Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study
Abstract
1. Background and Aims
2. Methods
2.1. Study Rationale and Study Population
2.2. Laboratory Parameters
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, T.; Fontana, M.; Martinez-Naharro, A.; Quarta, C.C.; Whelan, C.J.; Petrie, A.; Rowczenio, D.M.; Gilbertson, J.A.; Hutt, D.F.; Rezk, T.; et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation 2019, 140, 16–26. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Lohrmann, G.; Pipilas, A.; Mussinelli, R.; Gopal, D.M.; Berk, J.L.; Connors, L.H.; Vellanki, N.; Hellawell, J.; Siddiqi, O.K.; Fox, J.; et al. Stabilization of Cardiac Function With Diflunisal in Transthyretin (ATTR) Cardiac Amyloidosis. J. Card. Fail. 2020, 26, 753–759. [Google Scholar] [CrossRef]
- Ioannou, A.; Fontana, M.; Gillmore, J.D. RNA Targeting and Gene Editing Strategies for Transthyretin Amyloidosis. BioDrugs 2023, 37, 127–142. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Dobner, S.; Bernhard, B.; Asatryan, B.; Windecker, S.; Stortecky, S.; Pilgrim, T.; Grani, C.; Hunziker, L. SGLT2 inhibitor therapy for transthyretin amyloid cardiomyopathy: Early tolerance and clinical response to dapagliflozin. ESC Heart Fail. 2023, 10, 397–404. [Google Scholar] [CrossRef]
- Lang, F.M.; Teruya, S.; Weinsaft, A.; Cuomo, M.; Santos, A.M.; Nalbandian, A.; Bampatsias, D.; Maurer, M.S. Sodium-glucose cotransporter 2 inhibitors for transthyretin amyloid cardiomyopathy: Analyses of short-term efficacy and safety. Eur. J. Heart Fail. 2024, 26, 938–947. [Google Scholar] [CrossRef]
- Steinhardt, M.J.; Cejka, V.; Chen, M.; Bauerlein, S.; Schafer, J.; Adrah, A.; Ihne-Schubert, S.M.; Papagianni, A.; Kortum, K.M.; Morbach, C.; et al. Safety and Tolerability of SGLT2 Inhibitors in Cardiac Amyloidosis-A Clinical Feasibility Study. J. Clin. Med. 2024, 13, 283. [Google Scholar] [CrossRef]
- Minguito-Carazo, C.; Sanchez Munoz, E.; Rodriguez Manero, M.; Martinez-Sande, J.L.; Fidalgo Andres, M.L.; Garcia Seara, J.; Gonzalez Rebollo, J.M.; Rodriguez Santamarta, M.; Gonzalez Melchor, L.; Gonzalez Ferrero, T.; et al. Impact of initiation of SGLT2 inhibitor treatment on the development of arrhythmias in patients with implantable cardiac devices. Rev. Esp. Cardiol. 2024, 77, 481–489. [Google Scholar] [CrossRef]
- Porcari, A.; Cappelli, F.; Nitsche, C.; Tomasoni, D.; Sinigiani, G.; Longhi, S.; Bordignon, L.; Masri, A.; Serenelli, M.; Urey, M.; et al. SGLT2 Inhibitor Therapy in Patients With Transthyretin Amyloid Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2411–2422. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, B.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A.; et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018, 137, 961–972. [Google Scholar] [CrossRef]
- Magdi, M.; Mostafa, M.R.; Abusnina, W.; Al-Abdouh, A.; Doss, R.; Mohamed, S.; Ekpo, C.P.; Alweis, R.; Baibhav, B. A systematic review and meta-analysis of the prevalence of transthyretin amyloidosis in heart failure with preserved ejection fraction. Am. J. Cardiovasc. Dis. 2022, 12, 102–111. [Google Scholar]
- Oghina, S.; Bougouin, W.; Bezard, M.; Kharoubi, M.; Komajda, M.; Cohen-Solal, A.; Mebazaa, A.; Damy, T.; Bodez, D. The Impact of Patients With Cardiac Amyloidosis in HFpEF Trials. JACC Heart Fail. 2021, 9, 169–178. [Google Scholar] [CrossRef]
- Suissa, S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol. Drug Saf. 2007, 16, 241–249. [Google Scholar] [CrossRef]
- Zhou, Z.; Rahme, E.; Abrahamowicz, M.; Pilote, L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: A comparison of methods. Am. J. Epidemiol. 2005, 162, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.V.; Schneeweiss, S.; Initiative, R.-D.; Franklin, J.M.; Desai, R.J.; Feldman, W.; Garry, E.M.; Glynn, R.J.; Lin, K.J.; Paik, J.; et al. Emulation of Randomized Clinical Trials With Nonrandomized Database Analyses: Results of 32 Clinical Trials. JAMA 2023, 329, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Manzi, G.; Pierucci, N.; Laviola, D.; Piro, A.; D’Amato, A.; Filomena, D.; Matteucci, A.; Severino, P.; Miraldi, F.; et al. SGLT2i effect on atrial fibrillation: A network meta-analysis of randomized controlled trials. J. Cardiovasc. Electrophysiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Spencer-Bonilla, G.; Njoroge, J.N.; Pearson, K.; Witteles, R.M.; Aras, M.A.; Alexander, K.M. Racial and Ethnic Disparities in Transthyretin Cardiac Amyloidosis. Curr. Cardiovasc. Risk Rep. 2021, 15, 12170. [Google Scholar] [CrossRef]
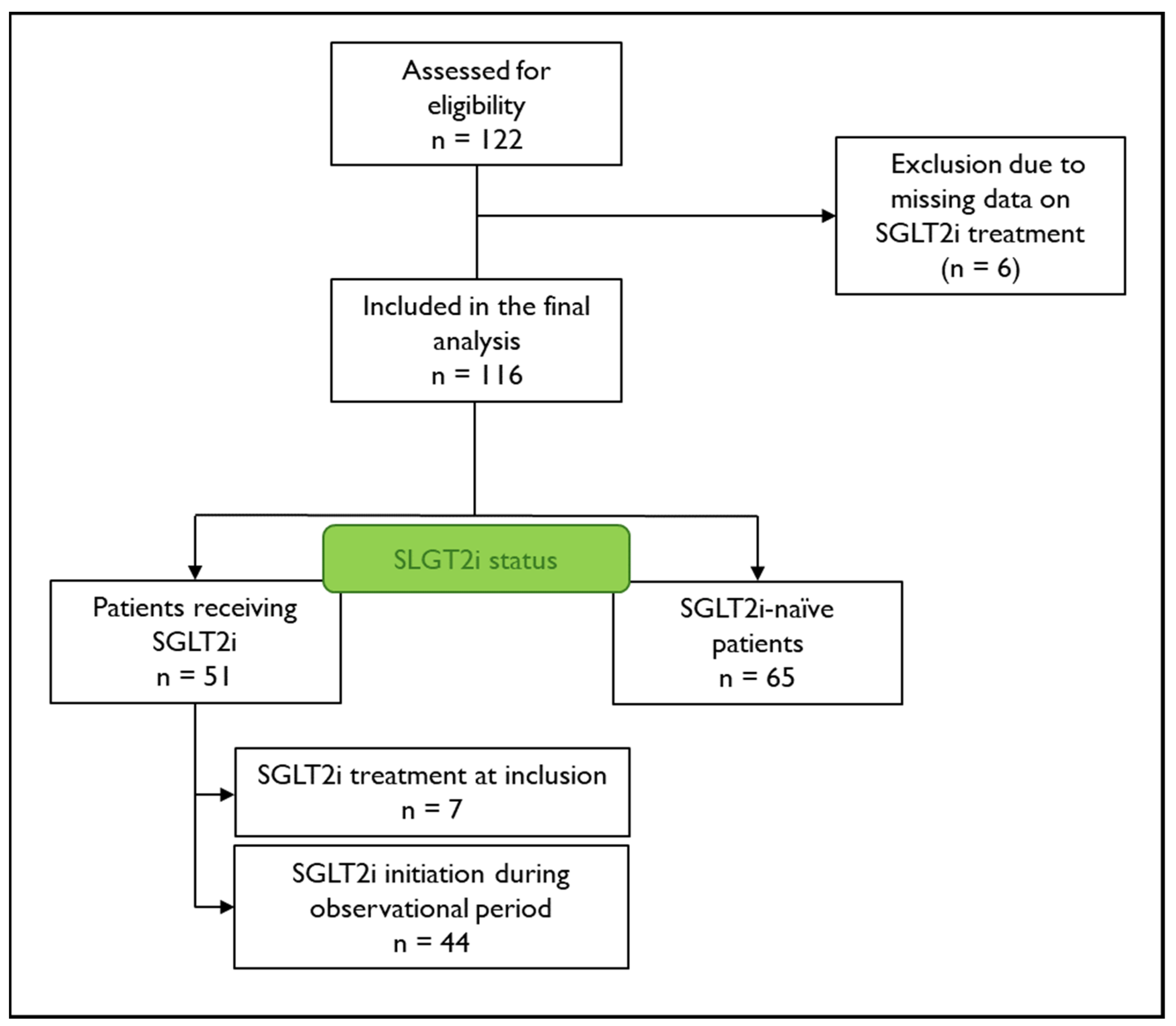
| All | SGLT2i Therapy | SGLT2i-Naïve | p-Value | |
|---|---|---|---|---|
| n = 116 | n = 51 | n = 65 | ||
| Age, years | 80 (76–82) | 80 (76–82) | 80 (77–83) | 0.732 |
| Female, n (%) | 17 (15) | 5 (10) | 12 (18) | 0.191 |
| Ethnicity Caucasian, n (%) | 116 (100) | 51 (100) | 65 (100) | - |
| Diabetes mellitus, n (%) | 23 (20) | 10 (20) | 13 (20) | 0.958 |
| NYHA-class, n (%) | ||||
| I | 12 (12) | 5 (10) | 7 (13) | 0.989 |
| II | 25 (24) | 12 (24) | 13 (24) | |
| II–III | 22 (21) | 10 (20) | 12 (22) | |
| III | 40 (39) | 20 (41) | 20 (37) | |
| IV | 4 (4) | 2 (4) | 2 (4) | |
| Angina pectoris symptoms, n (%) | ||||
| Typical | 20 (19) | 9 (18) | 11 (20) | 0.708 |
| Atypical | 12 (12) | 7 (14) | 5 (9) | |
| Tafamidis therapy, n (%) | 17 (15) | 8 (16) | 9 (14) | 0.781 |
| Loop diuretic, n (%) | 61 (53) | 32 (63) | 29 (44) | 0.327 |
| Thiazide diuretic, n (%) | 12 (10) | 3 (6) | 9 (14) | 0.083 |
| Potassium-sparing diuretic, n (%) | 41 (35) | 25 (49) | 16 (25) | 0.038 |
| Body mass index, kg/m2 | 24.7 (22.8–26.2) | 24.9 (22.6–26.6) | 24.4 (22.9–26.2) | 0.860 |
| Systolic blood pressure, mmHg | 132 (120–147) | 129 (118–152) | 132 (121–146) | 0.902 |
| Diastolic blood pressure, mmHg | 78 (73–86) | 78 (73–86) | 79 (71–85) | 0.927 |
| Heart rate, bpm | 70 (60–78) | 73 (60–81) | 70 (61–75) | 0.474 |
| LVEF, % | 51 (45–57) | 49 (43–52) | 54 (49–59) | <0.001 |
| NT-proBNP, pg/mL | 2845 (1519–5033) | 3224 (1949–4738) | 2717 (1183–5049) | 0.401 |
| hsTrop-T, pg/mL | 57 (35–83) | 59 (36–87) | 56 (31–81) | 0.543 |
| Estimated GFR, mL/min/1.73 m2 | 58 (46–69) | 58 (39–68) | 59 (48–70) | 0.478 |
| Creatinine, mg/dL | 1.2 (1.0–1.4) | 1.2 (1.0–1.6) | 1.1 (1.0–1.3) | 0.343 |
| CRP, mg/L | 2.3 (1.3–4.2) | 2.9 (1.3–4.3) | 2.0 (1.3–4.2) | 0.591 |
| All | SGLT2i Therapy | SGLT2i-Naïve | p-Value | |
|---|---|---|---|---|
| n = 116 | n = 51 | n = 65 | ||
| NYHA-class, n (%) | 0.258 | |||
| I | 12 (11) | 3 (6) | 9 (14) | |
| II | 30 (27) | 13 (27) | 17 (27) | |
| II–III | 18 (16) | 5 (10) | 13 (20) | |
| III | 50 (44) | 27 (55) | 23 (36) | |
| IV | 2 (2) | 1 (2) | 1 (2) | |
| Tafamidis therapy, n (%) | ||||
| At baseline | 39 (34) | 30 (59) | 9 (14) | <0.001 |
| During follow-up | 103 (89) | 48 (94) | 55 (85) | 0.107 |
| Loop diuretic, n (%) | 71 (69) | 42 (82) | 29 (56) | 0.004 |
| Thiazide diuretic, n (%) | 12 (12) | 3 (6) | 9 (17) | 0.071 |
| Potassium-sparing diuretic, n (%) | 46 (46) | 30 (61) | 16 (31) | 0.002 |
| Systolic blood pressure, mmHg | 133 (120–149) | 135 (120–157) | 132 (121–146) | 0.626 |
| Diastolic blood pressure, mmHg | 81 (70–86) | 84 (69–90) | 79 (71–85) | 0.194 |
| Heart rate, bpm | 71 (62–78) | 73 (64–81) | 70 (62–76) | 0.168 |
| LVEF, % | 51 (44–57) | 46 (39–53) | 54 (49–59) | 0.002 |
| NT-proBNP, pg/mL | 3001 (1488–5227) | 3384 (1976–6809) | 2718 (1183–5050) | 0.661 |
| hsTrop-T, pg/mL | 57 (35–85) | 59 (36–90) | 56 (31–81) | 0.687 |
| Estimated GFR, mL/min/1.73 m2 | 57 (42–69) | 52 (37–64) | 59 (48–70) | 0.068 |
| Creatinine, mg/dL | 1.2 (1.0–1.5) | 1.3 (1.0–1.7) | 1.1 (1.0–1.3) | 0.400 |
| All | SGLT2i Therapy | SGLT2i-Naïve | p-Value | |
|---|---|---|---|---|
| n = 116 | n = 51 | n = 65 | ||
| Outcomes, n (%) | ||||
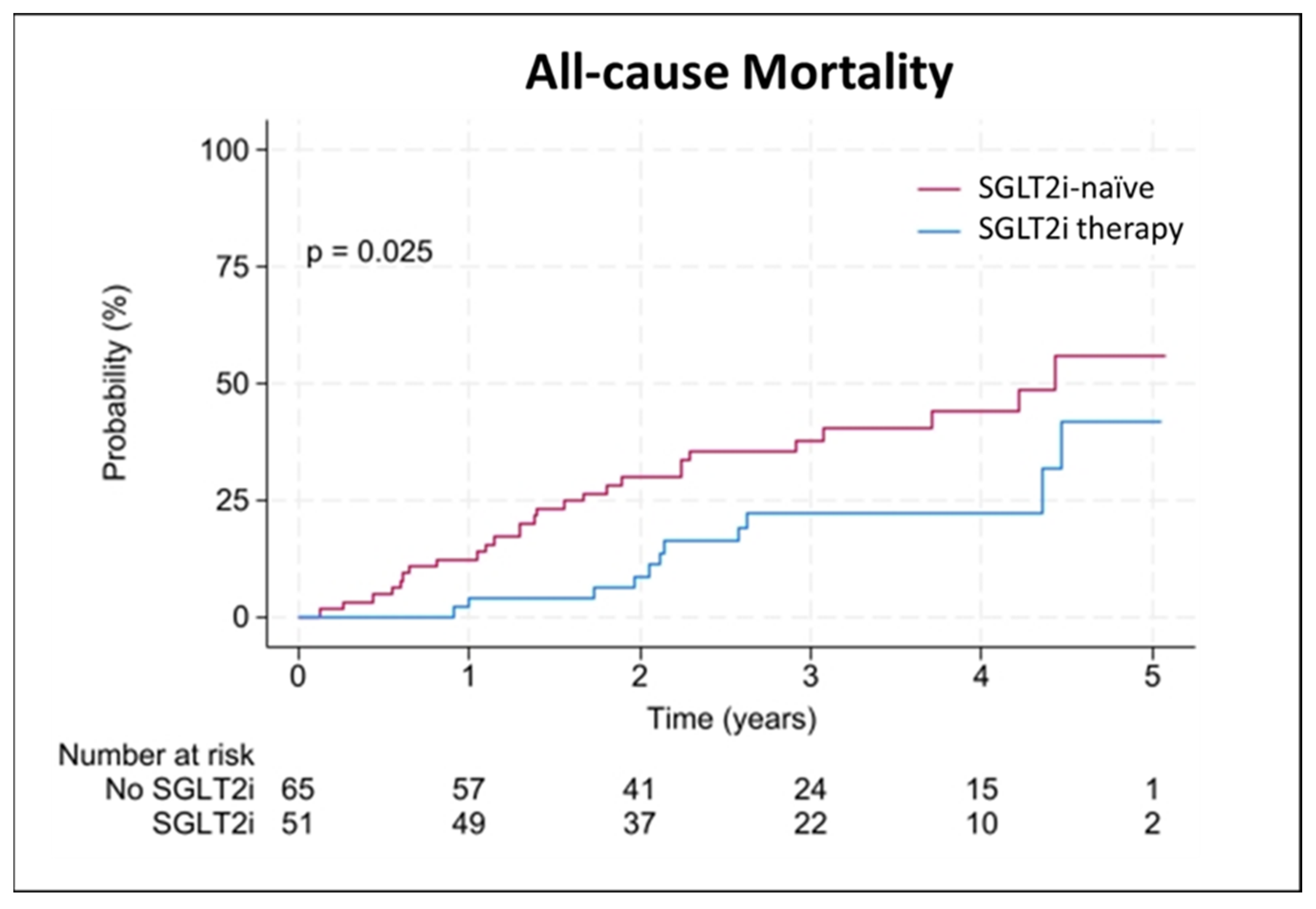
| All-cause mortality | 38 (33) | 11 (22) | 27 (42) | 0.023 |
| Cardiovascular death | 14 (12) | 4 (8) | 10 (15) | 0.216 |
| WHF hospitalization | 32 (28) | 18 (35) | 14 (22) | 0.100 |
| Observation time, years | ||||
| All-cause mortality | 2.6 (1.7–3.7) | 2.7 (2.0–3.7) | 2.5 (1.6–3.7) | |
| Cardiovascular death | 3.0 (2.2–4.1) | 2.9 (2.0–3.7) | 3.0 (2.3–4.2) | |
| WHF hospitalization | 2.0 (1.2–3.2) | 1.8 (1.0–3.0) | 2.2 (1.4–3.6) |
| Univariable | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| SLGT2i | 0.457 | 0.227–0.922 | 0.029 | 0.177 | 0.062–0.504 | 0.001 |
| Age | 1.023 | 0.940–1.113 | 0.598 | |||
| Sex, male | 0.428 | 0.151–1.217 | 0.112 | |||
| eGFR | 0.991 | 0.961–1.022 | 0.562 | |||
| NT-proBNP(log) | 2.164 | 1.221–3.837 | 0.008 | |||
| LVEF | 0.991 | 0.949–1.035 | 0.689 | |||
| Tafamidis | 1.844 | 0.794–4.285 | 0.155 | |||
| Univariable | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| SLGT2i | 1.075 | 0.524–2.206 | 0.843 | 0.839 | 0.352–1.999 | 0.692 |
| Age | 1.035 | 0.953–1.123 | 0.415 | |||
| Sex, male | 0.275 | 0.101–0.753 | 0.012 | |||
| eGFR | 1.009 | 0.980–1.039 | 0.556 | |||
| NT-proBNP(log) | 2.012 | 1.158–3.495 | 0.013 | |||
| LVEF | 1.002 | 0.959–1.048 | 0.917 | |||
| Tafamidis | 0.979 | 0.445–2.155 | 0.959 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwegel, N.; Toferer, C.; Zach, D.K.; Santner, V.; Höller, V.; Lugitsch, J.; Wallner, M.; Gollmer, J.; Aziz, F.; von Lewinski, D.; et al. Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study. J. Clin. Med. 2024, 13, 5966. https://doi.org/10.3390/jcm13195966
Schwegel N, Toferer C, Zach DK, Santner V, Höller V, Lugitsch J, Wallner M, Gollmer J, Aziz F, von Lewinski D, et al. Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study. Journal of Clinical Medicine. 2024; 13(19):5966. https://doi.org/10.3390/jcm13195966
Chicago/Turabian StyleSchwegel, Nora, Christina Toferer, David K. Zach, Viktoria Santner, Viktoria Höller, Jakob Lugitsch, Markus Wallner, Johannes Gollmer, Faisal Aziz, Dirk von Lewinski, and et al. 2024. "Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study" Journal of Clinical Medicine 13, no. 19: 5966. https://doi.org/10.3390/jcm13195966
APA StyleSchwegel, N., Toferer, C., Zach, D. K., Santner, V., Höller, V., Lugitsch, J., Wallner, M., Gollmer, J., Aziz, F., von Lewinski, D., Kolesnik, E., Ablasser, K., Zirlik, A., Sourij, H., & Verheyen, N. (2024). Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study. Journal of Clinical Medicine, 13(19), 5966. https://doi.org/10.3390/jcm13195966








