Elevated Circulating Levels of Gut Microbe-Derived Trimethylamine N-Oxide Are Associated with Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Source, and Study Population
2.2. Metabolomics
2.3. Statistical Analysis
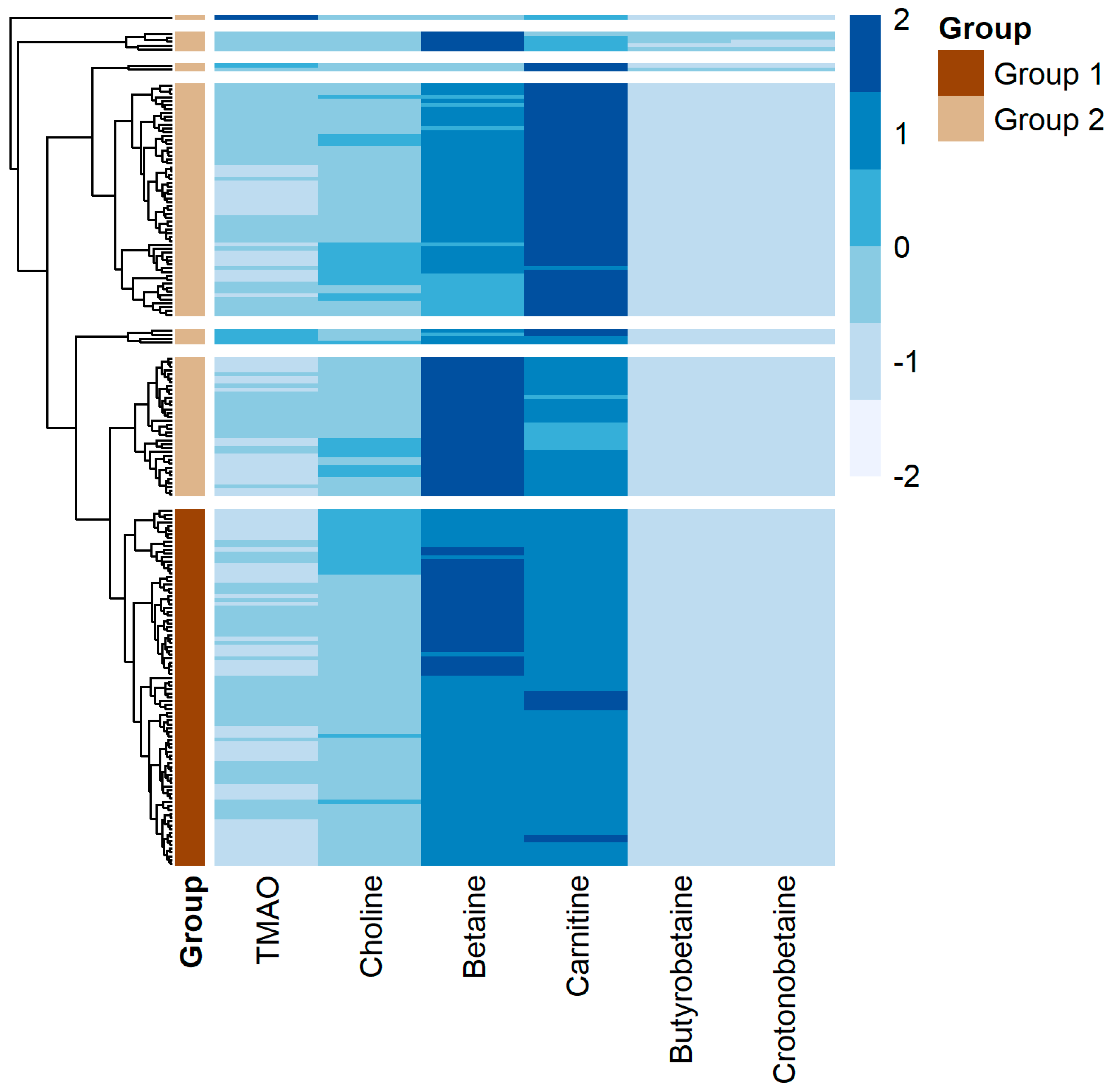
3. Results
4. Discussion
4.1. Involvement of TMAO in SSc
4.2. Sex-Related Differences in TMAO Levels
4.3. TMAO and Obesity
4.4. TMAO-Related Metabolites and SSc
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Varga, J.; Hinchcliff, M. Connective tissue diseases: Systemic sclerosis: Beyond limited and diffuse subsets? Nat. Rev. Rheumatol. 2014, 10, 200–202. [Google Scholar] [CrossRef]
- Bellocchi, C.; Volkmann, E.R. Update on the Gastrointestinal Microbiome in Systemic Sclerosis. Curr. Rheumatol. Rep. 2018, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, K.; Alrawi, Z.; Persson, A.; Jonsson, G.; Marsal, J. Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease. Arthritis Res. Ther. 2016, 18, 278. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Hoffmann-Vold, A.M.; Chang, Y.L.; Jacobs, J.P.; Tillisch, K.; Mayer, E.A.; Clements, P.J.; Hov, J.R.; Kummen, M.; Midtvedt, Ø.; et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol. 2017, 4, e000134. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Chang, Y.L.; Barroso, N.; Furst, D.E.; Clements, P.J.; Gorn, A.H.; Roth, B.E.; Conklin, J.L.; Getzug, T.; Borneman, J.; et al. Association of Systemic Sclerosis with a Unique Colonic Microbial Consortium. Arthritis Rheumatol. 2016, 68, 1483–1492. [Google Scholar] [CrossRef]
- Denton, C.P.; Murray, C. Cause or effect? Interpreting emerging evidence for dysbiosis in systemic sclerosis. Arthritis Res. Ther. 2019, 21, 81. [Google Scholar] [CrossRef]
- Seim, H.; Schulze, J.; Strack, E. Catabolic pathways for high-dosed L(-)- or D(+)-carnitine in germ-free rats? Biol. Chem. 1985, 366, 1017–1021. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metab. Clin. Exp. 1992, 41, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-EspinolaEspinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151 e5. [Google Scholar] [CrossRef]
- Kim, S.J.; Bale, S.; Verma, P.; Wan, Q.; Ma, F.; Gudjonsson, J.E.; Hazen, S.L.; Harms, P.W.; Tsou, P.-S.; Khanna, D.; et al. Gut microbe-derived metabolite trimethylamine N-oxide activates PERK to drive fibrogenic mesenchymal differentiation. iScience 2022, 25, 104669. [Google Scholar] [CrossRef]
- Gupta, N.; Buffa, J.A.; Roberts, A.B.; Sangwan, N.; Skye, S.M.; Li, L.; Ho, K.J.; Varga, J.; DiDonato, J.A.; Tang, W.W.; et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1239–1255. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.W.; Wu, Y.; Hazen, S.L.; et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E., 3rd; Polhemus, D.J.; Gupta, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J. Mol. Cell Cardiol. 2019, 134, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Stec, A.; Maciejewska, M.; Paralusz-Stec, K.; Michalska, M.; Giebułtowicz, J.; Rudnicka, L.; Sikora, M. The Gut Microbial Metabolite Trimethylamine N-Oxide is Linked to Specific Complications of Systemic Sclerosis. J. Inflamm. Res. 2023, 16, 1895–1904. [Google Scholar] [CrossRef]
- Ernster, V. Nested case-control studies. Prev. Med. 1994, 23, 587–590. [Google Scholar] [CrossRef]
- Richardson, C.; Agrawal, R.; Lee, J.; Almagor, O.; Nelson, R.; Varga, J.; Cuttica, M.J.; Dematte, J.D.; Chang, R.W.; Hinchcliff, M.E. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Semin. Arthritis Rheum. 2016, 46, 109–114. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; A DiDonato, J.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef]
- Romano, K.A.; Nemet, I.; Saha, P.P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut Microbiota-Generated Phenylacetylglutamine. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ni, Y.; Bazeley, P.; Grandhi, S.; Wang, J.; Li, S.T.; Hazen, S.L.; Tang, W.H.W.; LaFramboise, T. Mitochondrial DNA Content Is Linked to Cardiovascular Disease Patient Phenotypes. J. Am. Heart Assoc. 2021, 19, e018776. [Google Scholar] [CrossRef] [PubMed]
- Senthong, V.; Li, X.S.; Hudec, T.; Coughlin, J.; Wu, Y.; Levison, B.; Wang, Z.; Hazen, S.L.; Tang, W.W. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated with Atherosclerotic Burden. J. Am. Coll. Cardiol. 2016, 67, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Levison, B.S.; Hazen, J.E.; Donahue, L.; Li, X.M.; Hazen, S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014, 455, 35–40. [Google Scholar] [CrossRef]
- Jang, J.W.; Capaldi, E.; Smith, T.; Verma, P.; Varga, J.; Ho, K.J. Trimethylamine N-oxide: A meta-organismal axis linking the gut and fibrosis. Mol. Med. 2024, 30, 128. [Google Scholar] [CrossRef]
- Hausmann, A.J.; McMahan, Z.H.; Volkmann, E.R. Understanding the gastrointestinal microbiome in systemic sclerosis: Methodological advancements and emerging research. Curr. Opin. Rheumatol. 2024. [Google Scholar] [CrossRef]
- Wei, J.; Bhattacharyya Toutellotee, W.G.; Varga, J. Emerging concepts and implications for targeted therapy. Autoimmun. Rev. 2011, 19, 267–275. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef]
- Garcia-Perez, I.; Villasenor, A.; Wijeyesekera, A.; Posma, J.M.; Jiang, Z.; Stamler, J.; Aronson, P.; Unwin, R.; Barbas, C.; Elliott, P.; et al. Urinary metabolic phenotyping the slc26a6 (chloride-oxalate exchanger) null mouse model. J. Proteome Res. 2012, 11, 4425–4435. [Google Scholar] [CrossRef]
- Gavaghan McKee, C.L.; Wilson, I.D.; Nicholson, J.K. Metabolic phenotyping of nude and normal (Alpk:ApfCD, C57BL10J) mice. J. Proteome Res. 2006, 5, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.G.; Bailey, N.J.; Bollard, M.E.; Haselden, J.; Waterfield, C.; Holmes, E.; Nicholson, J. Sexual dimorphism in urinary metabolite profiles of Han Wistar rats revealed by nuclear-magnetic-resonance-based metabonomics. Anal. Biochem. 2005, 343, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Roi, C.M.; Pescari, D.; Velea-Barta, O.-A.; Mozos, I.; Stoian, D. Connections between serum Trimethylamine N-Oxide (TMAO), a gut-derived metabolite, and vascular biomarkers evaluating arterial stiffness and subclinical atherosclerosis in children with obesity. Front. Endocrinol. 2023, 14, 1253584. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients. 2018, 10, 1971. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Wang, Z.A.; Fretts, A.M.; McKnight, B.; Nemet, I.; Biggs, M.L.; Sotoodehnia, N.; de Oliveira Otto, M.C.; Psaty, B.M.; et al. Association of Trimethylamine N-Oxide and Related Metabolites in Plasma and Incident Type 2 Diabetes: The Cardiovascular Health Study. JAMA Netw. Open 2021, 4, e2122844. [Google Scholar] [CrossRef]
- Shimizu, M.; Cashman, J.R.; Yamazaki, H. Transient trimethylaminuria related to menstruation. BMC Med. Genet. 2007, 8, 2. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 1998, 18, 39–61. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Graziadio, C.; Maisto, M.; Pivari, F.; Falco, A.; Tenore, G.C.; Colao, A.; Savastano, S. Association of the Chronotype Score with Circulating Trimethylamine N-Oxide (TMAO) Concentrations. Nutrients 2021, 13, 1671. [Google Scholar] [CrossRef]
- Kuhn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. (CCLM) 2017, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]

| Control Group n = 400 | SSc Group n = 200 | p Value | |
|---|---|---|---|
| Age, years (median [IQR]) | 53.63 [45.86, 62.98] | 52.00 [43.75, 60.00] | 0.040 * |
| Female (%) | 383 (95.6) | 169 (84.5) | 0.565 |
| Caucasian (%) | 363 (92.8) | 165 (82.5) | <0.001 * |
| BMI (median [IQR]) | 29.66 [26.01, 34.01] | 24.76 [21.35, 28.37] | <0.001 * |
| eGFR, mL/min/1.73 m2 (median [IQR]) | 93.08 [81.76, 103.82] | 92.62 [75.83, 106.06] | 0.469 |
| Creatinine, mg/dL (median [IQR]) | 0.76 [0.67, 0.83] | 0.77 [0.68, 0.88] | 0.200 |
| History of cardiovascular disease (%) | 14 (3.5) | 6 (3.2) | 1.000 |
| History of diabetes mellitus (%) | 12 (3.0) | 5 (2.6) | 1.000 |
| SSc classification | |||
| Limited cutaneous (%) | 87 (43.5) | ||
| Diffuse cutaneous (%) | 109 (54.5) | ||
| Stiff skin syndrome (%) | 4 (2.0) | ||
| Disease duration, months (median [IQR]) | 46.00 [17.50, 97.50] | ||
| Autoantibodies | |||
| Anti-nuclear antibody (%) | 190 (96.4) | ||
| Anti-centromere antibody (%) | 39 (20.1) | ||
| Anti-topoisomerase I antibody (%) | 43 (22.4) | ||
| Anti-RNA polymerase III antibody (%) | 56 (34.1) | ||
| MRSS (median [IQR]) | 8.00 [4.00, 18.00] | ||
| FVC, liters (median [IQR]) | 2.84 [2.45, 3.38] | ||
| DLCO, mL/mm Hg/min (median [IQR]) | 14.60 [11.10, 18.35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.J.; Muhammad, L.N.; Khanh, L.N.; Li, X.S.; Carns, M.; Aren, K.; Kim, S.-J.; Verma, P.; Hazen, S.L.; Varga, J. Elevated Circulating Levels of Gut Microbe-Derived Trimethylamine N-Oxide Are Associated with Systemic Sclerosis. J. Clin. Med. 2024, 13, 5984. https://doi.org/10.3390/jcm13195984
Ho KJ, Muhammad LN, Khanh LN, Li XS, Carns M, Aren K, Kim S-J, Verma P, Hazen SL, Varga J. Elevated Circulating Levels of Gut Microbe-Derived Trimethylamine N-Oxide Are Associated with Systemic Sclerosis. Journal of Clinical Medicine. 2024; 13(19):5984. https://doi.org/10.3390/jcm13195984
Chicago/Turabian StyleHo, Karen J., Lutfiyya N. Muhammad, Linh Ngo Khanh, Xinmin S. Li, Mary Carns, Kathleen Aren, Seok-Jo Kim, Priyanka Verma, Stanley L. Hazen, and John Varga. 2024. "Elevated Circulating Levels of Gut Microbe-Derived Trimethylamine N-Oxide Are Associated with Systemic Sclerosis" Journal of Clinical Medicine 13, no. 19: 5984. https://doi.org/10.3390/jcm13195984
APA StyleHo, K. J., Muhammad, L. N., Khanh, L. N., Li, X. S., Carns, M., Aren, K., Kim, S.-J., Verma, P., Hazen, S. L., & Varga, J. (2024). Elevated Circulating Levels of Gut Microbe-Derived Trimethylamine N-Oxide Are Associated with Systemic Sclerosis. Journal of Clinical Medicine, 13(19), 5984. https://doi.org/10.3390/jcm13195984






