Capillary Blood Docosahexaenoic Acid Levels Predict Electrocardiographic Markers in a Sample Population of Premenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Ethical Approval and Participants
2.3. Procedures
2.4. Blood Sample Collection
2.5. Capillary Blood Lipid Transmethylation, Fatty Methyl Ester Extraction and Analysis
2.6. Diet Analysis
2.7. Electrocardiographic Reading Analysis
2.8. Statistical Analysis
3. Results
3.1. Anthropometric Data
3.2. Electrocardiographic Analysis
3.3. Blood Fatty Acid Profile
3.4. ECG Partial Correlations
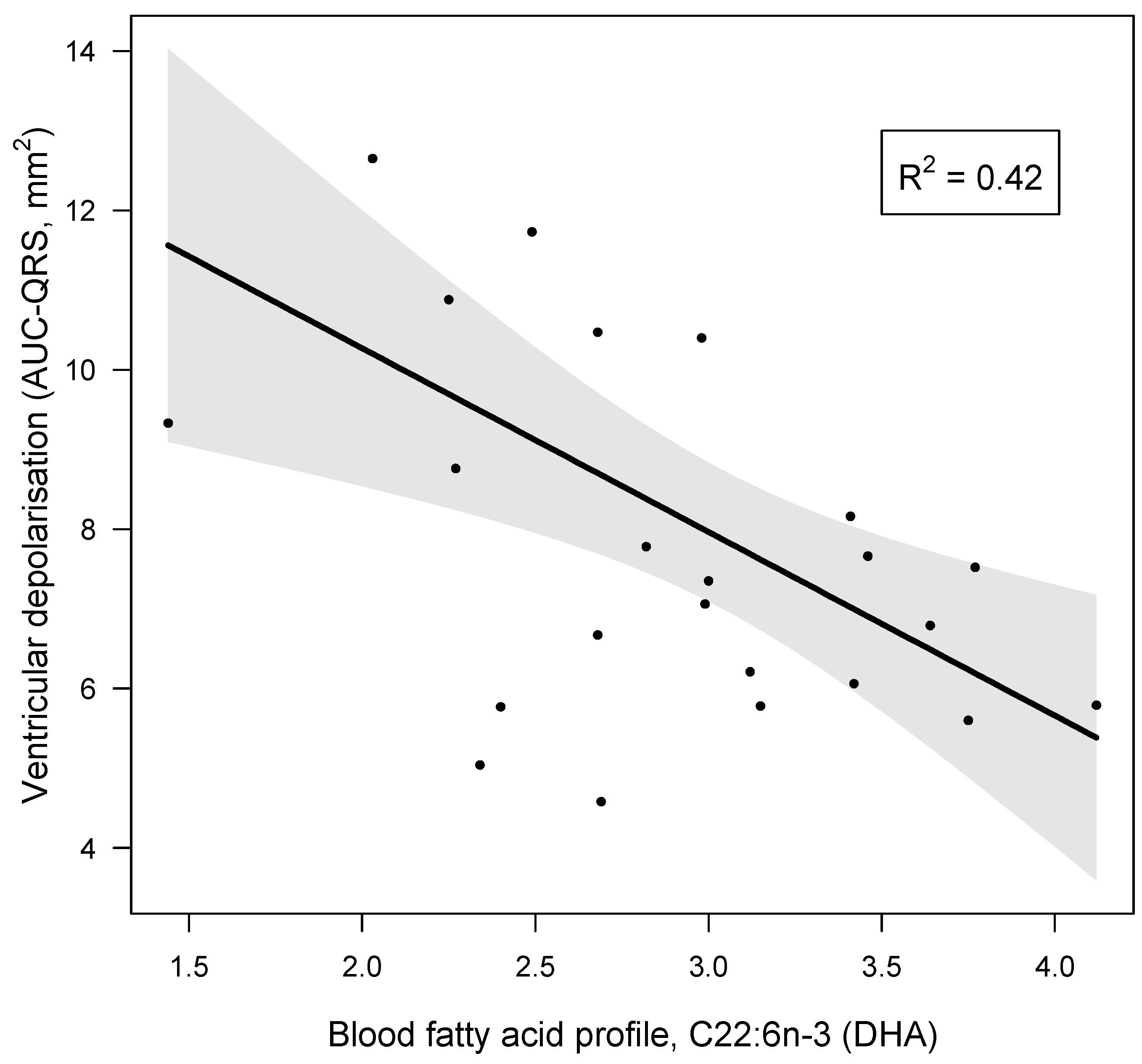
3.5. Blood DHA and AUC-QRS
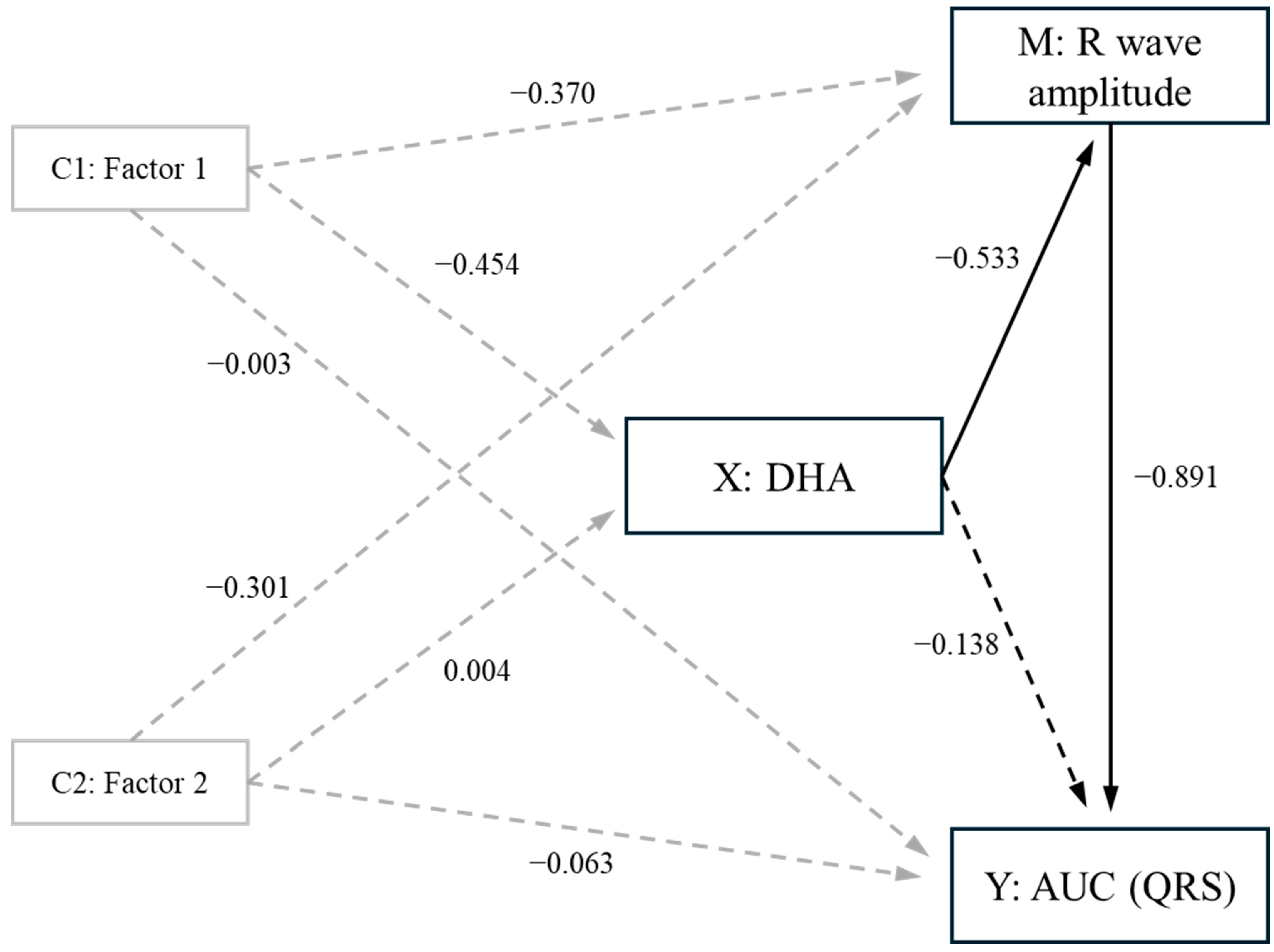
3.6. Mediation Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, P.; Yu, S.; Wang, J.; Zou, S.; Yao, D.S.; Xiaochen, Y. Global burden, trends, and inequalities of ischemic heart disease among young adults from 1990 to 2019: A population-based study. Front. Cardiovasc. Med. 2023, 10, 1274663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Erratum in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef]
- Office for National Statistics. Deaths Registered in England and Wales. Available online: https://www.ons.gov.uk (accessed on 9 June 2024).
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.F.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Couillard, C.; Ruel, G.; Archer, W.R.; Pomerleau, S.; Bergeron, J.; Couture, P.; Lamarche, B.; Bergeron, N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J. Clin. Endocrinol. Metab. 2005, 90, 6454–6459. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Engin, A. Endothelial dysfunction in obesity. Adv. Exp. Med. Biol. 2017, 960, 345–379. [Google Scholar]
- WHO Obesity and Overweight Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 September 2022).
- Congdon, P.; Amugsi, D. Editorial: The obesity epidemic: Causes, context, prevention. Front. Public Health 2022, 10, 1030180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fried, S.K.; Rao, S.P. Sugars, hypertriglyceridemia, and cardiovascular disease. Am. J. Clin. Nutr. 2003, 78, 873S–880S. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.; Abramson, J.L.; Vaccarino, V.; Gillespie, C.; Vos, M.B. Caloric sweetener consumption and dyslipidaemia among US adults. J. Am. Med. Assoc. 2010, 303, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Gerber, P.A.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.J.; Stamler, J.; Van Horn, L.; Robertson, C.E.; Chan, Q.; Dyer, A.R.; Huang, C.C.; Rodriguez, B.L.; Zhao, L.; Daviglus, M.L.; et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: International study of macro/micronutrients and blood pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iqbal, M.P. Trans fatty acids—A risk factor for cardiovascular disease. Pak. J. Med. Sci. 2014, 30, 194–197. [Google Scholar] [CrossRef]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef]
- Daniel, N.; Rossi Perazza, L.; Varin, T.V.; Trottier, J.; Marcotte, B.; St-Pierre, P.; Barbier, O.; Chassaing, B.; Marette, A. Dietary fat and low fiber in purified diets differently impact the gut-liver axis to promote obesity-linked metabolic impairments. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G1014–G1033. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.H.; Chen, Y.Y.; et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, L.; Wang, J.; Xiong, K.; Xu, L.; Zhang, B.; Ma, A. Intake of Fish and Marine n-3 Polyunsaturated Fatty Acids and Risk of Cardiovascular Disease Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 2342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dontas, A.S.; Zerefos, N.S.; Panagiotakos, D.B.; Vlachou, C.; Valis, D.A. Mediterranean diet and prevention of coronary heart disease in the elderly. Clin. Interv. Aging 2007, 2, 109–115, Erratum in Clin. Interv. Aging 2008, 3, 397. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Adherence to the Healthy Eating Index-2015 and Other Dietary Patterns May Reduce Risk of Cardiovascular Disease, Cardiovascular Mortality, and All-Cause Mortality. J. Nutr. 2020, 150, 312–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teicholz, N. A short history of saturated fat: The making and unmaking of a scientific consensus. Curr. Opin. Endocrinol. Diabetes Obes. 2023, 30, 65–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2020, 22, 330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, X.; Xia, J.; Zhou, Y.; Wang, Y.; Xia, H.; Wang, S.; Liao, W.; Sun, G. The Effect of MUFA-Rich Food on Lipid Profile: A Meta-Analysis of Randomized and Controlled-Feeding Trials. Foods 2022, 11, 1982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgiadi, A.; Kersten, S. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 2012, 3, 127–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berland, C.; Cansell, C.; Hnasko, T.S.; Magnan, C.; Luquet, S. Dietary triglycerides as signaling molecules that influence reward and motivation. Curr. Opin. Behav. Sci. 2016, 9, 126–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757, Erratum in Circulation 2003, 107, 512. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers-An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef]
- Santos, H.O.; May, T.L.; Bueno, A.A. Eating more sardines instead of fish oil supplementation: Beyond omega-3 polyunsaturated fatty acids, a matrix of nutrients with cardiovascular benefits. Front. Nutr. 2023, 10, 1107475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, A.; Sharma, A.; Chen, M.; Toy, R.; Mottola, G.; Conte, M.S. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE 2014, 9, e113480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peña-de-la-Sancha, P.; Muñoz-García, A.; Espínola-Zavaleta, N.; Bautista-Pérez, R.; Mejía, A.M.; Luna-Luna, M.; López-Olmos, V.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Carreón-Torres, E.; et al. Eicosapentaenoic and Docosahexaenoic Acid Supplementation Increases HDL Content in n-3 Fatty Acids and Improves Endothelial Function in Hypertriglyceridemic Patients. Int. J. Mol. Sci. 2023, 24, 5390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreno, C.; Macías, A.; Prieto, A.; De la Cruz, A.; González, T.; Valenzuela, C. Effects of n-3 polyunsaturated fatty acids on cardiac ion channels. Front. Physiol. 2012, 9, 245. [Google Scholar] [CrossRef]
- Billman, G.E.; Harris, W.S. Effect of dietary omega-3 fatty acids on the heart rate and the heart rate variability responses to myocardial ischemia or submaximal exercise. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2288–H2299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Christensen, J.H.; Christensen, M.S.; Dyerberg, J.; Schmidt, E.B. Heart rate variability and fatty acid content of blood cell membranes: A dose-response study with n-3 fatty acids. Am. J. Clin. Nutr. 1999, 70, 331–337. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H., Jr.; Abuissa, H.; Sastre, A.; Steinhaus, D.M.; Harris, W.S. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am. J. Cardiol. 2006, 97, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar] [PubMed]
- Boldarine, V.T.; Joyce, E.; Pedroso, A.P.; Telles, M.M.; Oyama, L.M.; Bueno, A.A.; Ribeiro, E.B. Oestrogen replacement fails to fully revert ovariectomy-induced changes in adipose tissue monoglycerides, diglycerides and cholesteryl esters of rats fed a lard-enriched diet. Sci. Rep. 2021, 11, 3841. [Google Scholar] [CrossRef]
- Wilson, F.N.; Macleod, A.; Barker, P.S. The potential variations produced by the heart beat at the apices of Einthoven’s triangle. Am. Heart J. 1931, 7, 207–211. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 May 2024).
- Tibbe, T.D.; Montoya, A.K. Correcting the bias correction for the bootstrap confidence interval in mediation analysis. Front. Psychol. 2022, 13, 810258. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/bitstream/handle/10665/44583/?sequence=1 (accessed on 17 September 2022).
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Zhang, Z.M.; Gregg, R.E.; Haisty, W.K., Jr.; ZVitolins, M.; Curtis, A.B.; Warren, J.; Horaĉek, M.B.; Zhou, S.H.; Soliman, E.Z. Normal standards for computer-ECG programs for prognostically and diagnostically important ECG variables derived from a large ethnically diverse female cohort: The Women’s Health Initiative (WHI). J. Electrocardiol. 2013, 46, 707–716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meek, S.; Morris, F. Introduction. II—Basic terminology. BMJ 2002, 324, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Vepsäläinen, T.; Laakso, M.; Lehto, S.; Juutilainen, A.; Airaksinen, J.; Rönnemaa, T. Prolonged P wave duration predicts stroke mortality among type 2 diabetic patients with prevalent non-major macrovascular disease. BMC Cardiovasc. Disord. 2014, 14, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rijnbeek, P.R.; van Herpen, G.; Bots, M.L.; Man, S.; Verweij, N.; Hofman, A.; Hillege, H.; Numans, M.E.; Swenne, C.A.; Witteman, J.C.; et al. Normal values of the electrocardiogram for ages 16–90 years. J. Electrocardiol. 2014, 47, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.H.; Evans, D.W. Correlation of left ventricular mass determined by echocardiography with vectorcardiographic and electrocardiographic voltage measurements. Br. Heart J. 1974, 36, 981–987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio AL, P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Gersovitz, M.; Madden, J.P.; Smiciklas-Wright, H. Validity of the 24-hr. dietary recall and seven-day record for group comparisons. J. Am. Diet. Assoc. 1978, 73, 48–55. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef]
- Sheppard, K.W.; Cheatham, C.L. Omega-6/omega-3 fatty acid intake of children and older adults in the U.S.: Dietary intake in comparison to current dietary recommendations and the Healthy Eating Index. Lipids Health Dis. 2018, 17, 43. [Google Scholar] [CrossRef]
- Marangoni, F.; Colombo, C.; Martiello, A.; Negri, E.; Galli, C. The fatty acid profiles in a drop of blood from a fingertip correlate with physiological, dietary and lifestyle parameters in volunteers. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Ghebremeskel, K.; Geppert, J.; Khalil, F. Effect of storage temperature and length on fatty acid composition of fingertip blood collected on filter paper. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Sparkes, C.; Sinclair, A.J.; Gibson, R.A.; Else, P.L. Fingertip Whole Blood as an Indicator of Omega-3 Long-Chain Polyunsaturated Fatty Acid Changes during Dose-Response Supplementation in Women: Comparison with Plasma and Erythrocyte Fatty Acids. Nutrients 2021, 13, 1419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makrides, M.; Neumann, M.A.; Byard, R.W.; Simmer, K.; Gibson, R.A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 1994, 60, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzaki, N.; Eda, A.; Kameoka, R.; Nakashima, Y. Effects of Intake of Maternal Dietary Elaidic Acids during Pregnancy and Lactation on the Fatty Acid Composition of Plasma, Erythrocyte Membrane, and Brain in Rat Pups. J. Nutr. Metab. 2013, 2013, 701818. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Petridou, A.; Mougios, V. Comparison of the phospholipid and triacylglycerol fatty acid profile of rat serum, skeletal muscle and heart. Physiol. Res. 2006, 55, 259–265. [Google Scholar] [CrossRef]
- Nodera, M.; Suzuki, H.; Yamada, S.; Kamioka, M.; Kaneshiro, T.; Kamiyama, Y.; Takeishi, Y. Association of Serum n-3/n-6 Polyunsaturated Fatty Acid Ratio with T-Wave Alternans in Patients with Ischemic Heart Disease. Int. Heart J. 2015, 56, 613–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McLennan, P.L. Cardiac physiology and clinical efficacy of dietary fish oil clarified through cellular mechanisms of omega-3 polyunsaturated fatty acids. Eur. J. Appl. Physiol. 2014, 114, 1333–1356. [Google Scholar] [CrossRef]
- Ramadeen, A.; Connelly, K.A.; Leong-Poi, H.; Hu, X.; Fujii, H.; Laurent, G.; Domenichiello, A.F.; Bazinet, R.P.; Dorian, P. Docosahexaenoic Acid, but Not Eicosapentaenoic Acid, Supplementation Reduces Vulnerability to Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2012, 5, 978–983. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef]
- Sherrard, G. Are Anthropometric Biomarkers, Nutrient Intake and Blood Fatty Acid Composition Associated with the Electrical Activity of the Heart in a Sample Population of Healthy Women? Master’s Thesis, School of Science and the Environment, University of Worcester, Worcester, UK, 2023. Available online: https://eprints.worc.ac.uk/14186/ (accessed on 9 August 2024).
| Anthropometric Parameters | Mean (SD) or Median (IQR) | Typical Values and Expected Ranges |
|---|---|---|
| Age # | 38.00 (IQR 35.00, 39.00) | |
| Height (cm) | 166.73 (SD: 7.03) | |
| Weight (kg) | 71.96 (SD: 14.72) | |
| Waist circumference (cm) | 80.04 (SD: 8.55) | ≤80 [46] |
| Hip circumference (cm) | 104.28 (SD: 9.55) | |
| Waist–hip ratio | 0.77 (SD: 0.04) | <0.85 [46] |
| Body fat % | 36.29 (SD: 5.11) | |
| BMI (kg/m2) # | 24.50 (IQR 22.15, 27.25) | 18.5–24.9 |
| Haematocrit (%) | 39.52 (SD: 2.35) | 36–46 |
| Heart rate (bpm) | 67.91 (SD: 8.15) | 60–100 |
| SpO2 (%) | 98.00 (SD: 0.60) | 95–100 |
| SBP (mmHg) | 117.52 (SD: 10.68) | 90–120 |
| DBP (mmHg) # | 73.00 (IQR 70.50, 85.50) | 60–80 |
| MAP (mmHg) | 90.46 (SD: 8.46) | 70–100 |
| Electrocardiographic parameters | ||
| AUC for the QRS complex (mm2) | 7.74 (SD: 2.23) [47] | |
| QRS duration (ms) # | 84.80 (IQR 73.60, 89.60) [48] | 70–104 [48] |
| R wave amplitude (mV) | 1.08 (SD: 0.32) [47] | <2 mV [47] |
| PR interval (ms) | 149.13 (SD: 21.06) [49] | 118–212 [49] |
| P wave duration (ms) | 93.30 (SD: 12.80) [48] | <110 [48] |
| P wave amplitude (mV) | 0.14 (SD: 0.03) [47] | <0.25 mV [47] |
| QT interval (ms) | 393.06 (SD: 20.51) [47] | 388–450 [47] |
| QTc # | 408 (IQR 395.98, 432.97) [50] | 419 (377, 464) [50] |
| Fatty Acid (% of Total Fatty Acids) | Mean (SD) | Reference Values # |
|---|---|---|
| Total SFAs | 35.308 (SD: 2.829) | 36.8 (SD: 1.5) |
| Palmitic acid (C16:0) | 23.332 (SD: 2.119) | 22.7 (SD: 1.9) |
| Total MUFAs | 25.193 (SD: 3.000) | 24.3 (SD: 2.5) |
| Oleic acid (C18:1n-9) | 20.483 (SD: 2.374) | 20.0 (SD: 2.5) |
| Total PUFAs | 37.890 (SD 4.189) | |
| Total n-6 | 32.939 (SD: 3.685) | 33.2 (SD: 1.8) |
| Arachidonic acid (C20:4n-6) | 8.769 (SD: 1.501) | 8.1 (SD: 1.6) § |
| Total n-3 | 4.953 (SD: 0.788) | 4.5 (SD: 1.3) |
| Docosahexaenoic acid (C22:6n-3) | 2.909 (SD: 0.642) | 2.8 (SD: 1.1) |
| n-6:n:3 ratio | 6.752 (SD: 0.924) |
| Parameters | ECG Phase | ||
|---|---|---|---|
| AUC (QRS) | QRS Duration | R Wave Amplitude | |
| Blood fatty acids | |||
| C16:0 (%) | r = −0.495, p = 0.031, pw = 0.736 | ||
| C16:1n-7 (%) | r = −0.732, p = 0.006, pw = 0.998 * | ||
| C22:6n-3 (DHA) | r = −0.668, p = 0.007, pw = 0.983 * | r = −0.612, p = 0.016, pw = 0.940 * | |
| Total n-3 (%) | r = −0.615, p = 0.033, pw = 0.944 * | r = −0.561, p = 0.042, pw = 0.869 * | |
| n6:n3 ratio | r = 0.687, p = 0.004, pw = 0.990 * | r = 0.671, p = 0.006, pw = 0.984 * | |
| Dietary analysis | |||
| Total fat (g) | r = 0.678, p = 0.001, pw = 0.990 * | r = 0.527, p = 0.008, pw = 0.889 * | r = 0.539, p = 0.009, pw = 0.829 * |
| Saturated fat (g) | r = 0.523, p = 0.013, pw = 0.797 | ||
| Total Kcal | r = 0.568, p = 0.005, pw = 0.881 * | r = 0.567, p = 0.004, pw = 0.879 * | |
| Carbohydrates (g) | r = 0.428, p = 0.032, pw = 0.578 | r = 0.441, p = 0.017, pw = 0.609 | |
| Fibre (g) | r = 0.511, p = 0.012, pw = 0.772 | r = 0.491, p = 0.012, pw = 0.727 | |
| NSP (g) | r = 0.438, p = 0.027, pw = 0.602 | r = 0.424, p = 0.021, pw = 0.568 | |
| Sugars (g) | r = 0.444, p = 0.043, pw = 0.616 | ||
| Glucose (g) | r = 0.498, p = 0.025, pw = 0.743 | ||
| Potassium (mg) | r = 0.423, p = 0.005, pw = 0.566 | r = 0.449, p = 0.008, pw = 0.628 | |
| Calcium (mg) | r = 0.490, p = 0.008, pw = 0.725 | r = 0.423, p = 0.023, pw = 0.566 | |
| Magnesium (mg) | r = 0.570, p = 0.002, pw = 0.884 * | r = 0.478, p = 0.011, pw = 0.697 | |
| Carotene (µg) | r = 0.423, p = 0.049, pw = 0.566 | ||
| Vitamin E (mg) | r = 0.534, p = 0.011, pw = 0.819 * | ||
| Parameters | PR Interval (ms) | P Wave Duration (ms) | P Wave Amplitude (mV) | QTc (Bazzet Formula) |
|---|---|---|---|---|
| Weight (kg) | r = 0.851, p = 0.008, pw > 0.999 * | r = 0.716, p = 0.013, pw = 0.997 * | ||
| Blood fatty acids | ||||
| C14:0 (%) | r = −0.570, p = 0.011, pw = 0.874 * | r = −0.574, p = 0.007, pw = 0.890 * | ||
| C18:0 (%) | r = 0.641, p = 0.033, pw = 0.962 * | r = 0.752, p = 0.001, pw = 0.999 * | ||
| C24:0 (%) | r = 0.466, p = 0.008, pw = 0.669 | |||
| Total SFAs (%) | r = 0.544, p = 0.016, pw = 0.839 * | r = 0.600, p = 0.004, pw = 0.926 * | ||
| C18:1n-9 (%) | ||||
| Total MUFAs (%) | r = 0.486, p = 0.023, pw = 0.716 | |||
| C18:2n-6t (9t,12c) (%) | r = −0.542, p = 0.011, pw = 0.835 * | |||
| C20:3n-3 (%) | r = 0.494, p = 0.015, pw = 0.734 | |||
| Dietary analysis | ||||
| SFA (g) | r = −0.409, p = 0.033, pw = 0.532 | |||
| MUFAs (g) | r = −0.491, p = 0.004, pw = 0.727 | |||
| n-6:n-3 | r = −0.663, p = 0.029, pw = 0.981 * | |||
| Total KCal | r = −0.439, p = 0.005, pw = 0.239 | |||
| Parameter Estimates (Coefficients) | 95% Confidence Intervals | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Names | Estimate | SE | Lower | Upper | β | df | t-Value | p-Value | pw |
| (Intercept) | 7.741 | 0.381 | 6.961 | 8.496 | 0.018 | 19 | 20.291 | <0.001 | |
| C22:6n-3 (DHA) | −2.304 | 0.693 | −3.410 | −0.753 | −0.668 | 19 | −3.005 | 0.007 * | 0.983 |
| Component 1 | −0.888 | 0.444 | −1.618 | 0.0719 | −0.391 | 19 | −1.741 | 0.098 | 0.490 |
| Component 2 | −0.654 | 0.390 | −1.621 | 0.270 | −0.312 | 19 | −1.731 | 0.100 | 0.321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casagrande, B.P.; Sherrard, G.; Fowler, M.S.; Estadella, D.; Bueno, A.A. Capillary Blood Docosahexaenoic Acid Levels Predict Electrocardiographic Markers in a Sample Population of Premenopausal Women. J. Clin. Med. 2024, 13, 5957. https://doi.org/10.3390/jcm13195957
Casagrande BP, Sherrard G, Fowler MS, Estadella D, Bueno AA. Capillary Blood Docosahexaenoic Acid Levels Predict Electrocardiographic Markers in a Sample Population of Premenopausal Women. Journal of Clinical Medicine. 2024; 13(19):5957. https://doi.org/10.3390/jcm13195957
Chicago/Turabian StyleCasagrande, Breno P., George Sherrard, Mike S. Fowler, Débora Estadella, and Allain A. Bueno. 2024. "Capillary Blood Docosahexaenoic Acid Levels Predict Electrocardiographic Markers in a Sample Population of Premenopausal Women" Journal of Clinical Medicine 13, no. 19: 5957. https://doi.org/10.3390/jcm13195957
APA StyleCasagrande, B. P., Sherrard, G., Fowler, M. S., Estadella, D., & Bueno, A. A. (2024). Capillary Blood Docosahexaenoic Acid Levels Predict Electrocardiographic Markers in a Sample Population of Premenopausal Women. Journal of Clinical Medicine, 13(19), 5957. https://doi.org/10.3390/jcm13195957





