Microperimetry Sensitivity Correlates to Structural Macular Changes in Adolescents with Achromatopsia Unlike Other Visual Function Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Visual Acuity and Contrast Sensitivity
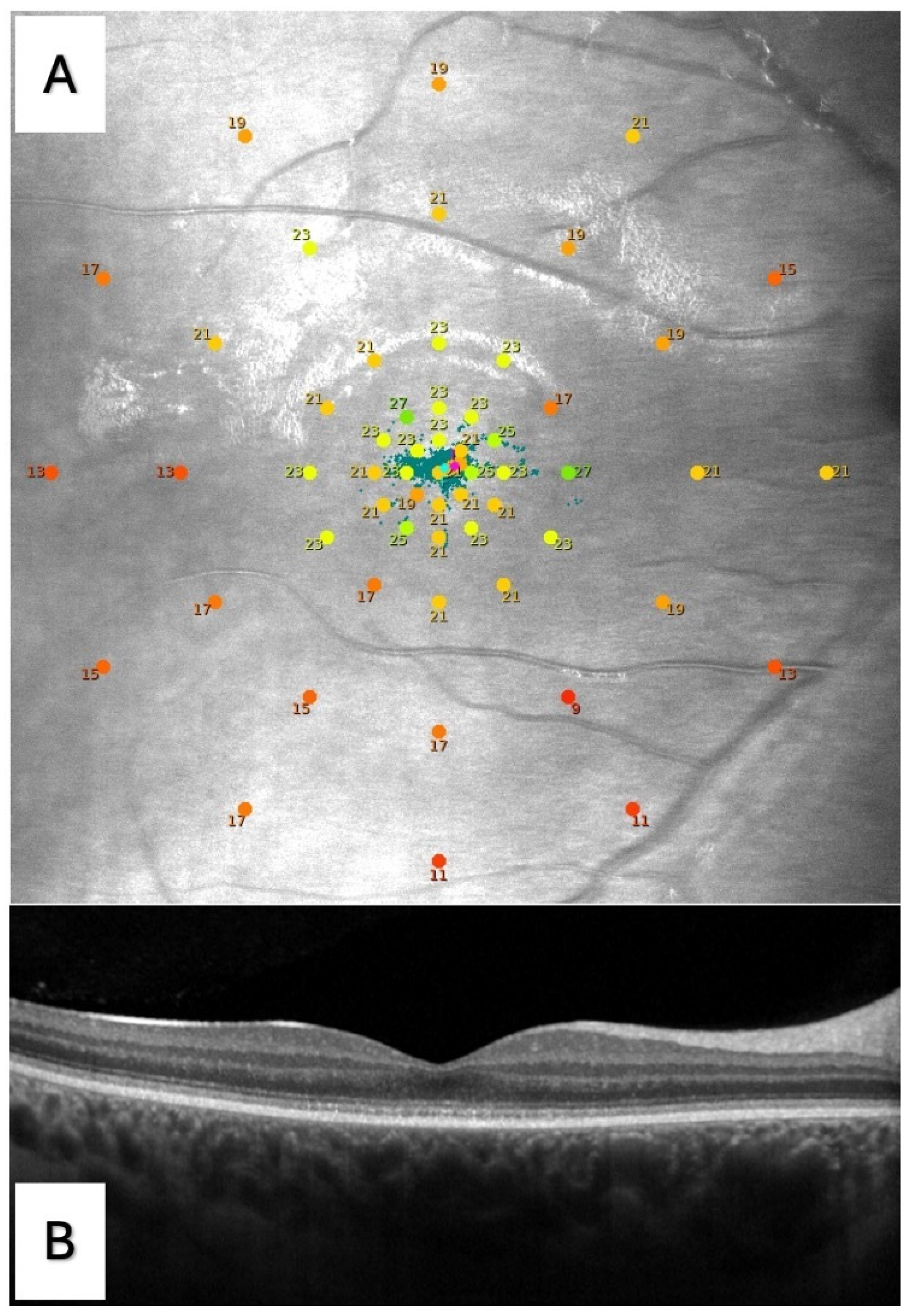
2.3. Microperimetry
2.4. Optical Coherence Tomography
2.5. Statistical Analysis
3. Results
3.1. Population
3.2. Morpho-Functional Correlations
3.3. Visual Functional Parameter Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharpe, L.T.; Stockman, A.; Jagle, H.; Nathans, J. Opsin genes, cone photopigments, color vision, and color blindness. In Color Vision: From Genes to Perception; Gegenfurtner, K., Sharpe, L.T., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 3–52. [Google Scholar]
- Kohl, S.; Zobor, D.; Chiang, W.; Weisschuh, N.; Staller, J.; Menendez, I.G.; Chang, S.; Beck, S.C.; Garrido, M.G.; Sothilingam, V.; et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet. 2015, 47, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Michaelides, M.; Aligianis, I.A.; Ainsworth, J.; Mollon, J.; Maher, E.; Moore, A.; Hunt, D.l. Achromatopsia caused by novel mutations in both CNGA3 and CNGB3. J. Med. Genet. 2004, 41, e20. [Google Scholar] [CrossRef]
- Kohl, S.; Varsanyi, B.; Antunes, G.A.; Baumann, B.; Hoyng, C.B.; Jägle, H.; Rosenberg, T.; Kellner, K.; Lorenz, B.; Salati, R.; et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet. 2005, 13, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Thiadens, A.A.H.J.; Somervuo, V.; van den Born, L.I.; Roosing, S.; van Schooneveld, M.J.; Kuijpers, R.W.A.M.; van Moll-Ramirez, N.; Cremers, F.P.M.; Hoyng, C.B.; Klaver, C.C.W. Progressive Loss of Cones in Achromatopsia: An Imaging Study Using Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5952–5957. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Wilde, C.; Aboshiha, J.; Cowing, J.; Han, C.; Langlo, C.S.; Chana, R.; Davidson, A.E.; Sergouniotis, P.I.; Bainbridge, J.W.; et al. Retinal Structure and Function in Achromatopsia: Implications for Gene Therapy. Ophthalmology 2014, 121, 234–245. [Google Scholar] [CrossRef]
- Aboshiha, J.; Dubis, A.M.; Cowing, J.; Fahy, R.T.A.; Sundaram, V.; Bainbridge, J.W.; Ali, R.R.; Dubra, A.; Nardini, M.; Webster, A.R.; et al. A Prospective Longitudinal Study of Retinal Structure and Function in Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5733–5743. [Google Scholar] [CrossRef]
- Zobor, D.; Werner, A.; Stanzial, F.; Benedicenti, F.; Rudolph, G.; Kellner, U.; Hamel, C.; Andréasson, S.; Zobor, G.; Strasser, T.; et al. The Clinical Phenotype of CNGA3-Related Achromatopsia: Pretreatment Characterization in Preparation of a Gene Replacement Therapy Trial. Investig. Ophthalmol. Vis. Sci. 2017, 58, 821–832. [Google Scholar] [CrossRef]
- Thomas, M.G.; Kumar, A.; Kohl, S.; Proudlock, F.A.; Gottlob, I. High-Resolution in Vivo Imaging in Achromatopsia. Ophthalmology 2011, 118, 882–887. [Google Scholar] [CrossRef]
- Thomas, M.G.; McLean, R.J.; Kohl, S.; Sheth, V.; Gottlob, I. Early Signs of Longitudinal Progressive Cone Photoreceptor Degeneration in Achromatopsia. Br. J. Ophthalmol. 2012, 96, 1232–1236. [Google Scholar] [CrossRef]
- Greenberg, J.P.; Sherman, J.; Zweifel, S.A.; Chen, R.W.S.; Duncker, T.; Kohl, S.; Baumann, B.; Wissinger, B.; Yannuzzi, L.A.; Tsang, S.H. Spectral-Domain Optical Coherence Tomography Staging and Autofluorescence Imaging in Achromatopsia. JAMA Ophthalmol. 2014, 132, 437–445. [Google Scholar] [CrossRef]
- Langlo, C.S.; Erker, L.R.; Parker, M.; Patterson, E.J.; Higgins, B.P.; Summerfelt, P.; Razeen, M.M.; Collison, F.T.; Fishman, G.A.; Kay, C.N.; et al. Repeatability and longitudinal assessment of foveal cone structure in cngb3-associated achromatopsia. Retina 2017, 37, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Hirji, N.; Georgiou, M.; Kalitzeos, A.; Bainbridge, J.W.; Kumaran, N.; Aboshiha, J.; Carroll, J.; Michaelides, M. Longitudinal Assessment of Retinal Structure in Achromatopsia Patients with Long-Term Follow-Up. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5735–5744. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, R.; Karali, M.; Melillo, P.; Di Iorio, V.; De Benedictis, A.; Iaccarino, G.; Testa, F.; Banfi, S.; Simonelli, F. Clinical and Molecular Characterization of Achromatopsia Patients: A Longitudinal Study. Int. J. Mol. Sci. 2021, 22, 1681. [Google Scholar] [CrossRef] [PubMed]
- Tekavčič Pompe, M.; Vrabič, N.; Volk, M.; Meglič, A.; Jarc-Vidmar, M.; Peterlin, B.; Hawlina, M.; Fakin, A. Disease Progression in CNGA3 and CNGB3 Retinopathy; Characteristics of Slovenian Cohort and Proposed OCT Staging Based on Pooled Data from 126 Patients from 7 Studies. Curr. Issues Mol. Biol. 2021, 43, 941–957. [Google Scholar] [CrossRef]
- Grissim, G.; Walesa, A.; Follett, H.M.; Higgins, B.P.; Goetschel, K.; Heitkotter, H.; Carroll, J. Longitudinal Assessment of OCT-Based Measures of Foveal Cone Structure in Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2024, 65, 16. [Google Scholar] [CrossRef]
- Triantafylla, M.; Papageorgiou, E.; Thomas, M.G.; McLean, R.; Kohl, S.; Sheth, V.; Tu, Z.; Proudlock, F.A.; Gottlob, I. Longitudinal Evaluation of Changes in Retinal Architecture Using Optical Coherence Tomography in Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2022, 63, 6. [Google Scholar] [CrossRef]
- Michalakis, S.; Gerhardt, M.; Rudolph, G.; Priglinger, S.; Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 2022, 26, 51–59. [Google Scholar] [CrossRef]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef]
- Michaelides, M.; Hirji, N.; Wong, S.C.; Besirli, C.G.; Zaman, S.; Kumaran, N.; Georgiadis, A.; Smith, A.J.; Ripamonti, C.; Gottlob, I.; et al. First-in-Human Gene Therapy Trial of AAV8-HCARp.HCNGB3 in Adults and Children With CNGB3-Associated Achromatopsia. Am. J. Ophthalmol. 2023, 253, 243–251. [Google Scholar] [CrossRef]
- McKyton, A.; Marks Ohana, D.; Nahmany, E.; Banin, E.; Levin, N. Seeing Color Following Gene Augmentation Therapy in Achromatopsia. Curr. Biol. 2023, 33, 3489–3494.e2. [Google Scholar] [CrossRef]
- Farahbakhsh, M.; Anderson, E.J.; Maimon-Mor, R.O.; Rider, A.; Greenwood, J.A.; Hirji, N.; Zaman, S.; Jones, P.R.; Schwarzkopf, D.S.; Rees, G.; et al. A Demonstration of Cone Function Plasticity after Gene Therapy in Achromatopsia. Brain 2022, 145, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Genead, M.A.; Fishman, G.A.; Rha, J.; Dubis, A.M.; Bonci, D.M.O.; Dubra, A.; Stone, E.M.; Neitz, M.; Carroll, J. Photoreceptor Structure and Function in Patients with Congenital Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7298. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Singh, N.; Kane, T.; Zaman, S.; Hirji, N.; Aboshiha, J.; Kumaran, N.; Kalitzeos, A.; Carroll, J.; Weleber, R.G.; et al. Long-Term Investigation of Retinal Function in Patients with Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2020, 61, 38. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, E.; Midena, E.; Longhin, E.; Parrozzani, R.; Frisina, R.; Frizziero, L. Muller Cells and Choriocapillaris in the Pathogenesis of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1159–1167. [Google Scholar] [CrossRef]
- Longhin, E.; Convento, E.; Pilotto, E.; Bonin, G.; Vujosevic, S.; Kotsafti, O.; Midena, E. Static and Dynamic Retinal Fixation Stability in Microperimetry. Can. J. Ophthalmol. 2013, 48, 375–380. [Google Scholar] [CrossRef]
- Reichel, F.F.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Sothilingam, V.; Kuehlewein, L.; Kahle, N.; et al. Three-Year Results of Phase I Retinal Gene Therapy Trial for CNGA3-Mutated Achromatopsia: Results of a Non Randomised Controlled Trial. Br. J. Ophthalmol. 2022, 106, 1567–1572. [Google Scholar] [CrossRef]
- Singh, S.R.; Vaidya, H.; Borrelli, E.; Chhablani, J. Foveal Photoreceptor Disruption in Ocular Diseases: An Optical Coherence Tomography-Based Differential Diagnosis. Surv. Ophthalmol. 2023, 68, 655–668. [Google Scholar] [CrossRef]
- Pilotto, E.; Guidolin, F.; Convento, E.; Spedicato, L.; Vujosevic, S.; Cavarzeran, F.; Midena, E. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br. J. Ophthalmol. 2013, 97, 622–626. [Google Scholar] [CrossRef]
- Sunness, J.S.; Gonzalez-Baron, J.; Applegate, C.A.; Bresaler, N.M.; Tian, Y.; Hawkins, B.; Barron, Y.; Bergman, A. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999, 106, 1768–1779. [Google Scholar] [CrossRef]
- Bissell, A.J.; Yalcinbayir, O.; Akduman, L. Bilateral Geographic Atrophy: Spontaneous Visual Improvement after Loss of Vision in the Fellow Eye. Acta Ophthalmol. Scand. 2005, 83, 514–515. [Google Scholar] [CrossRef]
- Sunness, J.S.; Applegate, C.A.; Gonzalez-Baron, J. Improvement of Visual Acuity over Time in Patients with Bilateral Geographic Atrophy from Age-Related Macular Degeneration. Retina 2000, 20, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Gillies, M.C.; Chew, E.Y.; Bird, A.C.; Heeren, T.F.C.; Peto, T.; Holz, F.G.; Scholl, H.P.N. Macular Telangiectasia Type 2. Prog. Retin. Eye Res. 2013, 34, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Radin, P.P.; Pilotto, E.; Ghirlando, A.; Convento, E.; Varano, M. Fixation Pattern and Macular Sensitivity in Eyes with Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. A Microperimetry Study. Semin. Ophthalmol. 2004, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Schönbach, E.M.; Ibrahim, M.A.; Strauss, R.W.; Birch, D.G.; Cideciyan, A.V.; Hahn, G.A.; Ho, A.; Kong, X.; Nasser, F.; Sunness, J.S.; et al. Fixation Location and Stability Using the MP-1 Microperimeter in Stargardt Disease: ProgStar Report No. 3. Ophthalmol. Retin. 2017, 1, 68–76. [Google Scholar] [CrossRef]
| Demographic Features (8 Subjects) | |
|---|---|
| Sex, M:F (N, %) | 2, 25:6, 75 |
| Age, years (mean ± SD) | 17 ± 2.7 |
| Genetic mutation (8 subjects) | (N, %) |
| CNGA3 | 3, 37.5 |
| CNGB3 | 5, 62.5 |
| OCT stage (15 eyes) | (N, %) |
| Stage 1 | 5, 33.2 |
| Stage 2 | 6, 40 |
| Stage 3 | 0, 0 |
| Stage 4 | 2, 13.3 |
| Stage 5 | 2, 13.3 |
| Functional Parameter | Mean ± SD |
|---|---|
| BCVA, logMAR | 0.7 ± 0.3 |
| LLVA, logMAR | 0.8 ± 0.3 |
| NVA, logMAR | 0.3 ± 0.2 |
| CS, logCS | 1.4 ± 0.2 |
| Microperimetry | |
| BCEA63, degree2 | 13.0 ± 16.0 |
| BCEA95, degree2 | 39.0 ± 47.9 |
| AS, dB | 21.2 ± 2.7 |
| C, dB | 20.4 ± 6.6 |
| R1, dB | 20.8 ± 5.9 |
| R2, dB | 21.1 ± 4.7 |
| R3, dB | 23.1 ± 2.0 |
| R4, dB | 21.5 ± 2.9 |
| R5, dB | 20.2 ± 2.7 |
| Functional Parameters | OCT Staging (Mean ± SD) | p-Value | |||
|---|---|---|---|---|---|
| 1 (n = 5) | 2 (n = 6) | 4 (n = 2) | 5 (n = 2) | ||
| BCEA63, degree2 | 18.7 ± 27.0 | 6.7 ± 6.6 | 17.0 ± 3.5 | 13.9 ± 1.4 | 0.8098 |
| BCEA95, degree2 | 56.0 ± 80.8 | 20.1 ± 19.7 | 51.1 ± 10.7 | 41.7 ± 4.3 | 0.8096 |
| AS, dB | 21.9 ± 2.1 | 22.5 ± 1.4 | 23.9 ± 0.9 | 17.0 ± 3.1 | 0.0694 |
| C, dB | 21.1 ± 1.8 | 23.3 ± 2.4 | 24.0 ± 1.4 | 8.5 ± 12.0 | 0.0286 |
| R1, dB | 21.9 ± 2.1 | 23.5 ± 1.0 | 23.4 ± 0.5 | 7.2 ± 5.7 | 0.0008 |
| R2, dB | 21.1 ± 1.8 | 23.9 ± 0.9 | 23.3 ± 0.5 | 10.5 ± 4.2 | 0.0014 |
| R3, dB | 21.8 ± 1.7 | 23.4 ± 1.1 | 25.5 ± 0.9 | 22.8 ± 4.1 | 0.1682 |
| R4, dB | 19.7 ± 2.9 | 21.7 ± 2.8 | 24.8 ± 1.4 | 22.3 ± 1.6 | 0.5558 |
| R5, dB | 18.7 ± 3.5 | 20.4 ± 2.4 | 22.4 ± 1.3 | 21.3 ± 1.2 | 0.5336 |
| BCVA, logMAR | 0.620 ± 0.390 | 0.633 ± 0.350 | 0.700 ± 0.000 | 0.800 ± 0.000 | 0.9865 |
| LLVA, logMAR | 0.820 ± 0.492 | 0.700 ± 0.253 | 0.700 ± 0.000 | 0.800 ± 0.000 | 0.9181 |
| NVA, logMAR | 0.280 ± 0.164 | 0.217 ± 0.098 | 0.200 ± 0.000 | 0.600 ± 0.000 | 0.2608 |
| CS, logCS | 1.260 ± 0.272 | 1.400 ± 0.122 | 1.425 ± 0.106 | 1.575 ± 0.106 | 0.6628 |
| Microperimetry Parameter | BCVA | LLVA | NVA | CS |
|---|---|---|---|---|
| BCEA63 | 0.0508 (B) * | <0.0001 * | 0.2750 | 0.9941 |
| BCEA95 | 0.0507 (B) * | <0.0001 * | 0.2753 | 0.9427 |
| AS | 0.5096 | 0.5124 | 0.0797 | 0.7608 |
| C | 0.6194 | 0.5462 | 0.0521 (B) † | 0.2888 |
| R1 | 0.7506 | 0.9414 | 0.0033 † | 0.1758 |
| R2 | 0.7914 | 0.9823 | 0.0025 † | 0.3006 |
| R3 | 0.3578 | 0.4930 | 0.9451 | 0.2175 |
| R4 | 0.0724 | 0.1680 | 0.6010 | 0.2199 |
| R5 | 0.0295 * | 0.0618 | 0.2755 | 0.6525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosmo, E.; Pilotto, E.; Convento, E.; Parolini, F.; Midena, E. Microperimetry Sensitivity Correlates to Structural Macular Changes in Adolescents with Achromatopsia Unlike Other Visual Function Tests. J. Clin. Med. 2024, 13, 5968. https://doi.org/10.3390/jcm13195968
Cosmo E, Pilotto E, Convento E, Parolini F, Midena E. Microperimetry Sensitivity Correlates to Structural Macular Changes in Adolescents with Achromatopsia Unlike Other Visual Function Tests. Journal of Clinical Medicine. 2024; 13(19):5968. https://doi.org/10.3390/jcm13195968
Chicago/Turabian StyleCosmo, Eleonora, Elisabetta Pilotto, Enrica Convento, Federico Parolini, and Edoardo Midena. 2024. "Microperimetry Sensitivity Correlates to Structural Macular Changes in Adolescents with Achromatopsia Unlike Other Visual Function Tests" Journal of Clinical Medicine 13, no. 19: 5968. https://doi.org/10.3390/jcm13195968
APA StyleCosmo, E., Pilotto, E., Convento, E., Parolini, F., & Midena, E. (2024). Microperimetry Sensitivity Correlates to Structural Macular Changes in Adolescents with Achromatopsia Unlike Other Visual Function Tests. Journal of Clinical Medicine, 13(19), 5968. https://doi.org/10.3390/jcm13195968









