer, M., Kufner, S., Xhepa, E., Hoppmann, P., Joner, M., Schunkert, H., Laugwitz, K.-L., Kastrati, A., & Cassese, S. (2024). A Prospective, Randomized Trial of Bioresorbable Polymer Drug-Eluting Stents versus Fully Bioresorbable Scaffolds in Patients Undergoing Coronary Stenting. Journal of Clinical Medicine, 13(19), 5949. https://doi.org/10.3390/jcm13195949






