Association between Visit-to-Visit Ultrafiltration Volume Variability, Vascular Biomarkers and Cardiovascular Parameters in Chronic Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Measurements
2.3. Echocardiography
2.4. Statistical Analysis
2.5. Ethical Consideration
3. Results
4. Discussion
5. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, J.; Bowe, B.; Mokdad, A.; Tsai, C.Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990–2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Brück, K.; Stel, V.; Gambaro, G.; Hallan, S.; Völcke, H.; Arnlov, J. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.; Agodoa, L.; Bhave, N.; Bragg-Gresham, J. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2017, 71, A7. [Google Scholar] [CrossRef]
- Rehm, M.; Haller, M.; Orth, V.; Kreimeier, U.; Jacob, M.; Dressel, H. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology 2001, 95, 849–856. [Google Scholar] [CrossRef]
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001, 19, 53–61. [Google Scholar] [CrossRef]
- Sarnak, M.; Levey, A.; Schoolwerth, A.; Cores, J.; Culleton, B.; Hamm, L. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Woo, J.; Wang, M.; Sea, M.; Ip, R.; Li, P. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J. Am. Soc. Nephrol. 2001, 12, 1927–1936. [Google Scholar] [CrossRef]
- Ok, E.; Asci, G.; Chazot, C.; Ozkahya, M.; Mees, E.J.D. Controversies and problems of volume control and hypertension in haemodialysis. Lancet 2016, 388, 285–293. [Google Scholar] [CrossRef]
- Canaud, B.; Chazot, C.; Koomans, J.; Collins, A. Fluid and hemodynamic management in hemodialysis patients: Challenges and opportunities. Braz. J. Nephrol. 2019, 41, 550–559. [Google Scholar] [CrossRef]
- Keane, D.F.; Raimann, J.G.; Zhang, H.; Willetts, J.; Thijssen, S.; Kotanko, P. The time of onset of intradialytic hypotension during a hemodialysis session associates with clinical parameters and mortality. Kidney Int. 2021, 99, 1408–1417. [Google Scholar] [CrossRef]
- Ohashi, Y.; Sakai, K.; Hase, H.; Joki, N. Dry weight targeting: The art and science of conventional hemodialysis. Semin. Dial. 2018, 31, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Yee, J.; Weiner, D.E.; Bansal, V.; Choi, M.J.; Brereton, L.; Berns, J.S.; Samaniego-Picota, M.; Scheel, P.; Rocco, M. Ultrafiltration rate thresholds in maintenance hemodialysis: An NKFKDOQI controversies report. Am. J. Kidney Dis. 2016, 68, 522–532. [Google Scholar] [CrossRef]
- Han, B.G.; Pak, D.; Kim, J.S.; Sohn, Y. The moderating effect of fluid overload on the relationship between the augmentation index and left ventricular diastolic function in patients with CKD. Sci. Rep. 2024, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Pecoits-Filho, R.; Barberato, S.H. Left ventricular mass in chronic kidney disease and ESRD. Clin. J. Am. Soc. Nephrol. 2009, 1, S79–S91. [Google Scholar] [CrossRef]
- Palacios, F.C.R.; Goyal, P.; Thompson, A.M.; Deschaine, B. Systolic blood pressure values might further risk-stratify the adverse outcomes of LVH in older patients with chronic kidney disease. Clin. Hypertens. 2016, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Slinin, Y.; Babu, M.; Ishani, A. Ultrafiltration rate in conventional hemodialysis: Where are the limits and what are the consequences? Semin. Dial. 2018, 31, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Csiky, B.; Sági, B.; Emmert, V.; Wittmann, I.; Sulyok, E. Cardiometabolic Effects of Irisin in Patients with End-Stage Renal Disease on Regular Hemo- or Peritoneal Dialysis. Blood Purif. 2022, 51, 450–457. [Google Scholar] [CrossRef]
- Csiky, B.; Sági, B.; Peti, A.; Lakatos, O.; Prémusz, V.; Sulyok, E. The impact of osteocalcin, osteoprotegerin and osteopontin on arterial stiffness in chronic renal failure patients on hemodialysis. Kidney Blood Press Res. 2017, 42, 1312–1321. [Google Scholar] [CrossRef]
- Dubin, R.; Owens, C.; Gasper, W.; Ganz, P.; Johansen, K. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodial. Int. 2011, 15, 350–358. [Google Scholar] [CrossRef]
- Hercog, C.; Mangrum, J.; Passman, R. Sudden cardiac death and dialysis patients. Semin. Dial. 2008, 21, 300–307. [Google Scholar] [CrossRef]
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the Endothelial Glycocalyx: From Structure to Function. Am. J. Pathol. 2020, 190, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hamm, L.; Mohler, E.; Hudaihed, A.; Arora, R.; Chen, C.S. Interrelationship of Multiple Endothelial Dysfunction Biomarkers with Chronic Kidney Disease. PLoS ONE 2015, 10, e0132047. [Google Scholar] [CrossRef]
- Liew, H.; Roberts, M.; Pope, A.; McMachon, L. Endothelial Glycocalyx damage in kidney disease correlates with uraemic toxins and endothelial dysfunction. BMC Nephrol. 2021, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, N.; Nie, L.; Lu, C.; Chen, H.; He, W.; Li, M.; Wang, Y.; Zhao, J.; Xiong, J. Visit-to-visit ultrafiltration volume variability predicts all-cause mortality in patients receiving hemodialysis. Ren. Fail. 2023, 45, 2194439. [Google Scholar] [CrossRef]
- Loutradis, C.; Sarafidis, P.A.; Papadopoulos, C.E.; Papagianni, A.; Zoccali, C. The Ebb and flow of echocardiographic cardiac function parameters in relationship to hemodialysis treatment in patients with ESRD. J. Am. Soc. Nephrol. 2018, 29, 1372–1381. [Google Scholar] [CrossRef]
- Wizemann, V.; Wabel, P.; Chamney, P.; Zaluska, W.; Moissl, U.; Rode, C.; Malecka-Masalska, T.; Marcelli, D. The mortality risk of overhydration in haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 1574–1579. [Google Scholar] [CrossRef]
- Yakar, B.; Demir, M.; Onalan, E.; Kaya, M.O. Arterial stiffness and its related factors in patients on hemodialysis. J. Coll. Physicians Surg. Pak. 2021, 31, 138–143. [Google Scholar] [CrossRef]
- Gadaen, R.J.R.; Kooman, J.P.; Cornelis, T.; van der Sande, F.M.; Winkens, B.J.; Broers, N.J.H. The Effects of Chronic Dialysis on Physical Status, Quality of Life, and Arterial Stiffness: A Longitudinal Study in Prevalent Dialysis Patients. Nephron 2021, 145, 44–54. [Google Scholar] [CrossRef]
- Padberg, J.S.; Wiesinger, A.; di Marco, G.S.; Reuter, S.; Grabner, A.; Kentrup, D.; Lukasz, A.; Oberleithner, H.; Pavenstädt, H.; Brand, M.; et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014, 234, 335–343. [Google Scholar] [CrossRef]
- Lilitkarntakul, P.; Dhaun, N.; Melville, V.; Blackwell, S.; Talwar, D.K.; Liebman, B.; Asai, T.; Pollock, J.; Goddard, J.; Webb, D.J. Blood pressure and not uraemia is the major determinant of arterial stiffness and endothelial dysfunction in patients with chronic kidney disease and minimal co-morbidity. Atherosclerosis 2011, 216, 217–225. [Google Scholar] [CrossRef]
- Singh, A.T.; Mothi, S.S.; Li, P.; Sabbisetti, V.; Waikar, S.S.; Mc Causland, F.R. Endothelin-1 and parameters of systolic blood pressure in hemodialysis. Am. J. Hypertens. 2021, 34, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schmidt, I.M.; Sabbisetti, V.; Tio, M.C.; Opotowsky, A.R.; Waikar, S.S. Plasma endothelin-1 and risk of death and hospitalization in patients undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Tian, J.; Lv, W.L.; Zhang, X.Y.; Zou, J.Z.; Fang, Y.; Yu, J.; Shen, B.; Liu, Z.H.; Ding, X.Q. Inappropriately elevated endothelin-1 plays a role in the pathogenesis of intradialytic hypertension. Hemodial. Int. 2015, 19, 279–286. [Google Scholar] [CrossRef]
- Hajal, J.; Joubran, N.; Sleilaty, G.; Chacra, D.; Saliba, Y.; Assaad, S.; Chelala, D.; Fares, N. Intradialytic hypotension: Beyond hemodynamics. Physiol. Res. 2019, 68, 793–805. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Qasem, A.; Schmitt, M.; Avolio, A.P.; Cockcroft, J.R.; Wilkinson, I.B. Endothelin-1 regulates arterial pulse wave velocity in vivo. J. Am. Coll. Cardiol. 2003, 42, 1975–1981. [Google Scholar] [CrossRef]
- Kato, K.; Nakashima, A.; Morishita, M.; Ohkido, I.; Yokoo, T. Parathyroid hormone levels and pulse wave velocity in hemodialysis patients. Ther. Apher. Dial. 2023, 27, 552–561. [Google Scholar] [CrossRef]
- Strózecki, P.; Adamowicz, A.; Nartowicz, E.; Odrowaz-Sypniewska, G.; Włodarczyk, Z.; Manitius, J. Parathormon, calcium, phosphorus, and left ventricular structure and function in normotensive hemodialysis patients. Ren. Fail. 2001, 23, 115–126. [Google Scholar] [CrossRef]
- Randon, R.B.; Rohde, L.E.; Comerlato, L.; Ribeiro, J.P.; Manfro, R.C. The role of secondary hyperparathyroidism in left ventricular hypertrophy of patients under chronic hemodialysis. Braz. J. Med. Biol. Res. 2005, 38, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Shizuku, J.; Yamashita, T.; Ohba, T.; Kabaya, T.; Nitta, K. Left atrial volume is an independent predictor of all-cause mortality in chronic hemodialysis patients. Intern. Med. 2012, 51, 1479–1485. [Google Scholar] [CrossRef]
- Charytan, D. Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 578–585. [Google Scholar] [CrossRef]
- Imaizumi, T.; Fujii, N.; Hamano, T.; Yang, W.; Taguri, M.; Kansal, M.; Mehta, R.; Shafi, T.; Taliercio, J.; Go, A.; et al. Excess risk of cardiovascular events in patients in the United States vs. Japan with chronic kidney disease is mediated mainly by left ventricular structure and function. Kidney Int. 2023, 103, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, A.; Tapoi, L.; Ureche, C.; Sascau, R.; Statescu, C.; Covic, A. Left atrial strain: A novel “biomarker” for chronic kidney disease patients? Echocardiography 2021, 38, 2077–2082. [Google Scholar] [CrossRef]
- Ozdogan, O.; Kayikcioglu, M.; Asci, G.; Ozkahya, M.; Toz, H.; Sezis, M.; Can, L.H.; Ok, E. Left atrial volume predicts mortality in low-risk dialysis population on long-term low-salt diet. Am. Heart J. 2010, 159, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Hensen, L.C.R.; Goossens, K.; Delgado, V.; Abou, R.; Rotmans, J.I.; Jukema, J.W.; Bax, J.J. Prevalence of left ventricular systolic dysfunction in pre-dialysis and dialysis patients with preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2018, 20, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, Y.; Zhang, F.; Steiger, S.; Guo, C.; Liu, N.; Lu, J.; Fan, G.; Wu, W.; Wu, M.; et al. Prediction of male coronary artery bypass grafting outcomes using body surface area eighted left ventricular end-diastolic diameter: Multicenter retrospective cohort study. Interact. J. Med. Res. 2023, 12, e45898. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Ogawa, T.; Iwabuchi, Y.; Otsuka, K.; Nitta, K. Left ventricular end- diastolic diameter is an independent predictor of mortality in hemodialysis patients. Ther. Apher. Dial. 2012, 16, 134–141. [Google Scholar] [CrossRef]
- Dohi, K. Echocardiographic assessment of cardiac structure and function in chronic renal disease. J. Echocardiogr. 2019, 17, 115–122. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barré, P.E. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J. Am. Soc. Nephrol. 1995, 5, 2024–2031. [Google Scholar] [CrossRef]
- Hee, L.; Nguyen, T.; Whatmough, M.; Descallar, J.; Chen, J.; Kapila, S.; French, J.K.; Thomas, L. Left atrial volume and adverse cardiovascular outcomes in unselected patients with and without CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 1369–1376.5. [Google Scholar] [CrossRef]
- Huang, T.H.; Chiu, H.; Wu, P.Y.; Huang, J.C.; Lin, M.Y.; Chen, S.C.; Chang, J.M. The association of echocardiographic parameters on renal outcomes in chronic kidney disease. Ren. Fail. 2021, 43, 433–444. [Google Scholar] [CrossRef]
- Hickson, L.J.; Negrotto, S.M.; Onuigbo, M.; Scott, C.G.; Rule, A.D.; Norby, S.M.; Albright, R.C.; Casey, E.T.; Dillon, J.J.; Pellikka, P.A.; et al. Echocardiography criteria for structural heart disease in patients with end-stage renal disease initiating hemodialysis. J. Am. Coll. Cardiol. 2016, 67, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
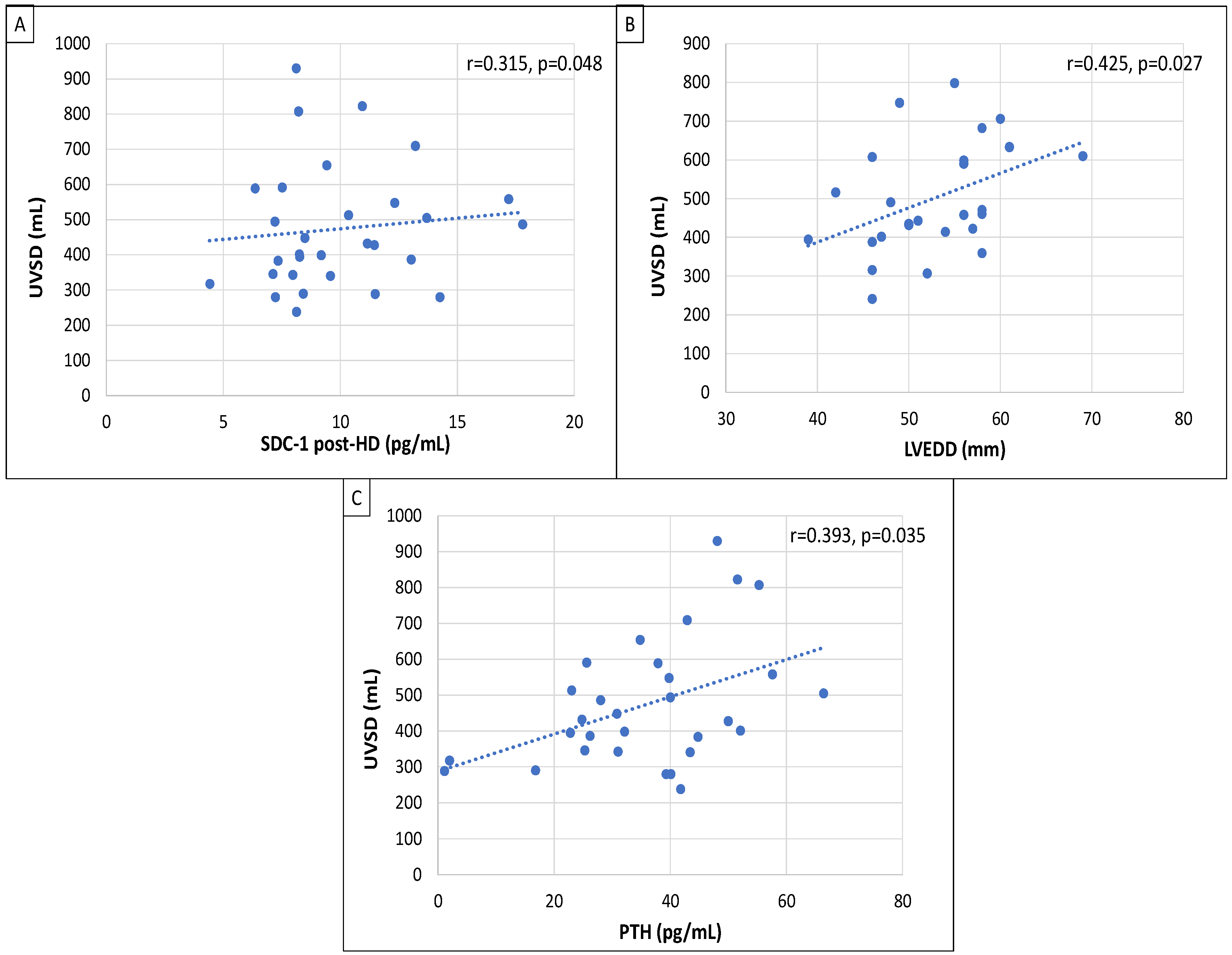
| HD Patients (n = 29) | UVSD < 500 mL (n = 17) | UVSD ≥ 500 mL (n = 12) | p | |
|---|---|---|---|---|
| Man/woman (n/%) | 15/14 (51/48) | 7/10 (41/59) | 8/4 (67/33) | 0.080 |
| Age (year) | 67 (57–71) | 69.5 (57–76) | 64 (57.5–67.75) | 0.066 |
| HT (n/%) | 29 (100) | 17 (100) | 12 (100) | 0.059 |
| DM (n/%) | 10 (34.4) | 4 (23) | 6 (50) | 0.057 |
| BMI (kg/m2) | 25.8 (23.5–31.5) | 25.1 (22.5–32) | 29.2 (25.1–32) | 0.250 |
| Hb (g/l) | 110.24 ± 11.22 | 111.80 ± 9.73 | 107.90 ± 12.27 | 0.190 |
| Ca (mmol/L) | 2.2± 0.15 | 2.19 ± 0.18 | 2.21 ± 0.09 | 0.410 |
| P (mmol/L) | 1.7 ± 0.48 | 1.68 ± 0.48 | 1.73 ± 0.45 | 0.630 |
| PTH (pmol/L) | 35.97 ± 15.27 | 32.11 ± 14.93 | 41.44 ± 13.31 | 0.340 |
| Glucose (mmol/L) | 6.4 ± 1.69 | 6.73 ± 1.72 | 5.93 ± 1.46 | 0.220 |
| Cholesterol (mmol/L) | 4.2 (3.7–4.8) | 4.2 (3.6–4.5) | 4.45 (3.87–4.95) | 0.251 |
| Triglyceride (mmol/L) | 1.48 (1.18–1.95) | 1.63 (1.27–2.03) | 1.32 (1.05–1.63) | 0.158 |
| HDL cholesterol (mmol/L) | 1.1 (0.93–1.46) | 1.13 (0.99–1.46) | 1.04 (0.92–1.35) | 0.237 |
| CRP (mg/L) | 4.45 ± 4.29 | 2.87 ± 2.45 | 6.70 ± 5.09 | 0.007 |
| Fe (umol/L) | 12.33 ± 3.81 | 12.51 ± 3.94 | 12.08 ± 3.42 | 0.710 |
| Ferritin (mg/L) | 311.97 ± 252.93 | 308.47 ± 192.07 | 316.91 ± 311.39 | 0.100 |
| ALP (U/L) | 80.1 ± 25.69 | 80.70 ± 16.51 | 79.10 ± 33.94 | 0.591 |
| Creat (umol/L) | 719.14 ± 191.97 | 698.05 ± 169.39 | 749.00 ± 209.33 | 0.092 |
| Dialysis vintage (months) | 59 ± 34 | 67.82 ± 37.10 | 46.50 ± 22.90 | 0.049 |
| Ultrafiltration volume (ml) single HD | 2413.79 ± 766.58 | 2211.00 ± 729.83 | 2450.00 ± 781.15 | 0.092 |
| Ultrafiltration volume/body weight (mL/kg) | 7.61 ± 2.14 | 7.66 ± 2.22 | 7.55 ± 2.20 | 0.173 |
| Overhydration assessed by bioimpedance (L) | 2.30 ± 1.54 | 2.21 ± 1.82 | 2.45 ± 1.16 | 0.094 |
| HD Patients (n = 29) | UVSD < 500 mL (n = 17) | UVSD ≥ 500 mL (n = 12) | p | |
|---|---|---|---|---|
| Brachial systolic/diastolic BP (mmHg) | ||||
| pre-HD | 141.4/73.3 ± 17.4/17.8 | 140.7/72.1 ± 19.8/10.2 | 142.4/75.0 ± 12.2/12.9 | 0.078 |
| mid-HD | 138.4/71.3 ± 19.2/10.1 | 143.1/71.0 ± 18.7/9.8 | 131.7/71.9 ± 16.9/10.8 | 0.056 |
| post-HD | 142.9/72.6± 20.2/10.0 | 147.2/71.7 ± 21.0/10.2 | 136.9/74.0 ± 16.3/9.2 | 0.067 |
| Aix (%) | ||||
| pre-HD | 33.90 ± 9.23 | 34.11 ± 8.83 | 33.58 ± 9.38 | 0.560 |
| mid-HD | 33.29 ± 10.91 | 34.52 ± 11.90 | 31.36 ± 8.18 | 0.672 |
| post-HD | 31.31 ± 10.02 | 30.29 ± 9.18 | 32.75 ± 10.55 | 0.131 |
| cfPWV (m/s) | ||||
| pre-HD | 13 (9.37–14.4) | 14.1 (10.3–15.4) | 10.75 (8.47–12.82) * | 0.032 |
| mid-HD | 12.25 (9.52–13.92) | 12.4 (9.5–17.5) | 10.45 (9.5–12.62) | 0.076 |
| post-HD | 13.77 (10–17.25) | 14.3 (10–17.4) | 13.9 (10.65–17.75) | 0.267 |
| Aorta systolic BP (mmHg) | ||||
| pre-HD | 130.86 ± 17.37 | 130.70 ± 19.24 | 131.08 ± 13.39 | 0.235 |
| mid-HD | 128.41 ± 18.00 | 133.29 ± 17.53 | 121.50 ± 15.47 | 0.041 |
| post-HD | 131.45 ± 20.68 | 134.05 ± 22.55 | 127.75 ± 15.93 | 0.076 |
| SDC-1 (ng/mL) | ||||
| pre-HD | 9.167 ± 2.491 | 8.64 ± 2.02 | 9.90 ± 2.78 | 0.234 |
| mid-HD | 9.425 ± 3.008 | 8.65 ± 2.52 | 10.43 ± 3.16 | 0.134 |
| post-HD | 9.954 ± 3.180 | 9.17 ± 2.53 | 11.05 ± 3.52 | 0.111 |
| ET-1 (pg/mL) | ||||
| pre-HD | 9.89 ± 3.48 | 10.79 ± 2.71 | 8.61 ± 3.88 | 0.049 |
| mid-HD | 9.92 ± 3.66 | 10.69 ± 2.65 | 8.82 ± 4.39 | 0.098 |
| post-HD | 9.61 ± 3.47 | 10.20 ± 2.63 | 8.77 ± 4.13 | 0.087 |
| Echocardiographic parameters | ||||
| LVEDD (mm) | 52.95 ± 6.53 | 51.0 ± 5.38 | 55.7 ± 7.28 | 0.037 |
| LVESD (mm) | 34.1 ± 7.62 | 32.0 ± 5.61 | 37.0 ± 9.29 | 0.091 |
| LVMI (g/m2) | 390.12 ± 136.97 | 350.29 ± 85.92 | 449.88 ± 180.47 | 0.038 |
| LVEF (%) | 59 (53–66) | 60 (55.5–66.5) | 53.5 (47–63) | 0.036 |
| RAV (mL/m2) | 47.63 ± 23.97 | 43.63 ± 19.82 | 53.28 ± 27.67 | 0.054 |
| LAV (mL/m2) | 53.90 ± 30.35 | 51.22 ± 25.12 | 57.69 ± 35.85 | 0.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sági, B.; Vas, T.; Jakabfi-Csepregi, R.K.; Sulyok, E.; Csiky, B. Association between Visit-to-Visit Ultrafiltration Volume Variability, Vascular Biomarkers and Cardiovascular Parameters in Chronic Hemodialysis Patients. J. Clin. Med. 2024, 13, 5958. https://doi.org/10.3390/jcm13195958
Sági B, Vas T, Jakabfi-Csepregi RK, Sulyok E, Csiky B. Association between Visit-to-Visit Ultrafiltration Volume Variability, Vascular Biomarkers and Cardiovascular Parameters in Chronic Hemodialysis Patients. Journal of Clinical Medicine. 2024; 13(19):5958. https://doi.org/10.3390/jcm13195958
Chicago/Turabian StyleSági, Balázs, Tibor Vas, Rita Klaudia Jakabfi-Csepregi, Endre Sulyok, and Botond Csiky. 2024. "Association between Visit-to-Visit Ultrafiltration Volume Variability, Vascular Biomarkers and Cardiovascular Parameters in Chronic Hemodialysis Patients" Journal of Clinical Medicine 13, no. 19: 5958. https://doi.org/10.3390/jcm13195958
APA StyleSági, B., Vas, T., Jakabfi-Csepregi, R. K., Sulyok, E., & Csiky, B. (2024). Association between Visit-to-Visit Ultrafiltration Volume Variability, Vascular Biomarkers and Cardiovascular Parameters in Chronic Hemodialysis Patients. Journal of Clinical Medicine, 13(19), 5958. https://doi.org/10.3390/jcm13195958







