Early Fever in Allogeneic Stem Cell Transplantation: Are Presepsin and YKL-40 Valuable Diagnostic Tools?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients and Design
2.2. Clinical Events
2.3. Biomarker Measurements
2.4. Statistical Analysis
3. Results
3.1. Patient and FE Characteristics
3.2. Biomarkers—Diagnostic Potential
3.2.1. Infection vs. FUO
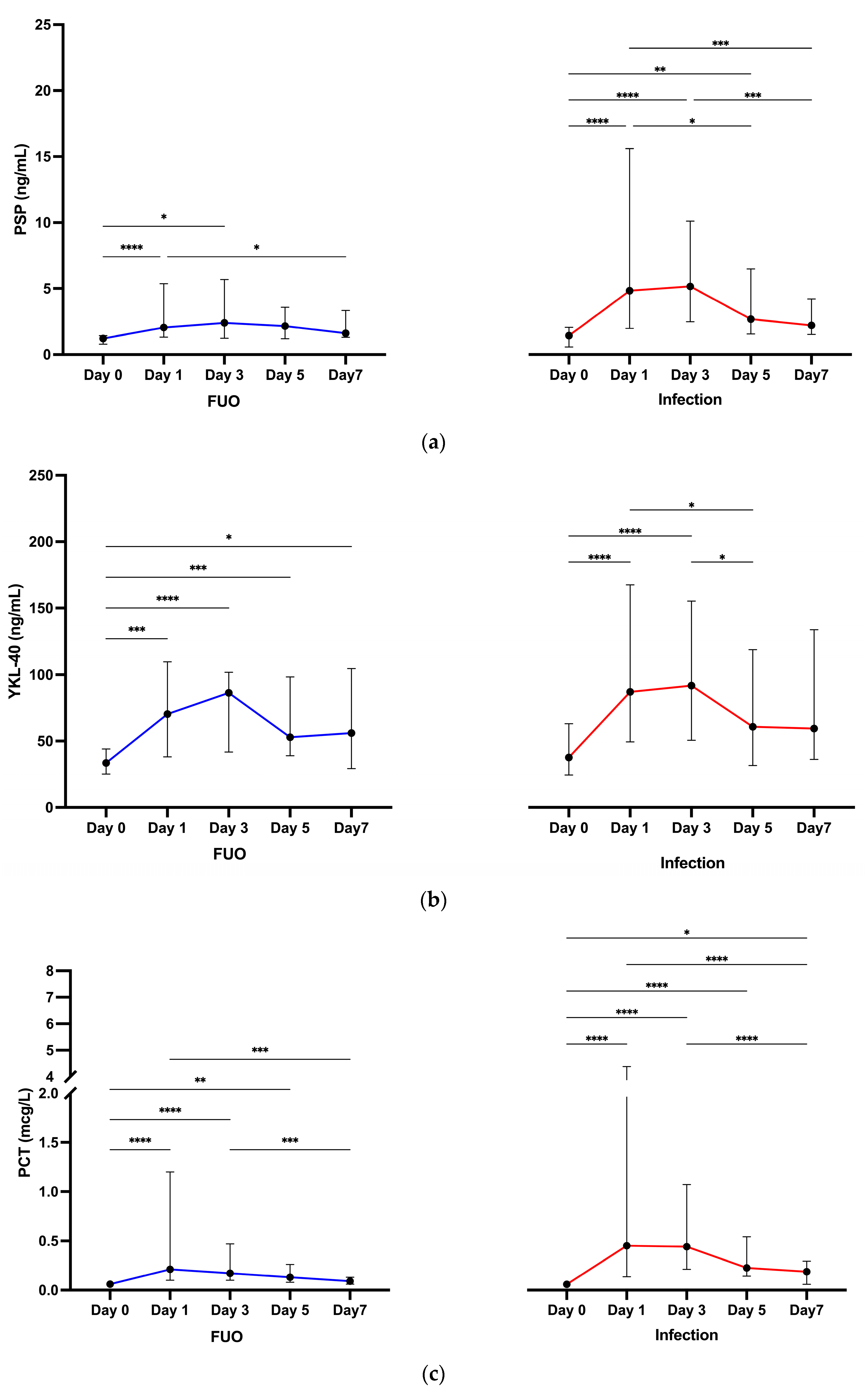
3.2.2. Biomarkers across Time Points
3.2.3. Associations between PSP, YKL-40, and PCT
3.3. Biomarkers—Diagnostic Utility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| ANC | Absolute neutrophil count |
| ATG | Anti-thymocyte globulin |
| AUC | Area under the curve |
| CD14 | Cluster of differentiation 14 |
| CDC | Centers for Disease Control and Prevention |
| CDI | Clinically documented infections |
| CHI3L1 | Chitinase-3-like protein 1 |
| CLEIA | Chemiluminescent enzyme immunoassay |
| CML | Chronic myeloid leukemia |
| CMV | Cytomegalovirus |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| G-CSF | Granulocyte colony-stimulating factor |
| DOR | Diagnostic odds ratio |
| EBMT | European Society for Blood and Marrow Transplantation |
| EBV | Epstein–Barr virus |
| ELISA | Enzyme-linked immunosorbent assay |
| FUO | Fever of unknown origin |
| GVHD | Graft-versus-host disease |
| HCT-CI | Hematopoietic cell transplantation comorbidity index |
| HSCT | Hematopoietic stem cell transplantation |
| IL | Interleukin |
| IQR | Interquartile range |
| ISCT | International Society for Cell and Gene Therapy |
| JACIE | Joint Accreditation Committee of the International Society for Cell and Gene Therapy and the European Society for Blood and Marrow Transplantation |
| LBP | Lipopolysaccharide binding protein |
| LH – | Negative likelihood ratio |
| LH + | Positive likelihood ratio |
| LPS | Lipopolysaccharide |
| MDI | Microbiologically documented infection |
| MDS | Myelodysplastic syndrome |
| MPN | Myeloproliferative neoplasms |
| PCR | Polymerase chain reaction |
| PCT | Procalcitonin |
| PSP | Presepsin |
| qSOFA | Quick Sepsis-Related Organ Failure Assessment |
| RIC | Reduced intensity conditioning |
| ROC | receiver operating characteristic |
| SD | Standard deviation |
| SIRS | Systemic inflammatory response syndrome |
| VIF | Variance inflation factor |
| WHO | World Health Organization |
References
- Styczyński, J.; Tridello, G.; Koster, L.; Iacobelli, S.; van Biezen, A.; van der Werf, S.; Mikulska, M.; Gil, L.; Cordonnier, C.; Ljungman, P.; et al. Death after Hematopoietic Stem Cell Transplantation: Changes over Calendar Year Time, Infections and Associated Factors. Bone Marrow Transplant. 2020, 55, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Weisser, M.; Theilacker, C.; Tschudin, S.S.; Babikir, R.; Bertz, H.; Götting, T.; Dettenkofer, M.; Kern, W.V.; Widmer, A.F. Secular Trends of Bloodstream Infections during Neutropenia in 15 181 Haematopoietic Stem Cell Transplants: 13-Year Results from a European Multicentre Surveillance Study (ONKO-KISS). Clin. Microbiol. Infect. 2017, 23, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An Overview of Infectious Complications after Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. (Eds.) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 259–363. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Takahashi, G.; Miyata, M.; Endo, S.; Shirakawa, K.; Furusako, S. Evaluation of a Newly Identified Soluble CD14 Subtype as a Marker for Sepsis. J. Infect. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef]
- Bas, S.; Gauthier, B.R.; Spenato, U.; Stingelin, S.; Gabay, C. CD14 Is an Acute-Phase Protein. J. Immunol. 2004, 172, 4470–4479. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Wen, W.; Zhang, X. Presepsin as a Novel Sepsis Biomarker. World J. Emerg. Med. 2014, 5, 16–19. [Google Scholar] [CrossRef]
- Bazil, V.; Strominger, J.L. Shedding as a Mechanism of Down-Modulation of CD14 on Stimulated Human Monocytes. J. Immunol. 1991, 147, 1567–1574. [Google Scholar] [CrossRef]
- Amanai, E.; Nakai, K.; Saito, J.; Hashiba, E.; Miura, T.; Morohashi, H.; Sakamoto, Y.; Mikami, A.; Hakamada, K.; Hirota, K. Usefulness of Presepsin for the Early Detection of Infectious Complications after Elective Colorectal Surgery, Compared with C-Reactive Protein and Procalcitonin. Sci. Rep. 2022, 12, 3960. [Google Scholar] [CrossRef]
- Liang, J.; Cai, Y.; Shao, Y. Comparison of Presepsin and Mid-Regional pro-Adrenomedullin in the Diagnosis of Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2023, 23, 288. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Leventogiannis, K.; Tavoulareas, G.; Mainas, E.; Toutouzas, K.; Mathas, C.; Prekates, A.; Sakka, V.; Panagopoulos, P.; Syrigos, K.; et al. Presepsin as a Diagnostic and Prognostic Biomarker of Severe Bacterial Infections and COVID-19. Sci. Rep. 2023, 13, 3814. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, J.; Park, D.W.; Seok, H.; Ahn, S.; Kim, J.; Park, J.; Cho, H.; Moon, S. Diagnostic and Prognostic Value of Presepsin and Procalcitonin in Non-Infectious Organ Failure, Sepsis, and Septic Shock: A Prospective Observational Study According to the Sepsis-3 Definitions. BMC Infect. Dis. 2022, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.X.; Yin, Q.; Zhao, Y.Z.; Li, C.S. Diagnostic Value and Prognostic Evaluation of Presepsin for Sepsis in an Emergency Department. Crit. Care 2013, 17, R244. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Sakanashi, D.; Mohri, T.; Watanabe, H.; Shiota, A.; Asai, N.; Kato, H.; Hagihara, M.; Murotani, K.; Yamagishi, Y.; et al. Can Presepsin Uniformly Respond to Various Pathogens?—An in Vitro Assay of New Sepsis Marker. BMC Immunol. 2020, 21, 33. [Google Scholar] [CrossRef]
- Behnes, M.; Bertsch, T.; Lepiorz, D.; Lang, S.; Trinkmann, F.; Brueckmann, M.; Borggrefe, M.; Hoffmann, U. Diagnostic and Prognostic Utility of Soluble CD 14 Subtype (Presepsin) for Severe Sepsis and Septic Shock during the First Week of Intensive Care Treatment. Crit. Care 2014, 18, 507. [Google Scholar] [CrossRef]
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and Prognostic Value of Presepsin in the Management of Sepsis in the Emergency Department: A Multicenter Prospective Study. Crit. Care 2013, 17, R168. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgrò, S.; Fanizza, C.; et al. Presepsin (Soluble CD14 Subtype) and Procalcitonin Levels for Mortality Prediction in Sepsis: Data from the Albumin Italian Outcome Sepsis Trial. Crit. Care 2014, 18, R6. [Google Scholar] [CrossRef]
- Bakula, D.; Vrkljan, N.; Ratkajec, V.; Glavcic, G.; Miler, M.; Pelajic, S.; Rogic, D.; Blazevic, N.; Pavic, T.; Hrabar, D. YKL-40 as a Biomarker in Various Inflammatory Diseases: A Review. Biochem. Medica 2024, 34, 42–56. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-Protein-1 Function and Its Role in Diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Liu, W.; He, C.H.; Jacoby, A.; Gornitzky, A.; Ma, B.; Flavell, R.; Lee, C.G.; Elias, J.A. Chitinase 3-like-1 Promotes Streptococcus Pneumoniae Killing and Augments Host Tolerance to Lung Antibacterial Responses. Cell Host Microbe 2012, 12, 34–46. [Google Scholar] [CrossRef]
- Kronborg, G.; Østergaard, C.; Weis, N.; Obel, N.; Johansen, J.S.; Price, P.A.; Nielsen, H.; Pedersen, S.S. Serum Level of YKL-40 Is Elevated in Patients with Streptococcus Pneumoniae Bacteremia and Is Associated with the Outcome of the Disease. Scand. J. Infect. Dis. 2002, 34, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Spoorenberg, S.M.C.; Vestjens, S.M.T.; Voorn, G.P.; van Moorsel, C.H.M.; Meek, B.; Zanen, P.; Rijkers, G.T.; Bos, W.J.W.; Grutters, J.C.; Ovidius study group. Course of SP-D, YKL-40, CCL18 and CA 15-3 in Adult Patients Hospitalised with Community-Acquired Pneumonia and Their Association with Disease Severity and Aetiology: A Post-Hoc Analysis. PLoS ONE 2018, 13, e0190575. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, C.; Johansen, J.S.; Benfield, T.; Price, P.A.; Lundgren, J.D. YKL-40 Is Elevated in Cerebrospinal Fluid from Patients with Purulent Meningitis. Clin. Vaccine Immunol. 2002, 9, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Sadanaga, T.; Nanni, L.; Wang, S.; Mizoguchi, A. Recently Updated Role of Chitinase 3-like 1 on Various Cell Types as a Major Influencer of Chronic Inflammation. Cells 2024, 13, 678. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Crisafi, G.M.; Holm, C.T.; Meyers, D.A.; Hawkins, G.A.; Bleecker, E.R.; Jarjour, N.; Cohn, L.; Chupp, G.L. Genetic Variation in Chitinase 3-like 1 (CHI3L1) Contributes to Asthma Severity and Airway Expression of YKL-40. J. Allergy Clin. Immunol. 2015, 136, 51–58.e10. [Google Scholar] [CrossRef]
- Hattori, N.; Oda, S.; Sadahiro, T.; Nakamura, M.; Abe, R.; Shinozaki, K.; Nomura, F.; Tomonaga, T.; Matsushita, K.; Kodera, Y.; et al. YKL-40 identified by proteomic analysis as a biomarker of sepsis. Shock 2009, 32, 393–400. [Google Scholar] [CrossRef]
- Johansen, J.S.; Krabbe, K.S.; Møller, K.; Pedersen, B.K. Circulating YKL-40 Levels during Human Endotoxaemia. Clin. Exp. Immunol. 2005, 140, 343–348. [Google Scholar] [CrossRef]
- Memar, M.Y.; Baghi, H.B. Presepsin: A Promising Biomarker for the Detection of Bacterial Infections. Biomed. Pharmacother. 2019, 111, 649–656. [Google Scholar] [CrossRef]
- He, C.H.; Lee, C.G.; Dela Cruz, C.S.; Lee, C.M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzog, E.L.; Rosenberg, S.A.; Li, Y.; et al. Chitinase 3-like 1 Regulates Cellular and Tissue Responses via IL-13 Receptor A2. Cell Rep. 2013, 4, 830–841. [Google Scholar] [CrossRef]
- Ebihara, Y.; Kobayashi, K.; Ishida, A.; Maeda, T.; Takahashi, N.; Taji, Y.; Asou, N.; Ikebuchi, K. Diagnostic Performance of Procalcitonin, Presepsin, and C-Reactive Protein in Patients with Hematological Malignancies. J. Clin. Lab. Anal. 2017, 31, e22147. [Google Scholar] [CrossRef]
- Koh, H.; Aimoto, M.; Katayama, T.; Hashiba, M.; Sato, A.; Kuno, M.; Makuuchi, Y.; Takakuwa, T.; Okamura, H.; Hirose, A.; et al. Diagnostic Value of Levels of Presepsin (Soluble CD14-Subtype) in Febrile Neutropenia in Patients with Hematological Disorders. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2016, 22, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Kostic, I.; Gurrieri, C.; Piva, E.; Semenzato, G.; Plebani, M.; Caputo, I.; Vianello, F. Comparison of Presepsin, Procalcitonin, Interleukin-8 and C-Reactive Protein in Predicting Bacteraemia in Febrile Neutropenic Adult Patients with Haematological Malignancies. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019047. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Shimizu, K.; Shigeta, M.; Okuno, T.; Minamiguchi, H.; Kito, K.; Hodohara, K.; Yamagishi, Y.; Andoh, A.; Fujiyama, Y.; et al. Plasma Presepsin Level Is an Early Diagnostic Marker of Severe Febrile Neutropenia in Hematologic Malignancy Patients. BMC Infect. Dis. 2017, 17, 27. [Google Scholar] [CrossRef]
- Moustafa, R.; Albouni, T.; Aziz, G. The Role of Procalcitonin and Presepsin in the Septic Febrile Neutropenia in Acute Leukemia Patients. PLoS ONE 2021, 16, e0253842. [Google Scholar] [CrossRef] [PubMed]
- Stoma, I.; Karpov, I.; Uss, A.; Rummo, O.; Milanovich, N.; Iskrov, I. Diagnostic Value of Sepsis Biomarkers in Hematopoietic Stem Cell Transplant Recipients in a Condition of High Prevalence of Gram-Negative Pathogens. Hematol. Oncol. Stem Cell Ther. 2017, 10, 15–21. [Google Scholar] [CrossRef]
- Foundation for the Accreditation of Cellular Therapy; Joined Accreditation Committee of ISCT and EBMT. FACT-JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration, 7th ed.; FACT/JAICE: Omaha, NE USA, 2018. [Google Scholar]
- Klastersky, J.; De Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of Febrile Neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef]
- Robinson, J.O.; Lamoth, F.; Bally, F.; Knaup, M.; Calandra, T.; Marchetti, O. Monitoring Procalcitonin in Febrile Neutropenia: What Is Its Utility for Initial Diagnosis of Infection and Reassessment in Persistent Fever? PLoS ONE 2011, 6, e18886. [Google Scholar] [CrossRef]
- Kanate, A.S.; Nagler, A.; Savani, B. Summary of Scientific and Statistical Methods, Study Endpoints and Definitions for Observational and Registry-Based Studies in Hematopoietic Cell Transplantation. Clin. Hematol. Int. 2019, 2, 2–4. [Google Scholar] [CrossRef]
- Pizzo, P.A.; Armstrong, D.; Bodey, G.; de Pauw, B.; Feld, R.; Glauser, M.; Gaya, H.; Karp, J.; Klastersky, J.; Todeschini, G.; et al. From the Immunocompromised Host Society: The Design, Analysis, and Reporting of Clinical Trials on the Empirical Antibiotic Management of the Neutropenic Patient: Report of a Consensus Panel. J. Infect. Dis. 1990, 161, 397–401. [Google Scholar] [CrossRef]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC Definitions for Nosocomial Infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Beekmann, S.E.; Diekema, D.J.; Doern, G.V. Determining the Clinical Significance of Coagulase-Negative Staphylococci Isolated From Blood Cultures. Infect. Control Hosp. Epidemiol. 2005, 26, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Griffiths, P.; Paya, C. Definitions of Cytomegalovirus Infection and Disease in Transplant Recipients. Clin. Infect. Dis. 2002, 34, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, X.; Zhang, X.; Milebe Nkoua, G.D.; Chen, F.; Liu, Y.; Chai, Y. The Roles of Tissue-Resident Macrophages in Sepsis-Associated Organ Dysfunction. Heliyon 2023, 9, e21391. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.M.; Malandra, M.; Corey, S.J. G-CSF and GM-CSF in Neutropenia. J. Immunol. 2015, 195, 1341–1349. [Google Scholar] [CrossRef]
- Michel, C.S.; Teschner, D.; Wagner, E.M.; Theobald, M.; Radsak, M.P. Diagnostic Value of sTREM-1, IL-8, PCT, and CRP in Febrile Neutropenia after Autologous Stem Cell Transplantation. Ann. Hematol. 2017, 96, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, A.; De Vroey, V.; Goossens, H.; Roelant, E.; Van De Velde, A.; Berneman, Z.; Schroyens, W.; Gadisseur, A. comparation of the Power of Procalcitonin and C-reactive Protein to Discriminate Between Different Aetiologies of Fever in Prolonged Profound Neutropenia: A Single-Centre Prospective Observational Study. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019023. [Google Scholar] [CrossRef]
- Cerasi, S.; Leardini, D.; Lisanti, N.; Belotti, T.; Pierantoni, L.; Zama, D.; Lanari, M.; Prete, A.; Masetti, R. The Role of Presepsin in Pediatric Patients with Oncological and Hematological Diseases Experiencing Febrile Neutropenia. Sci. Rep. 2023, 13, 6464. [Google Scholar] [CrossRef]
- Kondo, Y.; Umemura, Y.; Hayashida, K.; Hara, Y.; Aihara, M.; Yamakawa, K. Diagnostic Value of Procalcitonin and Presepsin for Sepsis in Critically Ill Adult Patients: A Systematic Review and Meta-Analysis. J. Intensive Care 2019, 7, 22. [Google Scholar] [CrossRef]
- Wu, C.C.; Lan, H.M.; Han, S.T.; Chaou, C.H.; Yeh, C.F.; Liu, S.H.; Li, C.H.; Blaney, G.N.; Liu, Z.Y.; Chen, K.F. Comparison of Diagnostic Accuracy in Sepsis between Presepsin, Procalcitonin, and C-Reactive Protein: A Systematic Review and Meta-Analysis. Ann. Intensive Care 2017, 7, 91. [Google Scholar] [CrossRef]
- Klouche, K.; Cristol, J.P.; Devin, J.; Gilles, V.; Kuster, N.; Larcher, R.; Amigues, L.; Corne, P.; Jonquet, O.; Dupuy, A.M. Diagnostic and Prognostic Value of Soluble CD14 Subtype (Presepsin) for Sepsis and Community-Acquired Pneumonia in ICU Patients. Ann. Intensive Care 2016, 6, 59. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A Promising Diagnostic Marker for Sepsis and Antibiotic Therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Tvedt, T.H.A.; Vo, A.K.; Bruserud, Ø.; Reikvam, H. Cytokine Release Syndrome in the Immunotherapy of Hematological Malignancies: The Biology behind and Possible Clinical Consequences. J. Clin. Med. 2021, 10, 5190. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Yokoi, H. Development of a Point-of-Care Assay System for Measurement of Presepsin (sCD14-ST). Clin. Chim. Acta 2011, 412, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, C.O.; Gherghe, M.; Costache, M.; Cretu, B.; Cirstoiu, C. The Role of Serum and Peritoneal Biomarkers in Predicting Sepsis and Septic Multiorgan Failure in Patients with Secondary Peritonitis. Cureus 2023, 15, e41724. [Google Scholar] [CrossRef]
- El-Madbouly, A.; El Sehemawy, A.; Eldesoky, N.; Abd Elgalil, H.M.; Ahmed, A. Utility of Presepsin, Soluble Triggering Receptor Expressed on Myeloid Cells-1, and Neutrophil CD64 for Early Detection of Neonatal Sepsis. Infect. Drug Resist. 2019, 12, 311–319. [Google Scholar] [CrossRef]
- Aliu-Bejta, A.; Atelj, A.; Kurshumliu, M.; Dreshaj, S.; Baršić, B. Presepsin Values as Markers of Severity of Sepsis. Int. J. Infect. Dis. 2020, 95, 1–7. [Google Scholar] [CrossRef]
- Väänänen, T.; Vuolteenaho, K.; Kautiainen, H.; Nieminen, R.; Möttönen, T.; Hannonen, P.; Korpela, M.; Kauppi, M.J.; Laiho, K.; Kaipiainen-Seppänen, O.; et al. Glycoprotein YKL-40: A Potential Biomarker of Disease Activity in Rheumatoid Arthritis during Intensive Treatment with csDMARDs and Infliximab. Evidence from the Randomised Controlled NEO-RACo Trial. PLoS ONE 2017, 12, e0183294. [Google Scholar] [CrossRef]
- Schoneveld, L.; Ladang, A.; Henket, M.; Frix, A.N.; Cavalier, E.; Guiot, J.; on behalf of the COVID-19 clinical investigators of the CHU de Liège. YKL-40 as a New Promising Prognostic Marker of Severity in COVID Infection. Crit. Care 2021, 25, 66. [Google Scholar] [CrossRef]
- Hedetoft, M.; Hansen, M.B.; Madsen, M.B.; Johansen, J.S.; Hyldegaard, O. Associations between YKL-40 and Markers of Disease Severity and Death in Patients with Necrotizing Soft-Tissue Infection. BMC Infect. Dis. 2021, 21, 1046. [Google Scholar] [CrossRef]
- Lai, T.; Chen, M.; Deng, Z.; Lǚ, Y.; Wu, D.; Li, D.; Wu, B. YKL-40 Is Correlated with FEV1 and the Asthma Control Test (ACT) in Asthmatic Patients: Influence of Treatment. BMC Pulm. Med. 2015, 15, 1. [Google Scholar] [CrossRef]
- Holmgaard, D.B.; Mygind, L.H.; Titlestad, I.L.; Madsen, H.; Pedersen, S.S.; Johansen, J.S.; Pedersen, C. Plasma YKL-40 and All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease. BMC Pulm. Med. 2013, 13, 77. [Google Scholar] [CrossRef]
- van Bilsen, J.H.M.; van Dongen, H.; Lard, L.R.; van der Voort, E.I.H.; Elferink, D.G.; Bakker, A.M.; Miltenburg, A.M.M.; Huizinga, T.W.J.; de Vries, R.R.P.; Toes, R.E.M. Functional Regulatory Immune Responses against Human Cartilage Glycoprotein-39 in Health vs. Proinflammatory Responses in Rheumatoid Arthritis. Proc. Natl. Acad. Sci. USA 2004, 101, 17180–17185. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Dodds, R.A.; Emery, J.G.; Kirkpatrick, R.B.; Rosenberg, M.; Gowen, M. Human Cartilage Glycoprotein 39 (HC Gp-39) mRNA Expression in Adult and Fetal Chondrocytes, Osteoblasts and Osteocytes by in-Situ Hybridization. Osteoarthr. Cartil. 2000, 8, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S.; Schultz, N.A.; Jensen, B.V. Plasma YKL-40: A Potential New Cancer Biomarker? Future Oncol. 2009, 5, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Volck, B.; Price, P.; Johansen, J.; Sørensen, O.; Benfield, T.; Nielsen, H.; Calafat, J.; Borregaard, N. YKL-40, a Mammalian Member of the Chitinase Family, Is a Matrix Protein of Specific Granules in Human Neutrophils. Proc. Assoc. Am. Physicians 1998, 110, 351–360. [Google Scholar] [PubMed]
- Kzhyshkowska, J.; Mamidi, S.; Gratchev, A.; Kremmer, E.; Schmuttermaier, C.; Krusell, L.; Haus, G.; Utikal, J.; Schledzewski, K.; Scholtze, J.; et al. Novel Stabilin-1 Interacting Chitinase-like Protein (SI-CLP) Is up-Regulated in Alternatively Activated Macrophages and Secreted via Lysosomal Pathway. Blood 2006, 107, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Plomgaard, P.; Krabbe, K.S.; Johansen, J.S.; Pedersen, B.K. IL-6, but Not TNF-α, Increases Plasma YKL-40 in Human Subjects. Cytokine 2011, 55, 152–155. [Google Scholar] [CrossRef]
- Junker, N.; Johansen, J.S.; Hansen, L.T.; Lund, E.L.; Kristjansen, P.E.G. Regulation of YKL-40 Expression during Genotoxic or Microenvironmental Stress in Human Glioblastoma Cells. Cancer Sci. 2005, 96, 183–190. [Google Scholar] [CrossRef]
- Malinda, K.M.; Ponce, L.; Kleinman, H.K.; Shackelton, L.M.; Millis, A.J. Gp38k, a Protein Synthesized by Vascular Smooth Muscle Cells, Stimulates Directional Migration of Human Umbilical Vein Endothelial Cells. Exp. Cell Res. 1999, 250, 168–173. [Google Scholar] [CrossRef]
- Kornblit, B.; Wang, T.; Lee, S.J.; Spellman, S.R.; Zhu, X.; Fleischhauer, K.; Müller, C.; Verneris, M.R.; Müller, K.; Johansen, J.S.; et al. YKL-40 in Allogeneic Hematopoietic Cell Transplantation after AML and Myelodysplastic Syndrome. Bone Marrow Transplant. 2016, 51, 1556–1560. [Google Scholar] [CrossRef]


| Patients | n = 54 |
|---|---|
| Age (mean ± SD) | 46.50 ± 13.3 |
| Gender, n (%) | |
| Female | 29 (53.71) |
| Male | 25 (46.29) |
| HCT-CI score, n (%) | |
| Low (0) | 20 (37.04) |
| Intermediate (1–2) | 23 (42.59) |
| High (≥3) | 11 (20.37) |
| Underlying disease, n (%) | |
| AML | 25 (46.30) |
| ALL | 11 (20.37) |
| Lymphoma | 8 (14.81) |
| MPN | 3 (5.56) |
| MDS | 2 (3.70) |
| Aplastic anemia | 1 (1.85) |
| Rest | 4 (7.41) |
| Donor type, n (%) | |
| Matched unrelated | 34 (62.96) |
| Haploidentical | 12 (22.22) |
| Matched related | 8 (14.81) |
| Conditioning regimen, n (%) | |
| Nonmyeloablative/RIC | 39 (72.22) |
| Myeloablative | 15 (27.78) |
| Febrile Episodes (FE) | n = 61 |
|---|---|
| Recurrent, n (%) | 7 (11.48) |
| Documented infection, n (%) | 38 (62.30) |
| MDI | 27 (44.26) |
| CDI | 11 (18.04) |
| FUO, n (%) | 23 (37.70) |
| Isolated pathogen, n (%) | |
| G− bacteria | 14 (51.85) |
| Viral | 5 (18.52) |
| G+ bacteria | 4 (14.81) |
| Polymicrobial | 3 (11.11) |
| Fungal | 1 (3.70) |
| Infection source, n (%) | |
| Gastrointestinal | 21 (55.26) |
| Genitourinary | 4 (10.53) |
| Respiratory | 2 (5.26) |
| Skin and soft tissues | 0 (0) |
| Unknown | 11 (28.95) |
| Bacteremia, n (%) | 10 (16.39) |
| Neutropenia, n (%) | 44 (72.13) |
| Duration of neutropenia before FE (days), median (range) | 4 (0–8) |
| Acute GVHD, n (%) | 7 (11.48) |
| Engraftment (days), median (range) | 16 (14–21) |
| ATG, n (%) | 18 (29.51) |
| G-CSF, n (%) | 22 (36.07) |
| Infection severity, n (%) | |
| qSOFA score ≥ 2 | 7 (11.48) |
| Fatal outcome a | 1 (1.64) |
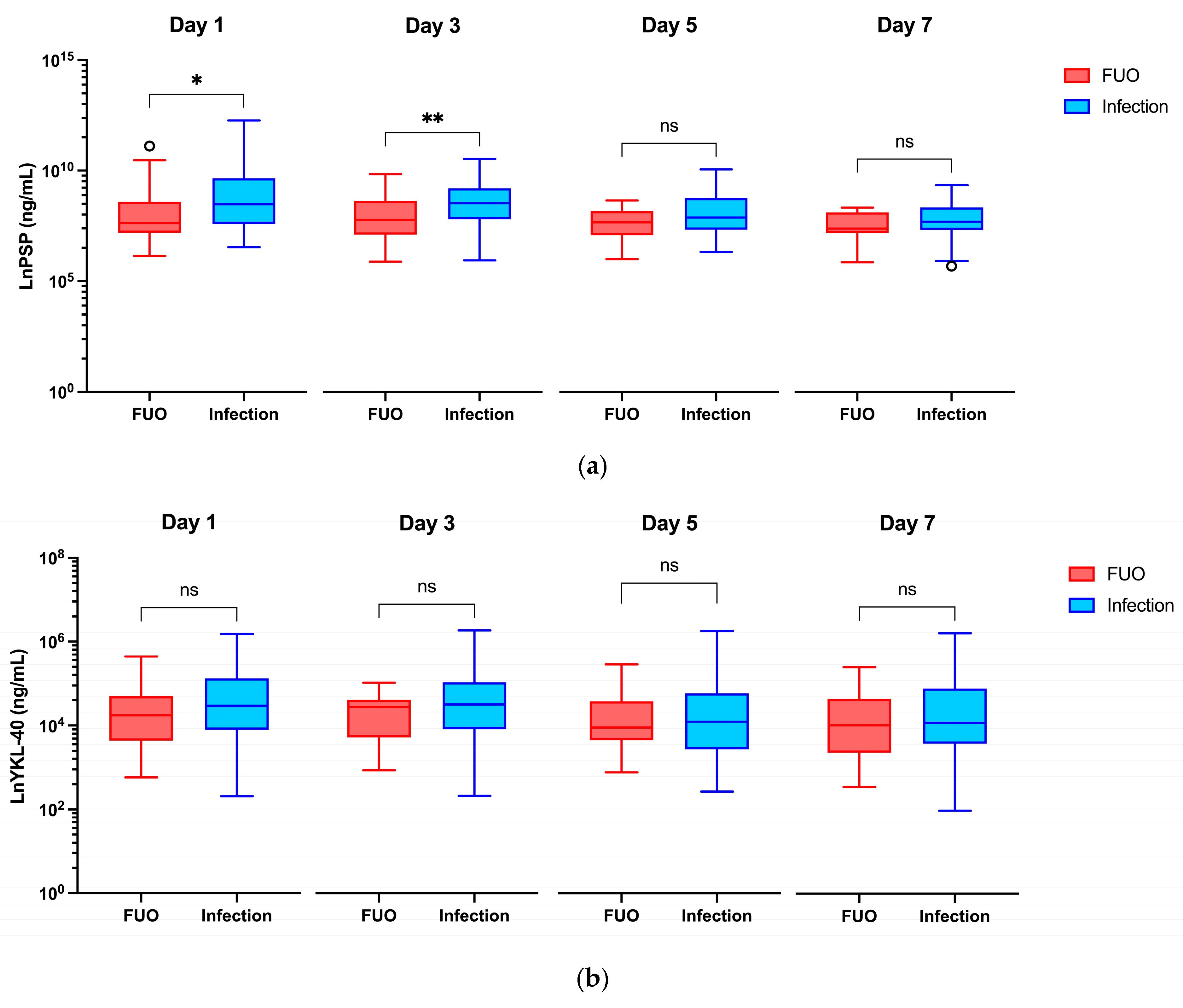
| Biomarker | Time Point (Day) | Infection (n = 38) | FUO (n = 23) | p-Value |
|---|---|---|---|---|
| PSP (ng/mL) | Day 0 | 1.442 (0.560–2.424) | 1.220 (0.762–1.560) | 0.483 |
| Day 1 | 4.835 (1.979–15.600) | 2.060 (1.322–5.383) | 0.042 * | |
| Day 3 | 5.160 (2.492–10.110) | 2.403 (1.236–5.686) | 0.009 ** | |
| Day 5 | 2.680 (1.553–6.489) | 2.168 (1.204–3.596) | 0.072 | |
| Day 7 | 2.207 (1.524–4.206) | 1.614 (1.320–3.354) | 0.174 | |
| YKL-40 (ng/mL) | Day 0 | 37.60 (21.80–70.65) | 26.90 (21.30–44.90) | 0.280 |
| Day 1 | 87.10 (49.40–167.60) | 70.30 (38.10–109.70) | 0.143 | |
| Day 3 | 91.80 (50.65–155.30) | 86.40 (41.80–101.90) | 0.215 | |
| Day 5 | 60.80 (31.60–118.70) | 52.90 (39.00–98.40) | 0.828 | |
| Day 7 | 59.40 (36.18–133.70) | 56.00 (29.30–104.70) | 0.393 | |
| PCT (μg/L) | Day 0 | N/D | N/D | N/A |
| Day 1 | 0.46 (0.14–4.53) | 0.23 (0.10–1.25) | 0.217 | |
| Day 3 | 0.45 (0.22–1.09) | 0.17 (0.10–0.47) | 0.005 ** | |
| Day 5 | 0.23 (0.15–0.47) | 0.13 (0.08–0.26) | 0.027 * | |
| Day 7 | 0.19 (0.06–0.30) | 0.09 (0.06–0.13) | 0.009 ** |
| Leukocyte | PSP | YKL-40 | PCT | ATG Therapy | |
|---|---|---|---|---|---|
| Leukocyte | |||||
| r | 1.00 | 0.38 | 0.13 | 0.40 | 0.50 |
| 95% CI | 0.14 to 0.58 | −0.13 to 0.38 | 0.16 to 0.60 | 0.28 to 0.67 | |
| p | 0.002 | 0.307 | 0.001 | <0.001 | |
| PSP | |||||
| r | 1.00 | 0.51 | 0.67 | 0.66 | |
| 95% CI | 0.29 to 0.68 | 0.50 to 0.79 | 0.48 to 0.78 | ||
| p | <0.001 | <0.001 | <0.001 | ||
| YKL-40 | |||||
| r | 1.00 | 0.45 | 0.41 | ||
| 95% CI | 0.22 to 0.64 | 0.17 to 0.60 | |||
| p | <0.001 | 0.001 | |||
| PCT | |||||
| r | 1.00 | 0.63 | |||
| 95% CI | 0.45 to 0.77 | ||||
| p | <0.001 | ||||
| ATG therapy | |||||
| r | 1.00 | ||||
| 95% CI | |||||
| p |
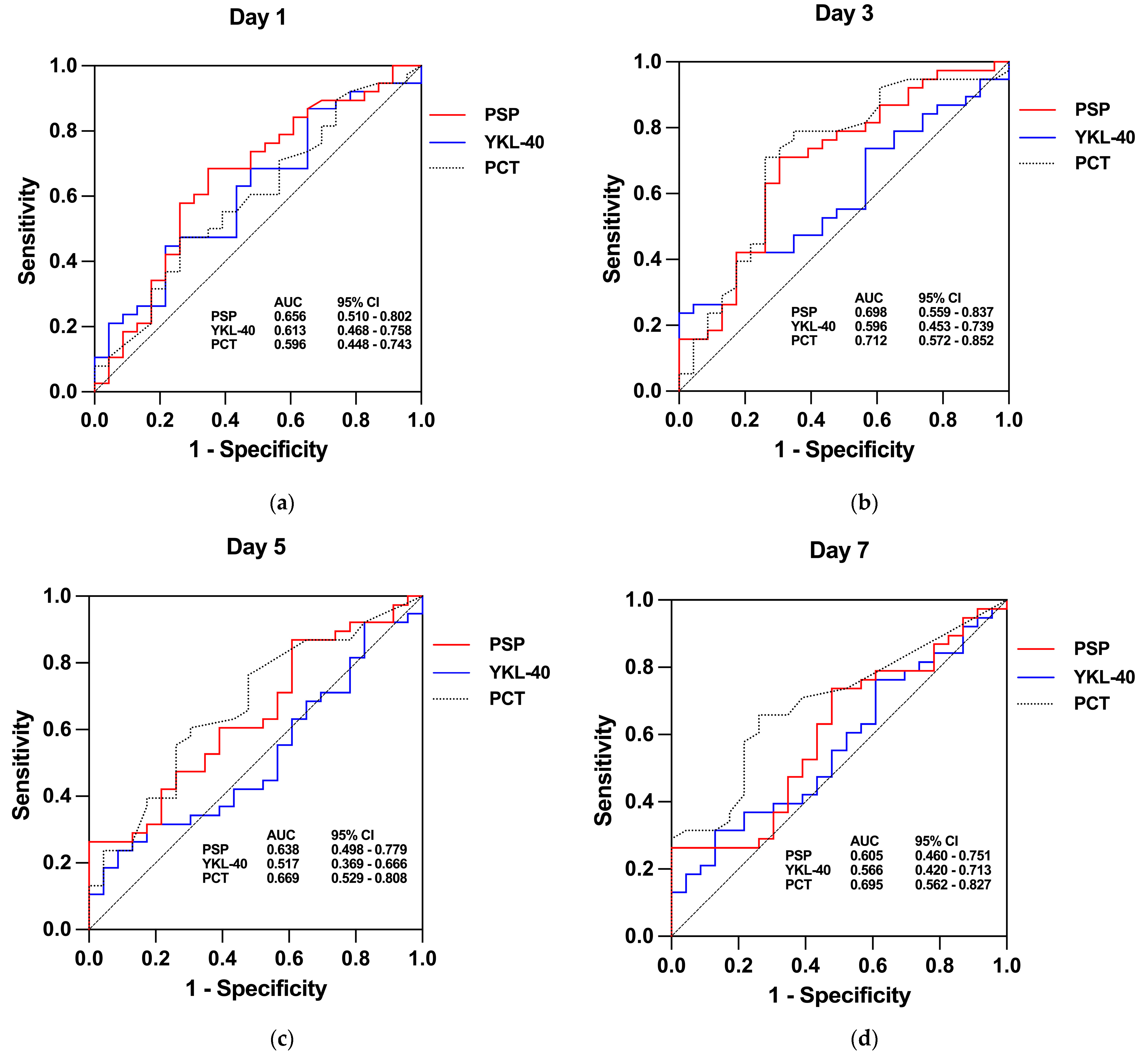
| Biomarker | Day | AUC (95% CI) | Cut-Off | Sensitivity (%) | Specificity (%) | Youden Index | LH+ | LH− | DOR | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| PSP (ng/mL) | Day 1 | 0.656 (0.510–0.802) | 2.910 | 68.42 | 65.22 | 0.34 | 1.967 | 0.484 | 4.063 | 0.042 * |
| Day 3 | 0.698 (0.559–0.837) | 3.144 | 71.05 | 69.57 | 0.41 | 2.335 | 0.416 | 5.612 | 0.010 * | |
| Day 5 | 0.638 (0.498–0.779) | 5.585 | 26.32 | 95.64 | 0.22 | 6.051 | 0.770 | 7.855 | 0.072 | |
| Day 7 | 0.605 (0.459–0.751) | 3.991 | 26.32 | 95.65 | 0.22 | 6.051 | 0.770 | 7.855 | 0.171 | |
| YKL-40 (ng/mL) | Day 1 | 0.613 (0.468–0.758) | 111.40 | 44.74 | 78.26 | 0.23 | 2.058 | 0.706 | 2.915 | 0.141 |
| Day 3 | 0.596 (0.453–0.739) | 108.40 | 42.11 | 82.61 | 0.25 | 2.422 | 0.701 | 3.456 | 0.211 | |
| Day 5 | 0.517 (0.369–0.666) | 31.45 | 23.68 | 91.30 | 0.15 | 2.722 | 0.836 | 3.256 | 0.823 | |
| Day 7 | 0.566 (0.420–0.712) | 119.80 | 31.58 | 86.96 | 0.19 | 2.422 | 0.787 | 3.078 | 0.388 | |
| PCT (μg/L) | Day 1 | 0.596 (0.448–0.743) | 0.57 | 47.37 | 73.91 | 0.21 | 1.816 | 0.712 | 2.550 | 0.214 |
| Day 3 | 0.712 (0.572–0.852) | 0.28 | 71.05 | 73.91 | 0.45 | 2.723 | 0.392 | 6.953 | 0.006 ** | |
| Day 5 | 0.669 (0.529–0.808) | 0.19 | 60.53 | 69.57 | 0.30 | 1.989 | 0.567 | 3.506 | 0.028 * | |
| Day 7 | 0.694 (0.562–0.827) | 0.12 | 65.79 | 73.91 | 0.40 | 2.522 | 0.463 | 5.448 | 0.011 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babel, J.; Košuta, I.; Vujaklija Brajković, A.; Lončar Vrančić, A.; Premužić, V.; Rogić, D.; Duraković, N. Early Fever in Allogeneic Stem Cell Transplantation: Are Presepsin and YKL-40 Valuable Diagnostic Tools? J. Clin. Med. 2024, 13, 5991. https://doi.org/10.3390/jcm13195991
Babel J, Košuta I, Vujaklija Brajković A, Lončar Vrančić A, Premužić V, Rogić D, Duraković N. Early Fever in Allogeneic Stem Cell Transplantation: Are Presepsin and YKL-40 Valuable Diagnostic Tools? Journal of Clinical Medicine. 2024; 13(19):5991. https://doi.org/10.3390/jcm13195991
Chicago/Turabian StyleBabel, Jakša, Iva Košuta, Ana Vujaklija Brajković, Ana Lončar Vrančić, Vedran Premužić, Dunja Rogić, and Nadira Duraković. 2024. "Early Fever in Allogeneic Stem Cell Transplantation: Are Presepsin and YKL-40 Valuable Diagnostic Tools?" Journal of Clinical Medicine 13, no. 19: 5991. https://doi.org/10.3390/jcm13195991





