Tumor Resection in Hepatic Carcinomas Restores Circulating T Regulatory Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Individuals
2.2. Phenotypic Characterization of Circulating T Cells and NK Cells
2.3. Flow Cytometry Data Acquisition and Analysis
2.4. Cell Purification by Fluorescence-Activated Cell Sorting
2.5. RNA Isolation and Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction
2.6. Statistical Analysis
3. Results
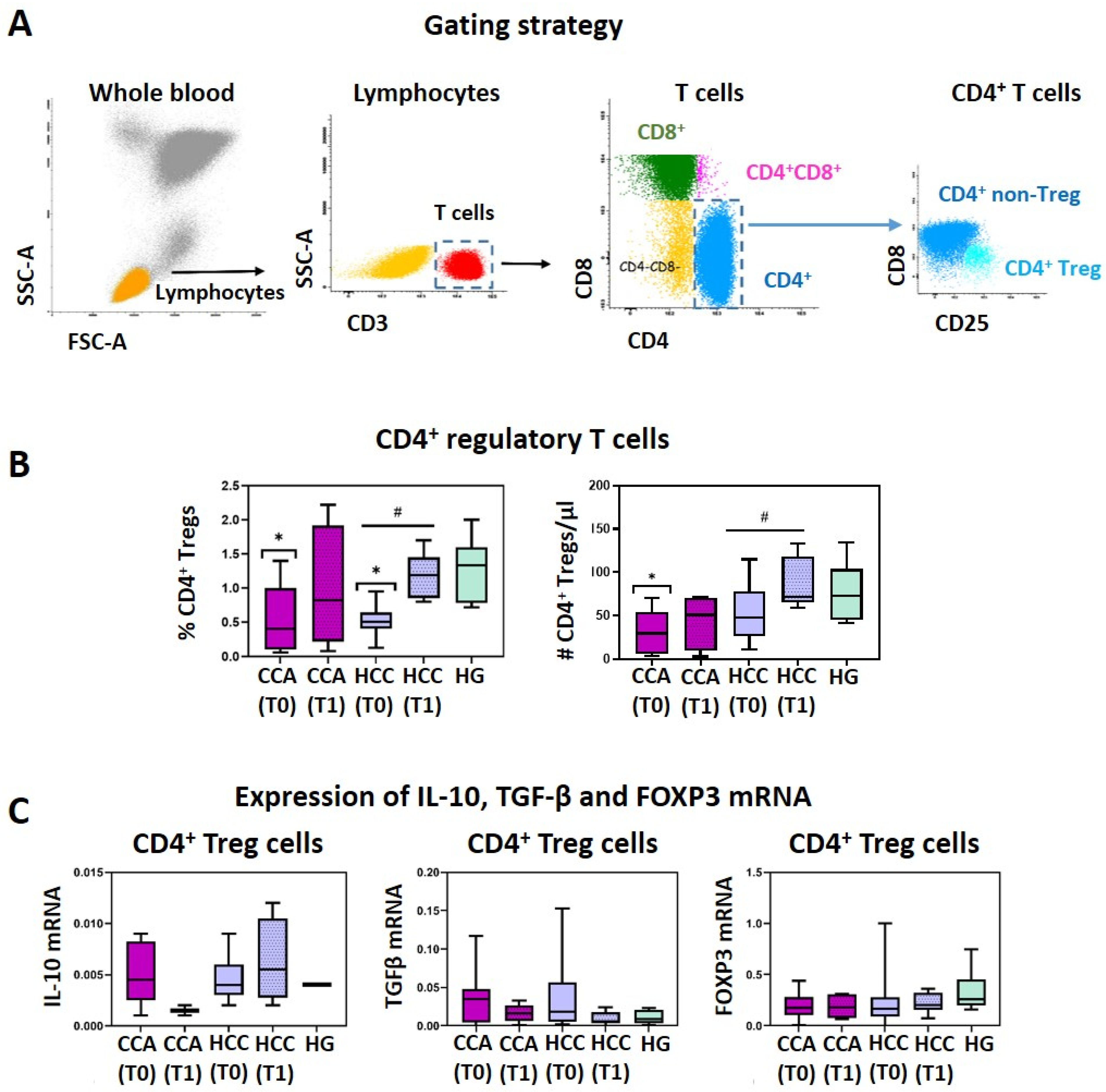
3.1. CCA and HCC Patients Display a Reduction of Circulating CD4+ Treg Cells That Is Restored after Surgical Tumor Resection
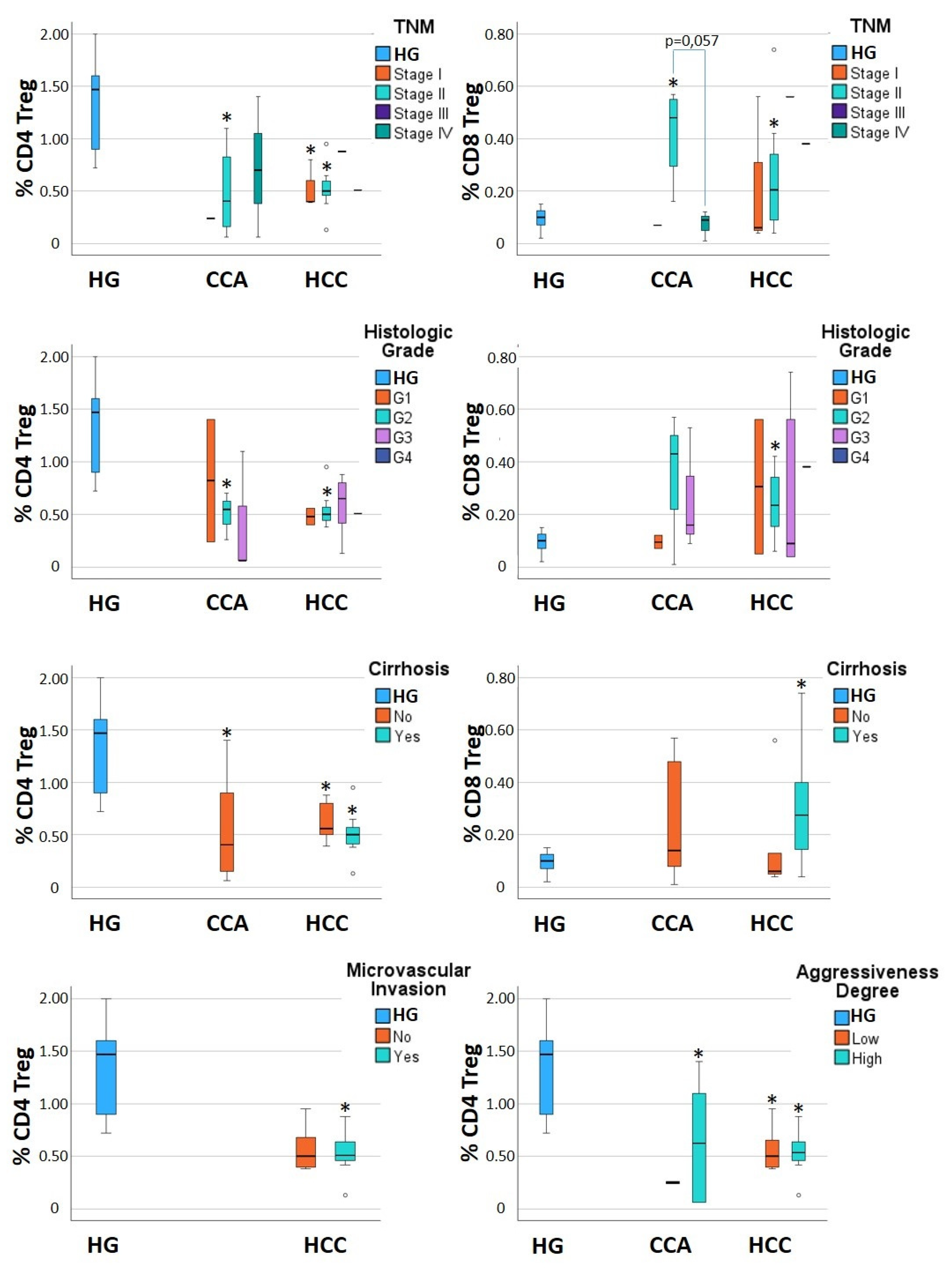
3.2. Alterations in CD4+ Tregs and CD8+ Tregs Are Detected When Considering Subgroups of CCA and HCC Patients
3.3. Alterations of Other T Cell Subsets and NK Cells in CCA and HCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shuai, Z.; Leung, M.W.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.; Eric Gershwin, M. Adaptive Immunity in the Liver. Cell Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and Surveillance for Hepatocellular Carcinoma: New Trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Kondili, L.A.; Lazarus, J.V.; Jepsen, P.; Murray, F.; Schattenberg, J.M.; Korenjak, M.; Craxì, L.; Buti, M. Inequities in Primary Liver Cancer in Europe: The State of Play. J. Hepatol. 2024, 80, 645–660. [Google Scholar] [CrossRef]
- Ghouri, Y.; Mian, I.; Blechacz, B. Cancer Review: Cholangiocarcinoma. J. Carcinog. 2015, 14, 1. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular Carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Acquired Resistance to Cancer Immunotherapy: Role of Tumor-Mediated Immunosuppression. Semin. Cancer Biol. 2020, 65, 13–27. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T Cells in Tumor Microenvironment: New Mechanisms, Potential Therapeutic Strategies and Future Prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Han, Y.; Cheng, H.; Liu, Q.; Ke, S.; Zhu, F.; Lu, Y.; Dai, X.; Wang, C.; et al. FOXP3+ Regulatory T Cell Perturbation Mediated by the IFNγ-STAT1-IFITM3 Feedback Loop Is Essential for Anti-Tumor Immunity. Nat. Commun. 2024, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Politou, M.; Boti, S.; Androutsakos, T.; Kontos, A.; Pouliakis, A.; Kapsimali, V.; Panayiotakopoulos, G.; Kordossis, T.; Karakitsos, P.; Sipsas, N.V. Regulatory T Cell Counts and Development of Malignancy in Patients with HIV Infection. Curr. HIV Res. 2020, 18, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Crispe, I.N. Hepatic T Cells and Liver Tolerance. Nat. Rev. Immunol. 2003, 3, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perea, A.L.; Arcia, E.D.; Rueda, C.M.; Velilla, P.A. Phenotypical Characterization of Regulatory T Cells in Humans and Rodents. Clin. Exp. Immunol. 2016, 185, 281–291. [Google Scholar] [CrossRef]
- Unitt, E.; Rushbrook, S.M.; Marshall, A.; Davies, S.; Gibbs, P.; Morris, L.S.; Coleman, N.; Alexander, G.J.M. Compromised Lymphocytes Infiltrate Hepatocellular Carcinoma: The Role of T-Regulatory Cells. Hepatology 2005, 41, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Farmer, J.D.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin Is a Common Target of Cyclophilin-Cyclosporin A and FKBP-FK506 Complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Pallet, N.; Fernández-Ramos, A.A.; Loriot, M.-A. Impact of Immunosuppressive Drugs on the Metabolism of T Cells. Int. Rev. Cell Mol. Biol. 2018, 341, 169–200. [Google Scholar] [CrossRef]
- Ormandy, L.A.; Hillemann, T.; Wedemeyer, H.; Manns, M.P.; Greten, T.F.; Korangy, F. Increased Populations of Regulatory T Cells in Peripheral Blood of Patients with Hepatocellular Carcinoma. Cancer Res. 2005, 65, 2457–2464. [Google Scholar] [CrossRef]
- Liao, J.; Xiao, J.; Zhou, Y.; Liu, Z.; Wang, C. Effect of Transcatheter Arterial Chemoembolization on Cellular Immune Function and Regulatory T Cells in Patients with Hepatocellular Carcinoma. Mol. Med. Rep. 2015, 12, 6065–6071. [Google Scholar] [CrossRef]
- Ren, Z.; Yue, Y.; Zhang, Y.; Dong, J.; Liu, Y.; Yang, X.; Lin, X.; Zhao, X.; Wei, Z.; Zheng, Y.; et al. Changes in the Peripheral Blood Treg Cell Proportion in Hepatocellular Carcinoma Patients after Transarterial Chemoembolization with Microparticles. Front. Immunol. 2021, 12, 624789. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sierra, C.; Martins, R.; Coucelo, M.; Abrantes, A.M.; Oliveira, R.C.; Tralhão, J.G.; Botelho, M.F.; Furtado, E.; Domingues, M.R.; Paiva, A.; et al. Elevated Soluble TNFα Levels and Upregulated TNFα mRNA Expression in Purified Peripheral Blood Monocyte Subsets Associated with High-Grade Hepatocellular Carcinoma. J. Inflamm. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sierra, C.; Martins, R.; Laranjeira, P.; Coucelo, M.; Abrantes, A.M.; Oliveira, R.C.; Tralhão, J.G.; Botelho, M.F.; Furtado, E.; Domingues, M.R.; et al. Functional and Phenotypic Characterization of Tumor-Infiltrating Leukocyte Subsets and Their Contribution to the Pathogenesis of Hepatocellular Carcinoma and Cholangiocarcinoma. Transl. Oncol. 2019, 12, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Unitt, E.; Marshall, A.; Gelson, W.; Rushbrook, S.M.; Davies, S.; Vowler, S.L.; Morris, L.S.; Coleman, N.; Alexander, G.J.M. Tumour Lymphocytic Infiltrate and Recurrence of Hepatocellular Carcinoma Following Liver Transplantation. J. Hepatol. 2006, 45, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luan, W.; Warren, L.; Fiel, M.I.; Blank, S.; Kadri, H.; Mandeli, J.; Hiotis, S.P. Prognostic Role of Immune Cells in Hepatitis B-Associated Hepatocellular Carcinoma Following Surgical Resection Depends on Their Localization and Tumor Size. J. Immunother. 2016, 39, 36–44. [Google Scholar] [CrossRef]
- Alvisi, G.; Termanini, A.; Soldani, C.; Portale, F.; Carriero, R.; Pilipow, K.; Costa, G.; Polidoro, M.; Franceschini, B.; Malenica, I.; et al. Multimodal Single-Cell Profiling of Intrahepatic Cholangiocarcinoma Defines Hyperactivated Tregs as a Potential Therapeutic Target. J. Hepatol. 2022, 77, 1359–1372. [Google Scholar] [CrossRef]
- Bai, Q.; Li, R.; He, X.; Hong, X.; Yan, Y.; Zhao, Z.; Lin, H.; Tacke, F.; Engelmann, C.; Hu, T. Single-Cell Landscape of Immune Cells during the Progression from HBV Infection to HBV Cirrhosis and HBV-Associated Hepatocellular Carcinoma. Front. Immunol. 2023, 14, 1320414. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Liao, W.; Yang, H.; Xu, H.; Du, S.; Zhao, H.; Lu, X.; Sang, X.; Mao, Y. Clinicopathologic and Prognostic Significance of Regulatory T Cells in Patients with Hepatocellular Carcinoma: A Meta-Analysis. Oncotarget 2017, 8, 39658–39672. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, N.; Kuang, S.; Zhang, J.; Zhao, H.; Wu, J.; Liu, M.; Wang, L. The Clinicopathological Significance and Relapse Predictive Role of Tumor Microenvironment of Intrahepatic Cholangiocarcinoma after Radical Surgery. Cancer 2023, 129, 393–404. [Google Scholar] [CrossRef]
- Feng, Q.; Lu, H.; Wu, L. Identification of M2-like Macrophage-Related Signature for Predicting the Prognosis, Ecosystem and Immunotherapy Response in Hepatocellular Carcinoma. PLoS ONE 2023, 18, e0291645. [Google Scholar] [CrossRef]
- He, J.; Miao, R.; Chen, Y.; Wang, H.; Liu, M. The Dual Role of Regulatory T Cells in Hepatitis B Virus Infection and Related Hepatocellular Carcinoma. Immunology 2023, 171, 445–463. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Tian, W.; Zheng, B.; Zeng, T. Identification of Cancer-Associated Fibroblasts Signature for Predicting the Prognosis and Immunotherapy Response in Hepatocellular Carcinoma. Medicine 2023, 102, e35938. [Google Scholar] [CrossRef]
- Devaud, C.; Darcy, P.K.; Kershaw, M.H. Foxp3 Expression in T Regulatory Cells and Other Cell Lineages. Cancer Immunol. Immunother. 2014, 63, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, P.; Charbonnier, L.-M.; Chatila, T.A. Regulatory T Cells: The Many Faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.-F.; Ding, Y.-H.; Ying, X.-H.; Wu, F.-Z.; Zhou, X.-M.; Zhang, D.-K.; Zou, H.; Ji, J.-S. Regulatory T Cells, Especially ICOS+ FOXP3+ Regulatory T Cells, Are Increased in the Hepatocellular Carcinoma Microenvironment and Predict Reduced Survival. Sci. Rep. 2016, 6, 35056. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef]
- Holmannová, D.; Koláčková, M.; Kondělková, K.; Kuneš, P.; Krejsek, J.; Andrýs, C. CD200/CD200R Paired Potent Inhibitory Molecules Regulating Immune and Inflammatory Responses; Part I: CD200/CD200R Structure, Activation, and Function. Acta Med. 2012, 55, 12–17. [Google Scholar] [CrossRef]
- Jenmalm, M.C.; Cherwinski, H.; Bowman, E.P.; Phillips, J.H.; Sedgwick, J.D. Regulation of Myeloid Cell Function through the CD200 Receptor. J. Immunol. 2006, 176, 191–199. [Google Scholar] [CrossRef]
- Gorczynski, R.M.; Lee, L.; Boudakov, I. Augmented Induction of CD4+CD25+ Treg Using Monoclonal Antibodies to CD200R. Transplantation 2005, 79, 1180–1183. [Google Scholar] [CrossRef]
- dos Santos Laranjeira, P.M. Immunomodulation by Mesenchymal Stromal Cells: Relevance to Mesenchymal Stromal Cells-Based Therapies. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2016. Available online: http://hdl.handle.net/10316/29602 (accessed on 18 September 2024).
- Lee, Y.H.; Chuah, S.; Nguyen, P.H.D.; Lim, C.J.; Lai, H.L.H.; Wasser, M.; Chua, C.; Lim, T.K.H.; Leow, W.Q.; Loh, T.J.; et al. IFNγ−IL-17+ CD8 T Cells Contribute to Immunosuppression and Tumor Progression in Human Hepatocellular Carcinoma. Cancer Lett. 2023, 552, 215977. [Google Scholar] [CrossRef]
- Lubezky, N.; Facciuto, M.; Harimoto, N.; Schwartz, M.E.; Florman, S.S. Surgical Treatment of Intrahepatic Cholangiocarcinoma in the USA. J. Hepato Biliary Pancreat. Sci. 2015, 22, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Shen, F.; Lau, W.Y. Significance of Presence of Microvascular Invasion in Specimens Obtained after Surgical Treatment of Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2018, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
| CCA n = 8 | HCC n = 20 | ||
|---|---|---|---|
| TNM | Stage I | 1 (13%) | 3 (15%) |
| Stage II | 4 (50%) | 15 (75%) | |
| Stage III | 0 (0%) | 1 (5%) | |
| Stage IV | 3 (38%) | 1 (5%) | |
| Histologic grade | G1 | 2 (25%) | 2 (10%) |
| G2 | 3 (38%) | 11 (55%) | |
| G3 | 3 (38%) | 6 (30%) | |
| G4 | 0 (0%) | 1 (5%) | |
| HBsAg | Positive | 0 (0%) | 1 (5%) |
| HCV | Positive | 0 (0%) | 6 (30%) |
| Microvascular invasion | Positive | - | 8 (40%) |
| Cirrhosis | Positive | 0 (0%) | 15 (75%) |
| Disease aggressiveness | Low degree | 2 (25%) | 10 (50%) |
| High degree | 6 (75%) | 10 (50%) | |
| Treatment | Liver transplant | 0 (0%) | 7 (35%) |
| Tumor resection | 8 (100%) | 13 (65%) |
| V450 | V500-c | FITC | PE | PE-Cy7 | APC | APC-H7 | |
|---|---|---|---|---|---|---|---|
| Tube 1 | CD4 | CD45 | CD127 | CD25 | CD3 | CD8 | |
| Clone | RPA-T4 | 2D1 | HIL-7R-M21 | B1.49.9 | SK7 | SK1 | |
| Commercial source | BD | BD | BD Pharmingen | Beckman Coulter | BD | BD | |
| Tube 2 | CD4 | CD45 | cyIFN-γ | cyIL-17 | CD56 | CD3 | CD8 |
| Clone | RPA-T4 | 2D1 | 4S.B3 | SCPL1362 | N901 | SK7 | SK1 |
| Commercial source | BD | BD | BD Pharmingen | BD Pharmingen | Beckman Coulter | BD | BD |
| CCA | HCC | HG | ||||
|---|---|---|---|---|---|---|
| CD4 Treg Mean ± SD | CD8 Treg Mean ± SD | CD4 Treg Mean ± SD | CD8 Treg Mean ± SD | CD4 Treg Mean ± SD | CD8 Treg Mean ± SD | |
| Total | 0.55 ± 0.49 * | 0.25 ± 0.22 | 0.71 ± 0.54 * | 0.37 ± 0.48 | 1.30 ± 0.44 | 0.16 ± 0.21 |
| TNM | ||||||
| Stage I | 0.24 ± NA | 0.07 ± NA | 0.53 ± 0.23 * | 0.22 ± 0.29 | ||
| Stage II | 0.49 ± 0.45 * | 0.42 ± 0.18 * | 0.76 ± 0.62 * | 0.38 ± 0.54 | ||
| Stage III | NA | NA | 0.88 ± NA | 0.56 ± NA | ||
| Stage IV | 0.72 ± 0.67 | 0.07 ± 0.06 | 0.51 ± NA | 0.38 ± NA | ||
| Histologic Grade | ||||||
| G1 | 0.82 ± 0.82 | 0.10 ± 0.04 | 0.48 ± 0.11 | 0.31 ± 0.36 | ||
| G2 | 0.50 ± 0.22 * | 0.34 ± 0.29 | 0.67 ± 0.41 | 0.44 ± 0.62 * | ||
| G3 | 0.41 ± 0.60 | 0.26 ± 0.24 | 0.89 ± 0.81 | 0.26 ± 0.31 | ||
| G4 | NA | NA | 0.51 ± NA | 0.38 ± NA | ||
| Liver Cirrhosis | ||||||
| No | 0.55 ± 0.49 | 0.25 ± 0.22 | 0.63 ± 0.21 * | 0.17 ± 0.22 | ||
| Yes | NA | NA | 0.74 ± 0.63 * | 0.44 ± 0.43 * | ||
| Microvascular Invasion | ||||||
| No | NA | NA | 0.86 ± 0.68 | 0.28 ± 0.23 | ||
| Yes | NA | NA | 0.53 ± 0.23 * | 0.49 ± 0.73 | ||
| Aggressiveness Degree | ||||||
| Low | 0.25 ± 0.01 * | 0.32 ± 0.35 | 0.68 ± 0.42* | 0.24 ± 0.17 | ||
| High | 0.65 ± 0.54 * | 0.22 ± 0.21 | 0.75 ± 0.67 * | 0.49 ± 0.65 | ||
| Recurrent CCA | Recurrence-Free CCA | HG | |||
|---|---|---|---|---|---|
| T0 n = 3 | T1 n = 2 | T0 n = 4 | T1 n = 2 | n = 10 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| % CD4+ Treg cells (within leukocytes) | 0.51 ± 0.77 | 0.55 ± 0.66 | 0.65 ± 0.36 a | 1.43 ± 1.12 | 1.30 ± 0.44 |
| [CD4+ Treg cells/µL] | 26 ± 38 | 66 ± 7 | 40 ± 18 | 75 ± 60 | 77 ± 33 |
| % CD8+ Treg cells (within CD8+ T cells) | 0.12 ± 0.04 | NA | 0.26 ± 0.26 | 0.17 ± 0.16 | 0.16 ± 0.21 |
| % CD8+ Treg cells (within leukocytes) | 0.0063 ± 0.0052 | NA | 0.0166 ± 0.0216 | 0.0112 ± 0.0061 | 0.0094 ± 0.0048 |
| [CD8+ Treg cells/µL] | 0.36 ± 0.24 | NA | 1.00 ± 1.14 | 0.51 ± 0.40 | 0.59 ± 0.25 |
| CCA n = 8 | HCC n = 20 | HG n = 10 | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| % Lymphocytes | 33 ± 14 | 37 ± 12 | 29 ± 14 | 28 ± 9 | 42 ± 8 | |
| [Lymphocytes/µL] | 1903 ± 585 a | 1253 ± 127 | 2730 ± 2798 | 2220 ± 904 | 2392 ± 500 | |
| % T cells | 22 ± 12 | 27 ± 11 | 20 ± 9 | 21 ± 9 | 29 ± 8 | |
| [T cells/µL] | 1305 ± 520 | 1281 ± 882 | 1619 ± 977 | 1631 ± 802 | 1539 ± 395 | |
| % CD4+ T cells | 57 ± 19 | 59 ± 16 | 61 ± 16 | 63 ± 15 | 66 ± 10 | |
| % IFN-γ+ | 15 ± 9 | 19 ± 6 | 23 ± 15 | 20 ± 14 | 19 ± 8 | |
| MFI IFN-γ+ | 6667 ± 2294 | 8862 ± 2543 | 6887 ± 3206 | 7286 ± 3823 | 8531 ± 1983 | |
| % IL-17+ | 0.90 ± 0.51 | 1.45 ± 0.94 | 1.42 ± 1.15 | 1.14 ± 0.82 b | 1.63 ± 1.45 | |
| MFI IL-17+ | 557 ± 110 | 619 ± 131 | 621 ± 173 | 514 ± 159 | 534 ± 124 | |
| % IFN-γ+IL-17+ | 0.11 ± 0.14 a | 0.15 ± 0.11 | 0.21 ± 0.28 | 0.16 ± 0.11 b | 0.29 ± 0.22 | |
| % CD8+ T cells | 39 ± 20 | 37 ± 17 | 32 ± 17 | 32 ± 16 | 28 ± 8 | |
| % IFN-γ+ | 43 ± 23 | 59 ± 19 | 52 ± 26 | 47 ± 35 | 47 ± 13 | |
| MFI IFN-γ+ | 5512 ± 2714 | 7189 ± 2313 | 5280 ± 3061 | 5487 ± 2886 | 6630 ± 1836 | |
| % IL-17+ | 0.12 ± 0.17 a | 0.08 ± 0.04 a | 0.15 ± 0.19 | 0.17 ± 0.15 b | 0.20 ± 0.12 | |
| MFI IL-17+ | 365 ± 162 | 540 ± 211 | 470 ± 152 | 385 ± 118 | 438 ± 190 | |
| % IFN-γ+IL-17+ | 0.08 ± 0.15 | 0.03 ± 0.02 | 0.08 ± 0.013 | 0.06 ± 0.06 | 0.09 ± 0.06 | |
| % CD56+ | 12 ± 6.7 | 9 ± 5 | 16 ± 14 | 17 ± 14 | 10 ± 9 | |
| % CD4+CD8+ T cells | 1.5 ± 0.8 | 1.2 ± 0.26 | 2.2 ± 1.0 | 1.5 ± 2.1 | 1.0 ± 0.8 | |
| % IFN-γ+ | 35 ± 25 | 44 ± 15 | 44 ± 29 | 32 ± 19 | 41 ± 16 | |
| MFI IFN-γ+ | 5545 ± 2485 | 6513 ± 2684 | 6049 ± 3258 | 5660 ± 3172 | 6941 ± 1858 | |
| % CD56+ | 13 ± 14 | 12 ± 6 | 14 ± 11 | 11 ± 8 | 12 ± 9 | |
| CCA n = 8 | HCC n = 20 | HG n = 10 | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| % NK cells | 2.6 ± 2.6 | 1.7 ± 0.9 | 3.2 ± 2.3 | 3.0 ± 1.7 | 2.3 ± 1.0 | |
| % CD56DIM NK cells | 82 ± 13 | 83 ± 12 | 91 ± 6 | 92 ± 4 | 88 ± 5 | |
| % IFNγ+ | 30 ± 27 | 54 ± 26 | 39 ± 29 | 35 ± 27 | 42 ± 25 | |
| MFI IFNγ | 1705 ± 766 | 2063 ± 888 | 1704 ± 761 | 1694 ± 583 | 1913 ± 604 | |
| % CD8+ | 4.1 ± 3.7 | 6.4 ± 4.0 | 3.4 ± 3.4 | 3.1 ± 3.8 | 2.3 ± 2.2 | |
| HLADR+ | 2.3 ± 1.6 | 2.1 ± 1.9 | 1.2 ± 1.5 | 0.9 ± 0.8 | 2.0 ± 1.8 | |
| % CD56BRIGHT NK cells | 17 ± 13 | 17 ± 13 | 8.7 ± 5.3 | 7.8 ± 4.4 | 12 ± 5 | |
| % IFNγ+ | 26 ± 29 | 34 ± 21 | 38 ± 30 | 23 ± 21 | 28 ± 14 | |
| MFI IFNγ | 2157 ± 952 | 1892 ± 997 | 1916 ± 1054 | 1805 ± 711 | 1939 ± 604 | |
| % CD8+ | 5.6 ± 10 | 4.0 ± 2.9 | 3.8 ± 4.1 | 5.8 ± 5.7 a | 1.3 ± 1.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Sierra, C.; Martins, R.; Coucelo, M.; Abrantes, A.M.; Caetano Oliveira, R.; Tralhão, J.G.; Botelho, M.F.; Furtado, E.; Domingues, M.R.; Paiva, A.; et al. Tumor Resection in Hepatic Carcinomas Restores Circulating T Regulatory Cells. J. Clin. Med. 2024, 13, 6011. https://doi.org/10.3390/jcm13196011
Martín-Sierra C, Martins R, Coucelo M, Abrantes AM, Caetano Oliveira R, Tralhão JG, Botelho MF, Furtado E, Domingues MR, Paiva A, et al. Tumor Resection in Hepatic Carcinomas Restores Circulating T Regulatory Cells. Journal of Clinical Medicine. 2024; 13(19):6011. https://doi.org/10.3390/jcm13196011
Chicago/Turabian StyleMartín-Sierra, Carmen, Ricardo Martins, Margarida Coucelo, Ana Margarida Abrantes, Rui Caetano Oliveira, José Guilherme Tralhão, Maria Filomena Botelho, Emanuel Furtado, Maria Rosário Domingues, Artur Paiva, and et al. 2024. "Tumor Resection in Hepatic Carcinomas Restores Circulating T Regulatory Cells" Journal of Clinical Medicine 13, no. 19: 6011. https://doi.org/10.3390/jcm13196011











