Acute Changes in Myocardial Work during Isometric Exercise in Hypertensive Patients with Ischemic Heart Disease: A Case–Control Study
Abstract
:1. Introduction
Study Hypothesis
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Main Results
4.2. Comparison with Previous Studies
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021, 42, 17–96, Erratum in Eur. Heart J. 2021, 42, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Fagard, R.H.; Coeckelberghs, E.; Vanhees, L. Impact of resistance training on blood pressure and other cardiovascular risk factors: A meta-analysis of randomized, controlled trials. Hypertension 2011, 58, 950–958. [Google Scholar] [CrossRef] [PubMed]
- López-Valenciano, A.; Ruiz-Pérez, I.; Ayala, F.; Sánchez-Meca, J.; Vera-Garcia, F.J. Updated systematic review and meta-analysis on the role of isometric resistance training for resting blood pressure management in adults. J. Hypertens. 2019, 37, 1320–1333. [Google Scholar] [CrossRef]
- Mcleod, J.C.; Stokes, T.; Phillips, S.M. Resistance Exercise Training as a Primary Countermeasure to Age-Related Chronic Disease. Front. Physiol. 2019, 10, 645. [Google Scholar] [CrossRef]
- Paluch, A.E.; Boyer, W.R.; Franklin, B.A.; Laddu, D.; Lobelo, F.; Lee, D.C.; McDermott, M.M.; Swift, D.L.; Webel, A.R.; Lane, A.; et al. Resistance Exercise Training in Individuals With and Without Cardiovascular Disease: 2023 Update: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e217–e231. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, M.; Li, J.; Zhang, H.; Liu, Q.; Zhao, L.; Wang, T.; Xu, H. Efficacy and Safety of Resistance Training for Coronary Heart Disease Rehabilitation: A Systematic Review of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 754794. [Google Scholar] [CrossRef]
- Marzolini, S.; Oh, P.I.; Brooks, D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: A meta-analysis. Eur. J. Prev. Cardiol. 2012, 19, 81–94. [Google Scholar] [CrossRef]
- Carlson, D.J.; Dieberg, G.; Hess, N.C.; Millar, P.J.; Smart, N.A. Isometric exercise training for blood pressure management: A systematic review and meta-analysis. Mayo Clin. Proc. 2014, 89, 327–334. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef] [PubMed]
- Kounoupis, A.; Papadopoulos, S.; Galanis, N.; Dipla, K.; Zafeiridis, A. Are Blood Pressure and Cardiovascular Stress Greater in Isometric or in Dynamic Resistance Exercise? Sports 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Goessler, K.; Buys, R.; Cornelissen, V.A. Low-intensity isometric handgrip exercise has no transient effect on blood pressure in patients with coronary artery disease. J. Am. Soc. Hypertens. 2016, 10, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Gois, M.O.; Simões, R.P.; Porta, A.; Kunz, V.C.; Pastre, C.M.; Catai, A.M. Cardiovascular responses to low-intensity isometric handgrip exercise in coronary artery disease: Effects of posture. Braz. J. Phys. Ther. 2020, 24, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.; ÖzdenTok, Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: A novel method ready for clinical testing. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef]
- D’Andrea, A.; Carbone, A.; Radmilovic, J.; Russo, V.; Fabiani, D.; Maio, M.D.; Ilardi, F.; Giallauria, F.; Caputo, A.; Cirillo, T.; et al. Myocardial Work Efficiency in Physiologic Left Ventricular Hypertrophy of Power Athletes. J. Cardiovasc. Echogr. 2022, 32, 154–159. [Google Scholar] [CrossRef]
- Meldrum, D.; Cahalane, E.; Conroy, R.; Fitzgerald, D.; Hardiman, O. Maximum voluntary isometric contraction: Reference values and clinical application. Amyotroph. Lateral Scler. 2007, 8, 47–55. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ven-tricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Edwards, J.J.; Coleman, D.A.; Ritti-Dias, R.M.; Farah, B.Q.; Stensel, D.J.; Lucas, S.J.E.; Millar, P.J.; Gordon, B.D.H.; Cornelissen, V.; Smart, N.A.; et al. Isometric Exercise Training and Arterial Hypertension: An Updated Review. Sports Med. 2024, 54, 1459–1497. [Google Scholar] [CrossRef]
- Taylor, K.A.; Wiles, J.D.; Coleman, D.D.; Sharma, R.; O’driscoll, J.M. Continuous Cardiac Autonomic and Hemodynamic Responses to Isometric Exercise. Med. Sci. Sports Exerc. 2017, 49, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.; Nagle, F. Isometric exercise: Cardiovascular responses in normal and cardiac populations. Cardiol. Clin. 1987, 5, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, R.P.; van der Bijl, P.; El Mahdiui, M.; Montero-Cabezas, J.M.; Kostyukevich, M.V.; Marsan, N.A.; Bax, J.J.; Delgado, V. Noninvasive Myocardial Work Indices 3 Months after ST-Segment Elevation Myocardial Infarction: Prevalence and Characteristics of Patients with Postinfarction Cardiac Remodeling. J. Am. Soc. Echocardiogr. 2020, 33, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- El Mahdiui, M.; van der Bijl, P.; Abou, R.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Global Left Ventricular Myocardial Work Efficiency in Healthy Individuals and Patients with Cardiovascular Disease. J. Am. Soc. Echocardiogr. 2019, 32, 1120–1127. [Google Scholar] [CrossRef]
- Azevedo, P.S.; Minicucci, M.F.; Santos, P.P.; Paiva, S.; Zornoff, L. Energy Metabolism in Cardiac Remodeling and Heart Failure. Cardiol. Rev. 2013, 21, 135–140. [Google Scholar] [CrossRef]
- Erevik, C.B.; Kleiven, Ø.; Frøysa, V.; Bjørkavoll-Bergseth, M.; Chivulescu, M.; Klæboe, L.G.; Dejgaard, L.; Auestad, B.; Skadberg, Ø.; Melberg, T.; et al. Myocardial inefficiency is an early indicator of exercise-induced myocardial fatigue. Front. Cardiovasc. Med. 2023, 9, 1081664. [Google Scholar] [CrossRef]
- MacDougall, J.D.; McKelvie, R.S.; Moroz, D.E.; Sale, D.G.; McCartney, N.; Buick, F. Factors affecting blood pressure during heavy weight lifting and static contractions. J. Appl. Physiol. 1992, 73, 1590–1597. [Google Scholar] [CrossRef]
- Anjos-Andrade, F.D.; Sousa, A.C.; Barreto-Filho, J.A.; Alves, E.O.; Nascimento-Júnior, A.C.; de Santana, N.O.; de Vasconcelos, F.L.; Garcez, F.B.; de Araujo, V.P.; de Araujo, A.C.; et al. Chronotropic incompetence and coronary artery disease. Acta Cardiol. 2010, 65, 631–638. [Google Scholar] [CrossRef]
- Haskell, W.L.; Savin, W.M.; Schroeder, J.S.; Alderman, E.A.; Ingles, N.B., Jr.; Daughters, G.T., 2nd; Stinson, E.B. Cardiovascular responses to handgrip isometric exercise in patients following cardiac transplantation. Circ. Res. 1981, 48 Pt 2, I156–I161. [Google Scholar]
- Nóbrega, A.C.; Williamson, J.W.; Garcia, J.A.; Mitchell, J.H. Mechanisms for increasing stroke volume during static exercise with fixed heart rate in humans. J. Appl. Physiol. 1997, 83, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F. Non-invasive assessment of left ventricular filling pressure. Eur. J. Heart Fail. 2018, 20, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; Hwang, I.C.; Park, J.J.; Park, J.B. Decreased Peak Left Atrial Longitudinal Strain Is Associated with Persistent Pulmonary Hypertension Associated with Left Heart Disease. J. Clin. Med. 2022, 11, 3510. [Google Scholar] [CrossRef] [PubMed]
- Coneglian, J.C.; Barcelos, G.T.; Bandeira, A.C.N.; Carvalho, A.C.A.; Correia, M.A.; Farah, B.Q.; Ritti-Dias, R.M.; Gerage, A.M. Acute Blood Pressure Response to Different Types of Isometric Exercise: A Systematic Review with Meta-Analysis. Rev. Cardiovasc. Med. 2023, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R. Influence of muscle mass on sympathetic neural activation during isometric exercise. J. Appl. Physiol. 1989, 67, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Irving, J.B.; Kerr, F.; Kirby, B.J. Static exercise in untreated systemic hypertension. Br. Heart J. 1973, 35, 413–421. [Google Scholar] [CrossRef]
- Larsen, A.I. Strength and aerobic exercise training in coronary artery disease; it’s not “either-or". Eur. J. Prev. Cardiol. 2017, 24, 1686–1691. [Google Scholar] [CrossRef]
- Lawrence, M.M.; Cooley, I.D.; Huet, Y.M.; Arthur, S.T.; Howden, R. Factors influencing isometric exercise training-induced reductions in resting blood pressure. Scand. J. Med. Sci. Sports 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Caminiti, G.; Volterrani, M.; Iellamo, F.; Marazzi, G.; Manzi, V.; D’Antoni, V.; Vadalà, S.; Di Biasio, D.; Catena, M.; Morsella, V.; et al. Changes in left atrial function following two regimens of combined exercise training in patients with ischemic cardiomyopathy: A pilot study. Front. Cardiovasc. Med. 2024, 11, 1377958. [Google Scholar] [CrossRef]
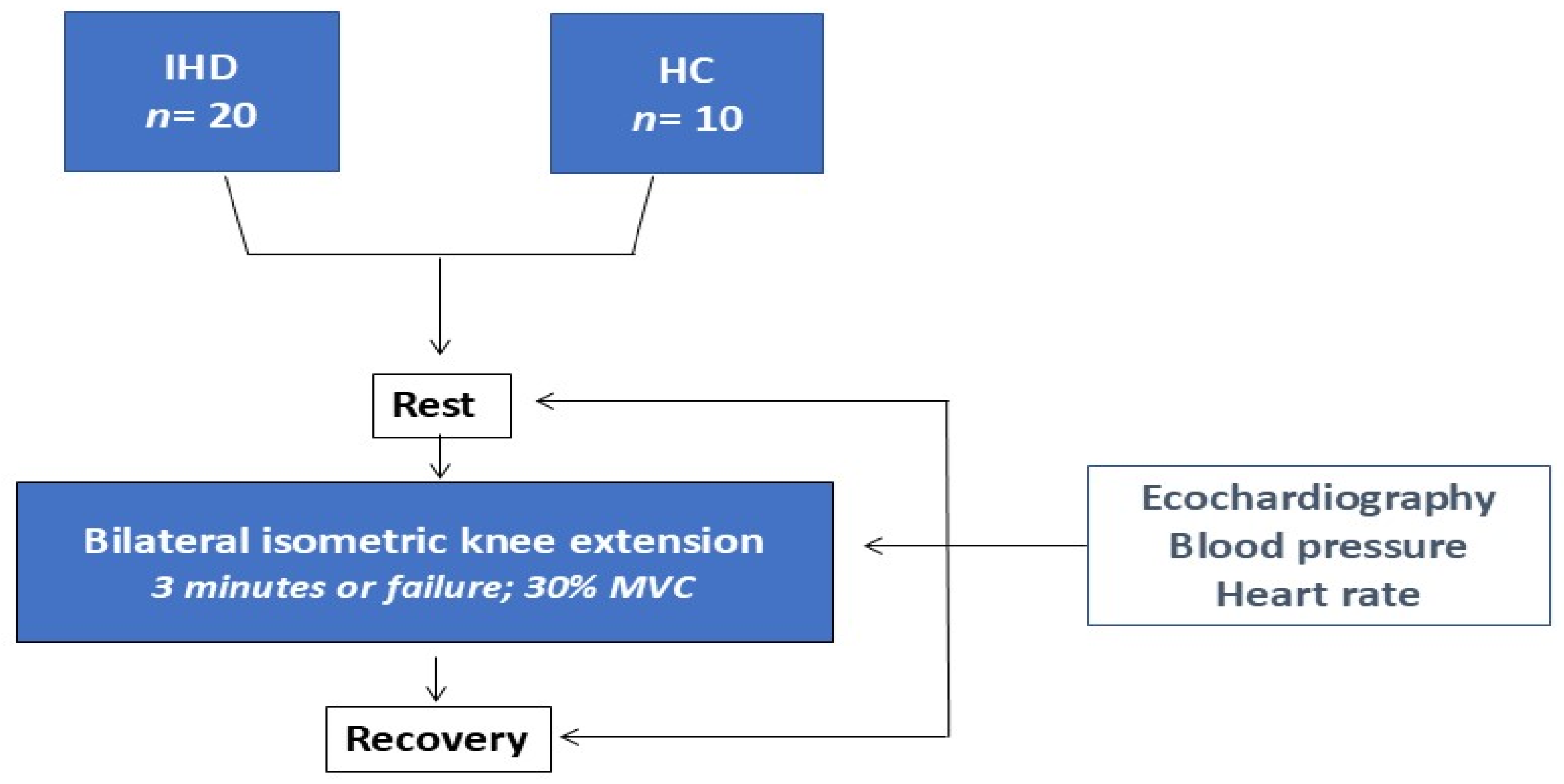

| IHD (n = 20) | HC (n = 10) | |
|---|---|---|
| Age, years | 63.4 ± 7.5 | 61.8 ± 4.8 |
| BMI, kg/m2 | 27.5 ± 6.3 | 26.7 ± 7.1 |
| Male/female | 16/4 | 7/3 |
| Previous PCI/CABG | 17/8 | - |
| EF, (%) | 50.3 ± 7.4 † | 58.4 ± 3.6 |
| NT-pro BNP | 95.0 ± 59.3 | 22 |
| Comorbidities | ||
| Carotid artery disease, n (%) | 8 (40) | - |
| Hypertension, n (%) | 20/(100) | - |
| Diabetes, n (%) | 5 (25) | - |
| Hypercholesterolemia, n (%) | 18 (90) | 4 (40) |
| Previous Smoke habit, n (%) | 13 (65) | 3 (30) |
| Treatment | ||
| Anti-platelet agents, n (%) | 20 (100) | |
| ACE-Is/ARBs, n (%) | 18 (90) | - |
| Betablockers, n (%) | 20 (100) | - |
| Diuretics, n (%) | 8 (40) | - |
| Ranolazine, n (%) | 4 (20) | |
| Ivabradine, n (%) | 1 (5) | |
| Statins, n (%) | 20 (100) | 3 (30) |
| Ezetimibe, n (%) | 12 (60) | 1 (10) |
| IHD (n = 20) | HC (n = 10) | |||||
|---|---|---|---|---|---|---|
| Rest | Peak | Recovery | Rest | Peak | Recovery | |
| HR, b/min | 68.7 ± 10.2 | 77.1 ± 14.6 | 63.1 ± 7.7 | 71.4 ± 12.0 | 91.1 ± 9.3 *,† | 75.1 ± 11.0 |
| SBP, mmHg | 124.6 ± 15.8 | 162.2 ± 34.6 *,† | 121.5 ± 14.6 | 124.1 ± 13 | 132.5 ± 11.2 | 120.7 ± 16.5 |
| DBP, mmHg | 77.8 ± 9.3 | 97.2 ± 20.2 *,† | 76.5 ± 6.0 | 75.0 ± 11.3 | 82.4 ± 7.6 | 76.3 ± 12.9 |
| DP | 8560.0 ± 952.7 | 12,505.6 ± 883.1 | 7666.6 ± 874.5 | 8860.7 ± 652.8 | 12,070.7 ± 1023.2 | 9064.5 ± 789.5 |
| Echocardiography | ||||||
| LVEDV, mL | 140.4 ± 33.4 | 122.3 ± 35.6 *,† | 137.1 ± 35.2 | 118.9 ± 18.2 | 119.4 ± 15.3 | 119.6 ± 16.1 |
| LVESV, mL | 70.5 ± 11.7 | 67.2 ± 16.1 | 72.2 ± 14.5 | 50.2 ± 7.9 | 49.3 ± 7.4 | 47.4 ± 6.5 |
| LVEF, % | 50.3 ± 7.4 | 49.7 ± 7.2 | 48.3 ± 5.6 | 58.4 ± 3.6 | 59.3 ± 3.2 | 59.5 ± 2.2 |
| LVGLS, % | −11.4 ± 3.8 | −7.9 ± 2.7 *,† | −12.2 ± 3.8 | −18.4 ± 2.4 | −18.6 ± 2.5 | −20.1 ± 2.6 |
| GWI, % | 1050.5 ± 412.6 | 1212.7 ± 356.4 *,† | 1145.6 ± 521.8 | 2033.0 ± 108.5 | 2025.7 ± 130.2 | 1573.1 ± 413.3 |
| GCW, % | 1501.3 ± 491.8 | 1958.1 ± 328.3 *,† | 1391.9 ± 469.0 | 2231.3 ± 85.8 | 2114.3 ± 126.7 | 2085.0 ± 348.6 |
| GWW, % | 289.7 ± 127.7 | 554.9 ± 304.7 *,† | 218.8 ± 128.7 | 52.7 ± 5.2 | 54.4 ± 3.1 | 58.5 ± 156.5 |
| GWE, % | 80.7 ± 11.7 | 66.4 ± 8.1 *,† | 82.3 ± 11.2 | 96.0 ± 2.9 | 95.3 ± 3.6 | 95.2 ± 9.6 |
| DT, ms | 248.7 ± 60.4 | 220.3 ± 57.6 *,† | 254.6 ± 56.3 | 194.8 ± 38.4 | 192.1 ± 53.6 | 192.4 ± 53.2 |
| E, cm/s | 48.1 ± 9.0 | 60.4 ± 15.2 | 48.5 ± 13.1 | 55.7 ± 13.4 | 55.4 ± 17.0 | 64.3 ± 12.9 |
| A, cm/s | 66.4 ± 16.1 | 77.5 ± 23.2 | 63.0 ± 15.2 | 48.6 ± 9.3 | 54.8 ± 9.8 | 51.2 ± 11.3 |
| E/A ratio | 0.75 ± 0.16 | 0.84 ± 15.2 | 0.78 ± 0.2 | 1.2 ± 0.3 | 1.5 ± 0.4 | 1.3 ± 0.4 |
| E′, cm/s | 7.4 ± 1.5 | 5.4 ± 4.4 *,† | 6.2 ± 3.1 | 11.7 ± 1.6 | 11.5 ± 2.8 | 11.9 ± 2.3 |
| E/E′ ratio | 6.7 ± 1.9 | 10.4 ± 7.4 *,† | 7.4 ± 2.4 | 5.2 ± 0.8 | 5.0 ± 1.1 | 6.4 ± 2.4 |
| TRV, m/s | 1.7 ± 0.4 | 1.7 ± 0.5 | 1.9 ± 0.5 | 1.7 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.4 |
| PALS, % | 19.7 ± 8.5 | 16.8 ± 6.7 | 22.2 ± 8.4 | 22.6 ± 4.7 | 22.4 ± 8.1 | 23.7 ± 9.4 |
| PACS, % | −8.9 ± 3.5 | −11.7 ± 6.7 | −12.3 ± 8.0 | −12.6 ± 4.5 | −12.5 ± 7.5 | −13.4 ± 6.1 |
| LAVI, mL/m2 | 25.6 ± 7.2 | 21.3 ± 7.8 | 22.9 ± 7.2 | 19.4 ± 3.2 | 20.25 ± 6.2 | 18.8 ± 3.5 |
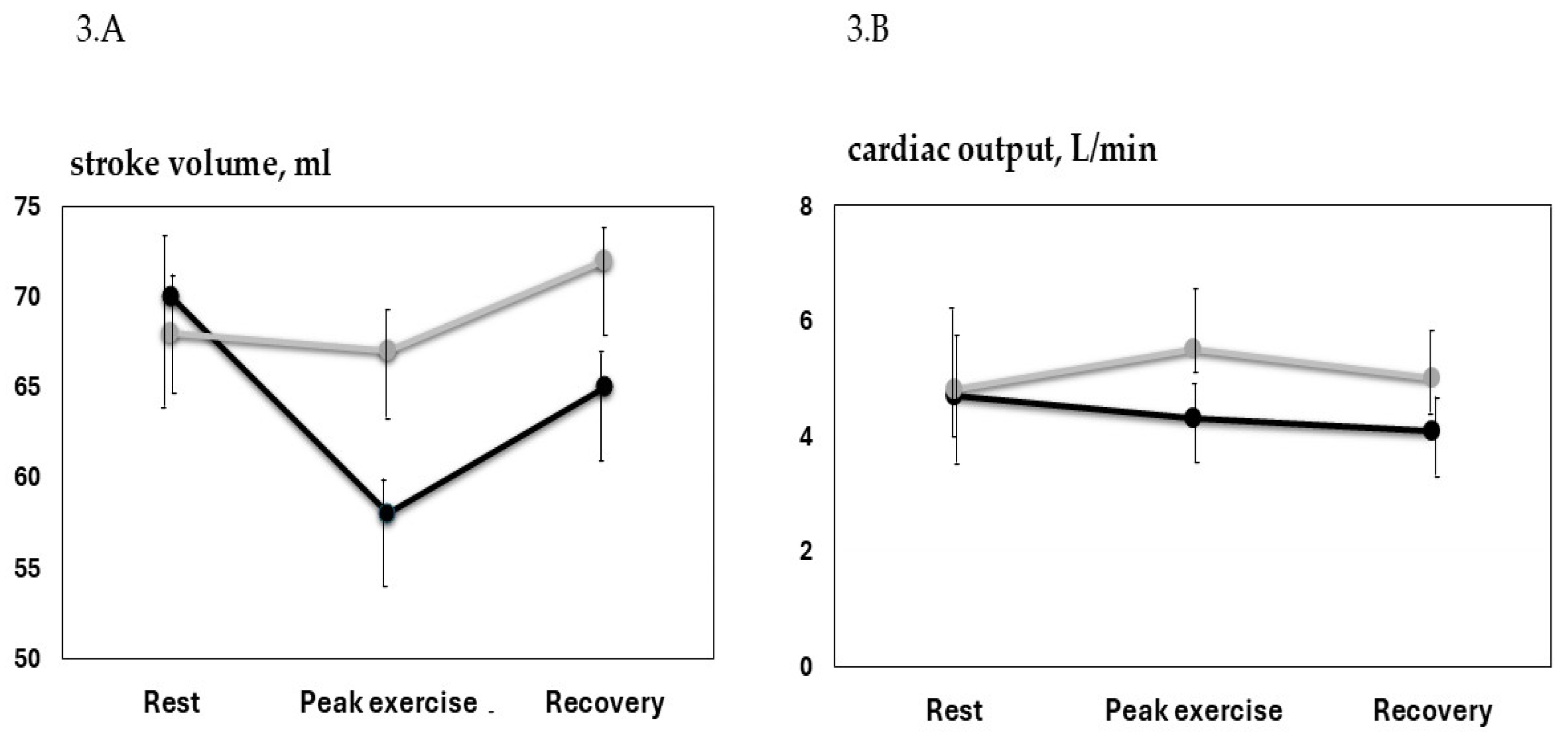
| SV, mL | 70.3 ± 14.3 | 58.5 ± 12.1 *,† | 64.7 ± 14.1 | 68.6 ± 11.8 | 67.1 ± 15.2 | 72.2 ± 6.1 |
| CO, L/min | 4.7 ± 1.1 | 4.3 ± 1.3 † | 4.1 ± 1.1 | 4.9 ± 0.8 | 5.5 ± 0.7 | 5.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminiti, G.; Volterrani, M.; Iellamo, F.; Marazzi, G.; D’Antoni, V.; Calandri, C.; Vadalà, S.; Catena, M.; Di Biasio, D.; Manzi, V.; et al. Acute Changes in Myocardial Work during Isometric Exercise in Hypertensive Patients with Ischemic Heart Disease: A Case–Control Study. J. Clin. Med. 2024, 13, 5955. https://doi.org/10.3390/jcm13195955
Caminiti G, Volterrani M, Iellamo F, Marazzi G, D’Antoni V, Calandri C, Vadalà S, Catena M, Di Biasio D, Manzi V, et al. Acute Changes in Myocardial Work during Isometric Exercise in Hypertensive Patients with Ischemic Heart Disease: A Case–Control Study. Journal of Clinical Medicine. 2024; 13(19):5955. https://doi.org/10.3390/jcm13195955
Chicago/Turabian StyleCaminiti, Giuseppe, Maurizio Volterrani, Ferdinando Iellamo, Giuseppe Marazzi, Valentino D’Antoni, Camilla Calandri, Sara Vadalà, Matteo Catena, Deborah Di Biasio, Vincenzo Manzi, and et al. 2024. "Acute Changes in Myocardial Work during Isometric Exercise in Hypertensive Patients with Ischemic Heart Disease: A Case–Control Study" Journal of Clinical Medicine 13, no. 19: 5955. https://doi.org/10.3390/jcm13195955









