Decongestion in Acute Heart Failure—Time to Rethink and Standardize Current Clinical Practice?
Abstract
:1. Introduction
2. Application of Diuretics, Diuretic Resistance and Early Monitoring of Natriuresis
- (1)
- The PUSH-AHF study [23] randomized in an open-label design 310 patients with AHF and planned IV loop diuretic administration (mean age 74 years, 45% females) either to natriuresis-guided therapy (urine sodium measured at 2, 6, 12, 18, 24 and 36 h) or usual care. Diuretic treatment was intensified if urine sodium was <70 mmol/L. The co-primary outcome, natriuresis at 24 h, was significantly higher in the intervention group (409 versus 345 mmol; p = 0.0061). However, the co-primary outcome of all-cause mortality or HF rehospitalization within 180 days remained unchanged (31% in both treatment arms; p = 0.70).
- (2)
- The ENACT-HF trial [24] enrolled 401 patients at 29 centers in a prospective, open-label, nonrandomized design. During the first phase, enrolled patients were treated according to usual care at their center. During the second phase, all centers switched to a standardized protocol emphasizing adequate initial dosing (doubling the oral home dose up to 200 mg furosemide equivalent IV) and further doubling the initial IV dose after 6 h if urinary sodium was <50 mmol/L or urinary output was <100 mL/hour, then given twice daily. The primary outcome of total natriuresis after 1 day was met, with a mean natriuresis of 174 mmol in the standard of care arm and 282 in the intervention arm (+64% change; mean ratio: 1.64, 95% confidence interval 1.37–1.95, p < 0.001). Furthermore, a reduction in the hospital duration was observed in the intervention group compared to the standard of care group (5.8 vs. 7.0 days, mean ratio: 0.87, 95% confidence interval 0.77–0.99, p = 0.036). Of note, weight loss and congestion score were not significantly different between groups. The treatment was also deemed safe, as there were no differences in the markers of renal dysfunction, hypokalemia or hypotension between the two treatment arms.
- (3)
- The ongoing ESCALATE trial [25]—an open labeled 1:1 randomized trial—includes a total of 450 AHF patients with hypervolemia of at least 10 pounds of estimated excess volume to a natriuresis-guided approach versus usual care. As a urine sodium concentration alone does not ensure a net negative sodium balance if not interpretated in relation to the urine creatinine, this study uses a natriuretic response prediction equation to predict the expected cumulative sodium excretion [26].
- (4)
- The ongoing DECONGEST trial (NCT05411991) has a similar design (randomized, open-label) investigating whether serial assessment of urinary sodium after diuretic administration improves decongestion versus usual care in AHF patients. It recommends combination diuretic therapy by the upfront use of (1) acetazolamide 500 mg once daily in the absence of hypernatremia (>145 mmol/L) or metabolic acidosis (bicarbonate < 22 mmol/L); and by the upfront use of (2) oral chlorthalidone 50 mg once daily if eGFR < 30 mL/min/1.73 m2 or hypernatremia (>145 mmol/L). The protocol further recommends a full nephron blockade with IV acetazolamide 500 mg once daily, IV bumetanide 4 mg twice daily, oral chlorthalidone 100 mg once daily, and IV canrenoate 200 mg once daily if the urinary sodium concentration remains <80 mmol/L with persistent signs of congestion.
3. Early Up-Titration of Guideline-Directed Medical Therapy and Post-Discharge Management
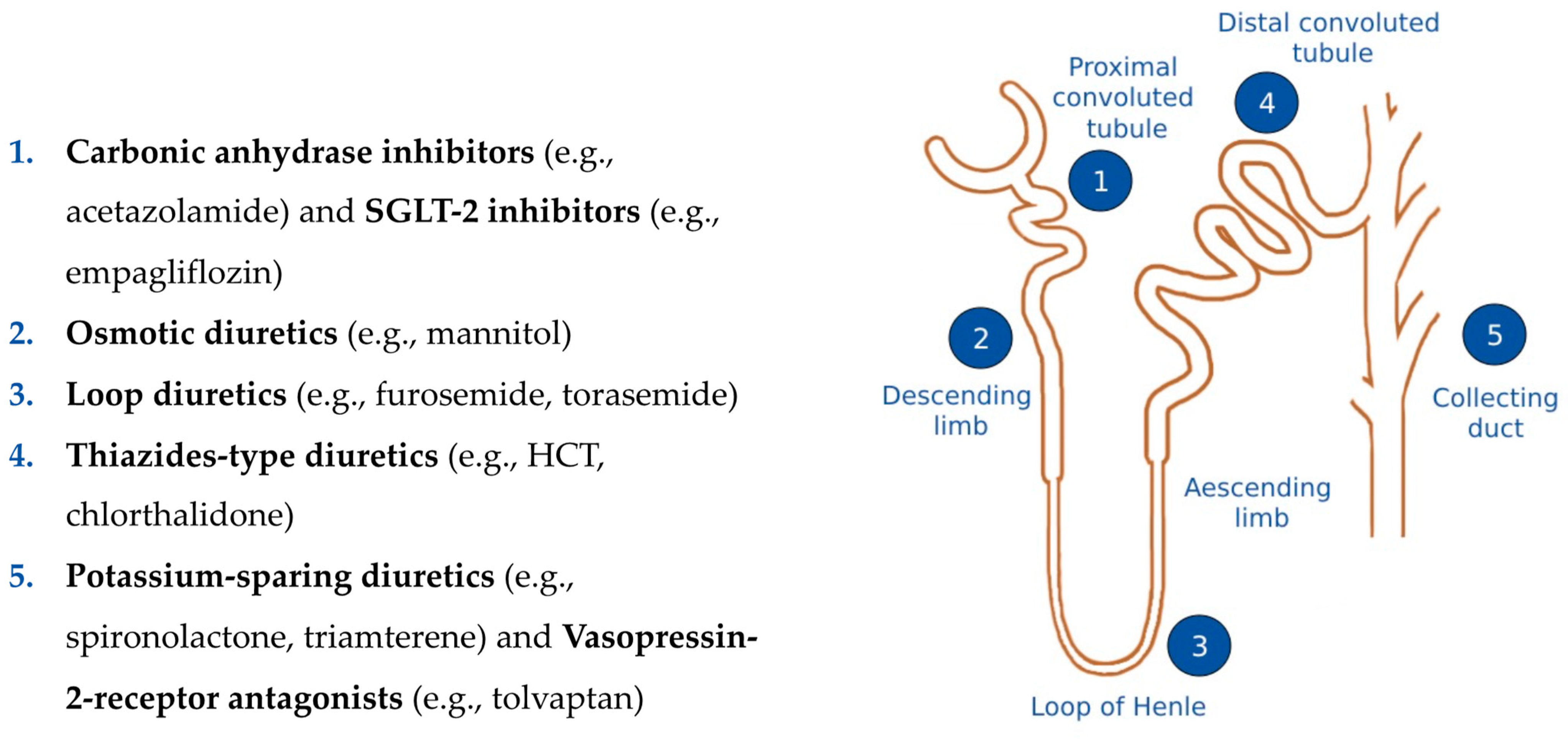
4. Pharmacokinetics, Pharmacodynamics and Related Clinical Issues of Different Diuretic Drugs
4.1. Loop Diuretics—Inhibitors of Na+-K+-2Cl– Symporter
4.2. Thiazide-Inhibitors of Na+-Cl− Symport
4.3. Inhibitors of Renal Epithelial Na+ Channels (K+-Sparing Diuretics)
4.4. Mineralocorticoid Receptor Antagonists (K+-Sparing Diuretics)
4.5. Carbonic Anhydrase Inhibitors
4.6. Sodium-Glucose Co-Transporter 2 (SGLT-2) Inhibitors
4.7. Vassopressin-2 Receptor Antagonists
5. Non-Pharmacological Interventions in the Prevention and Treatment of AHF
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. Acute heart failure congestion and perfusion status-impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2019, 21, 1338–1352. [Google Scholar] [CrossRef]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion during and after Hospitalization for Acute Heart Failure: Insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Davison, B.; Bettari, L.; Sun, H.; Edwards, C.; Lazzarini, V.; Piovanelli, B.; Carubelli, V.; Bugatti, S.; Lombardi, C.; et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ. Heart Fail. 2012, 5, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Harjola, V.; Mebazaa, A.; Rocca, H.B.; Martens, P.; Testani, J.M.; Tang, W.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Deniau, B.; Costanzo, M.R.; Sliwa, K.; Asakage, A.; Mullens, W.; Mebazaa, A. Acute heart failure: Current pharmacological treatment and perspectives. Eur. Heart J. 2023, 44, 4634–4649. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Beltrami, M.; Pirrotta, F.; Tavera, M.C.; Gennari, L.; Ruocco, G. Clinical, Laboratory and Lung Ultrasound Assessment of Congestion in Patients with Acute Heart Failure. J. Clin. Med. 2022, 11, 1642. [Google Scholar] [CrossRef]
- Alcidi, G.; Goffredo, G.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Brain Natriuretic Peptide Biomarkers in Current Clinical and Therapeutic Scenarios of Heart Failure. J. Clin. Med. 2022, 11, 3192. [Google Scholar] [CrossRef]
- Spinarova, L.; Vitovec, J. Neurohumoral changes in chronic heart failure. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2007, 151, 201–207. [Google Scholar] [CrossRef]
- Martens, P.; Mullens, W. How to tackle congestion in acute heart failure. Korean J. Intern. Med. 2018, 33, 462–473. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Verbrugge, F.; Nijst, P.; Tang, W. Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. Eur. Heart J. 2017, 38, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Mullan, B.P. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: Role for blood volume quantitation. JACC Heart Fail. 2014, 2, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S. Venous function and central venous pressure: A physiologic story. Anesthesiology 2008, 108, 735–748. [Google Scholar] [CrossRef]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically mediated changes in capacitance redistribution of the venous reservoir as a cause of decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Matsue, Y.; Damman, K.; Voors, A.A.; Kagiyama, N.; Yamaguchi, T.; Kuroda, S.; Okumura, T.; Kida, K.; Mizuno, A.; Oishi, S.; et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 3042–3051. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, S.H.; Oh, I.Y.; Choi, D.J.; Park, H.A.; Cho, H.J.; Lee, H.Y.; Cho, J.Y.; Kim, K.H.; Son, J.W.; et al. The Effect of Door-to-Diuretic Time on Clinical Outcomes in Patients with Acute Heart Failure. JACC Heart Fail. 2018, 6, 286–294, Erratum in JACC Heart Fail. 2018, 6, 812. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic Strategies in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H.W. Loop diuretic efficiency a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Nijst, P.; Dupont, M.; Penders, J.; Tang, W.H.; Mullens, W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ. Heart Fail. 2014, 7, 766–772. [Google Scholar] [CrossRef]
- Hodson, D.Z.; Griffin, M.; Mahoney, D.; Raghavendra, P.; Ahmad, T.; Turner, J.; Wilson, F.P.; Tang, W.H.W.; Rao, V.S.; Collins, S.P.; et al. Natriuretic Response Is Highly Variable and Associated with 6-Month Survival: Insights from the ROSE-AHF Trial. JACC Heart Fail. 2019, 7, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Shrestha, K.; Testani, J.M.; Verbrugge, F.H.; Dupont, M.; Mullens, W.; Tang, W.W. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J. Card. Fail. 2014, 20, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Postmus, D.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Damman, K. Natriuresis-guided diuretic therapy in acute heart failure: A pragmatic randomized trial. Nat. Med. 2023, 29, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Dauw, J.; Lelonek, M.; Zegri-Reiriz, I.; Paredes-Paucar, C.P.; Zara, C.; George, V.; Cobo-Marcos, M.; Knappe, D.; Shchekochikhin, D.; Lekhakul, A.; et al. Rationale and Design of the Efficacy of a Standardized Diuretic Protocol in Acute Heart Failure Study. ESC Heart Fail. 2021, 8, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Siddiqi, H.K.; Stevenson, L.W.; Bales, B.; Han, J.H.; Hart, K.; Imhoff, B.; Ivey-Miranda, J.B.; Jenkins, C.A.; Lindenfeld, J.; et al. Randomized controlled trial of urinE chemiStry guided aCute heArt faiLure treATmEnt (ESCALATE): Rationale and design. Am. Heart J. 2023, 265, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients with Heart Failure. Circ. Heart Fail. 2016, 9, e002370. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Huang, X.; Dorhout Mees, E.; Vos, P.; Hamza, S.; Braam, B. Everything we always wanted to know about furosemide but were afraid to ask. Am. J. Physiol.-Ren. Physiol. 2016, 310, F958–F971. [Google Scholar] [CrossRef]
- Greene, S.J.; Velazquez, E.J.; Anstrom, K.J.; Clare, R.M.; DeWald, T.A.; Psotka, M.A.; Ambrosy, A.P.; Stevens, G.R.; Rommel, J.J.; Alexy, T.; et al. Effect of Torsemide Versus Furosemide on Symptoms and Quality of Life Among Patients Hospitalized for Heart Failure: The TRANSFORM-HF Randomized Clinical Trial. Circulation 2023, 148, 124–134. [Google Scholar] [CrossRef]
- Mentz, R.J.; Anstrom, K.J.; Eisenstein, E.L.; Sapp, S.; Greene, S.J.; Morgan, S.; Testani, J.M.; Harrington, A.H.; Sachdev, V.; Ketema, F.; et al. Effect of Torsemide vs Furosemide after Discharge on All-Cause Mortality in Patients Hospitalized with Heart Failure: The TRANSFORM-HF Randomized Clinical Trial. JAMA 2023, 329, 214–223. [Google Scholar] [CrossRef]
- Ahmad Cheema, H.; Azeem, S.; Ejaz, A.; Khan, F.; Muhammad, A.; Shahid, A.; Nashwan, A.J.; Maqsood, M.H.; Dani, S.S.; Mentz, R.J.; et al. Efficacy and safety of torsemide versus furosemide in heart failure patients: A systematic review of randomized controlled trials. Clin. Cardiol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Osmanska, J.; Brooksbank, K.; Docherty, K.F.; Robertson, S.; Wetherall, K.; McConnachie, A.; Hu, J.; Gardner, R.S.; Clark, A.L.; Squire, I.B.; et al. A novel, small-volume subcutaneous furosemide formulation delivered by an abdominal patch infusor device in patients with heart failure: Results of two phase I studies. Eur. Heart J. Cardiovasc. Pharmacother. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Kjekshus, J.; Wikstrand, J.; Cleland, J.G.; Komajda, M.; Wedel, H.; Waagstein, F.; McMurray, J.J. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur. J. Heart Fail. 2016, 18, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G. The thiazide-sensitive Na+-Cl- cotransporter: Molecular biology, functional properties, and regulation by WNKs. Am. J. Physiol.-Ren. Physiol. 2009, 297, F838. [Google Scholar] [CrossRef] [PubMed]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J.; et al. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.W.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Martens, P.; Verbrugge, F.H.; Dauw, J.; Nijst, P.; Meekers, E.; Augusto, S.N.; Ter Maaten, J.M.; Heylen, L.; Damman, K.; Mebazaa, A.; et al. Pre-treatment bicarbonate levels and decongestion by acetazolamide: The ADVOR trial. Eur. Heart J. 2023, 44, 1995–2005. [Google Scholar] [CrossRef]
- Packer, M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus with That of Heart Failure. Circulation 2017, 136, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure-A Concise Review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, E.M.; Beusekamp, J.C.; Ter Maaten, J.M.; Figarska, S.M.; Danser, A.H.J.; van Veldhuisen, D.J.; van der Meer, P.; Heerspink, H.J.L.; Damman, K.; Voors, A.A. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur. J. Heart Fail. 2021, 23, 68–78. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Yeoh, S.E.; Osmanska, J.; Petrie, M.C.; Brooksbank, K.J.M.; Clark, A.L.; Docherty, K.F.; Foley, P.W.X.; Guha, K.; Halliday, C.A.; Jhund, P.S.; et al. Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics. Eur. Heart J. 2023, 44, 2966–2977. [Google Scholar] [CrossRef]
- Omar, M.; Jensen, J.; Burkhoff, D.; Frederiksen, P.H.; Kistorp, C.; Videbæk, L.; Poulsen, M.K.; Gustafsson, F.; Køber, L.; Borlaug, B.A.; et al. Effect of Empagliflozin on Blood Volume Redistribution in Patients with Chronic Heart Failure and Reduced Ejection Fraction: An Analysis from the Empire HF Randomized Clinical Trial. Circ. Heart Fail. 2022, 15, e009156. [Google Scholar] [CrossRef]
- Jensen, J.; Omar, M.; Kistorp, C.; Poulsen, M.K.; Tuxen, C.; Gustafsson, I.; Køber, L.; Gustafsson, F.; Fosbøl, E.; Bruun, N.E.; et al. Empagliflozin in heart failure patients with reduced ejection fraction: A randomized clinical trial (Empire HF). Trials 2019, 20, 374. [Google Scholar] [CrossRef]
- Cox, Z.L.; Collins, S.P.; Aaron, M.; Hernandez, G.A.; Iii, A.T.M.; Davidson, B.T.; Fowler, M.; Lindsell, C.J.; Frank, E.H., Jr.; Jenkins, C.A.; et al. Efficacy and safety of dapagliflozin in acute heart failure: Rationale and design of the DICTATE-AHF trial. Am. Heart J. 2021, 232, 116–124. [Google Scholar] [CrossRef]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Hung, R.; Lenihan, D.J.; Testani, J.M. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Fail. 2020, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.J.; Kohut, A.R.; Hasni, S.F.; Goldman, J.M.; Silverman, B.; Kelepouris, E.; Eisen, H.J.; Aggarwal, S. Readmission rate after ultrafiltration in acute decompensated heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2017, 22, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O’Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef]

| Trial Name | Clinical Setting | Trial Description | Treatment | Primary Endpoint | Primary Outcomes |
|---|---|---|---|---|---|
| PUSH-AHF [23] | AHF patients requiring treatment with IV loop diuretics | Pragmatic, single-center, randomized, controlled, open-label study | Natriuresis-guided therapy (urine sodium measured at 2, 6, 12, 18, 24 and 36 h) versus usual care | 24 h urinary sodium excretion after start of loop diuretic therapy and a combined endpoint of all-cause mortality or first HF rehospitalization at 6 months | The first primary endpoint was met, as natriuresis in the natriuresis-guided and usual care arms was 409 ± 178 mmol arm versus 345 ± 202 mmol, respectively (p = 0.0061). However, there were no significant differences between the two arms for the combined endpoint of time to all-cause mortality or first heart failure rehospitalization, which occurred in 46 (31%) and 50 (31%) of patients in the natriuresis-guided and usual care arms, respectively (hazard ratio 0.92, 95% confidence interval 0.62–1.38, p = 0.6980). |
| ENACT-HF [24] | AHF patients on chronic loop diuretic therapy, admitted to the hospital for IV loop diuretic therapy | Prospective, multicenter, open-label, nonrandomized, pragmatic trial | Early assessment of diuretic response with a spot urinary sodium measurement after 2 h and urine output after 6 h. Doubling the initial IV dose after 6 h if urinary sodium < 50 mmol/L or urinary output < 100 mL/h | Total natriuresis after 1 day | Mean natriuresis was 174 mmol in the standard of care arm and 282 in the protocol arm (+64% change; mean ratio: 1.64, 95% confidence interval 1.37–1.95, p < 0.001). Hospital duration was 7.0 days in the standard of care group and 5.8 days in the protocol group (mean ratio 0.87, 95% confidence interval: 0.77–0.99, p = 0.036). |
| ESCALATE [25] | Patients with AHF randomized to a diuretic strategy guided by urine chemistry or a usual care strategy | Randomized, double-blind clinical trial | Patients in both arms receive an open-label IV diuretic dose. Patients in the control arm have diuretic dosing based on diuretic response. Patients in the intervention arm have diuretic dosing guided by spot urine chemistry and the natriuretic response prediction equation calculator to achieve the established daily net negative goal. | A composite of the clinical state and global clinical status, assessed daily from randomization to day 14 | Trial is still ongoing |
| DECONGEST | AHF patients with signs of congestion | Pragmatic, multicenter, interventional, parallel-arm, randomized, open-label trial | Diuretic regimen, based on serial assessment of sodium concentration on spot urine samples and with low-threshold use of combination diuretic therapy versus usual care | Mortality, days in hospital and decongestion | Trial is still ongoing |
| Drug | Relative Potency | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Side Effects |
|---|---|---|---|---|---|---|---|
| Furosemide | 1 | 10–90 | 1–3 | 6–8 | 40–200 mg, 1–2 times daily; maximum 600 mg/d | ~65% R, ~35% M a | Hypokalemia, hypomagnesemia, hyperuricemia, hypocalcemia, hyponatremia, ototoxicity |
| Bumetanide | 40 | 80–100 | 1–3 | 6–8 | 0.5–4 mg 1–2 times daily; maximum 10 mg/d | ~62% R, ~38% M | |
| Tora- semide | 0.7 | 80–100 | 4–6 | 12–18 | 20–80 mg/d; maximum 200 mg/d | ~20% R, ~80% M |
| Drug | Relative Potency | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Side Effects |
|---|---|---|---|---|---|---|---|
| Thiazide diuretics | Hypokalemia, hypomagnesemia, hypercalcemia, hyponatremia, hyperuricemia, sulfonamide allergy | ||||||
| Hydrochloro thiazide | 1 | ~70 | ~2.5 | 6–12 | 12.5–100 mg/d Maximum: 200 mg/d | R | |
| Chlorothiazide (IV formulation available) | 0.1 | 9–56 (dose-dependent) | ~1.5 | 6–12 | 500–1000 mg/d Maximum: 1000 mg/d | R | |
| Thiazide-like diuretics | |||||||
| Metolazone | 10 | ~65 | 8–14 | ≥24 | 2.5–10 mg/d Maximum: 20 mg/d | ~80% R, ~10% B, ~10% M | |
| Chlorthalidone | 1 | ~65 | ~47 | 24–72 | 12.5–25 mg/d Maximum: 100 mg/d | ~65% R, ~10% B, ~25% U | |
| Indapamide | 20 | ~93 | ~14 | ≥24 | 2.5 mg/d Maximum: 5 mg/d | M | |
| Drug | Relative Potency | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Side Effects |
|---|---|---|---|---|---|---|---|
| Amiloride | 1 | 15–25 | ~21 | ~24 | 5–10 mg/d Maximum: 20 mg/d | R | Hyperkalemia |
| Triamterene | 0.1 | ~50 | ~4 | 7–9 | 100 mg twice daily Maximum: 300 mg/d | M |
| Drug | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Side Effects |
|---|---|---|---|---|---|---|
| Spironolactone | ~65 | ~1.6 | 48–72 | 25–50 mg/d Maximum: 200 mg/d | M | Hyperkalemia Spironolactone: gynecomastia |
| Eplerenone | 69 | ~5 | ~48 | 25 mg/d Maximum: 50 mg/d | M | |
| Finerenone | 44 | 2–3 | ~48 | 20 mg/d Maximum: 20 mg/d | M |
| Drug | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Side Effects |
|---|---|---|---|---|---|---|
| Acetazolamide | 70–90 | 3–9 | 8–12 | 250–500 mg/day | R | Metabolic acidosis Hypokalemia Hyponatremia Hyperchloremia Alkalinization of urine |
| Drug | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Dose Modifications |
|---|---|---|---|---|---|---|
| Empagliflozin | 78 | 12.4 | ~72 | 10 mg/d Maximum: 25 mg/d | ~ 50% M, ~ 50% R | eGFR ≥ 45 mL/min/1.73 m2: No dosage adjustment required eGFR 30–45 mL/min/1.73 m2: Do not initiate therapy; if already on it, discontinue therapy when eGFR persistently <45 mL/min/1.73 m2 eGFR < 30 mL/min/1.73 m2: Contraindicated |
| Dapagliflozin | 78 | ~12.9 | ~72 | 5 mg/d Maximum: 10 mg/d | 21% M, 79% R | eGFR ≥ 45 mL/min/1.73 m2: No dosage adjustment required eGFR 30 to <45 mL/min/1.73 m2: Not recommended eGFR < 30 mL/min/1.73 m2: Contraindicated |
| Canagliflozin | 65 | 10–13 (dose-dependent) | ~24 | 100 mg/d Maximum: 300 mg/d | 41.5% M, 7% as hydroxylated metabolite, 3.2% as O-glucuronide metabolite, <1% R, 30.5% as O-glucuronide metabolites | eGFR ≥ 60 mL/min/1.73 m2: No dosage adjustment necessary eGFR 45 to <60 mL/min/1.73 m2: 100 mg qDay eGFR 45 to <60 mL/min/1.73 m2 with albuminuria > 300 mg/day: 100 mg qDay eGFR < 30 mL/min/1.73 m2 or end-stage kidney disease on dialysis: Contraindicated |
| Drug | Oral Bioavailability (%) | Half-Life (h) | Duration of Action (h) | Typical Oral Doses | Route of Elimination | Side Effects |
|---|---|---|---|---|---|---|
| Tolvaptan | 56 (42–80) | 3 (15 mg)–12 (≥120 mg) | ~24 | 15–60 mg/d Maximum: 60 mg/d | M | Thirst, dry mouth, pollakiuria or polyuria, asthenia, constipation, hyperglycemia, pyrexia, anorexia |
| Conivaptan | NA | 5 | ~24 | IV route only; 20 mg as a loading dose over 30 min, followed by continuous infusion of 20 mg over 24 h for 2–4 days. Maximum: 40 mg/d | M | Infusion site reactions (e.g., erythema, pain, phlebitis), hypokalemia, headache, peripheral edema, vomiting, diarrhea, constipation, hypertension, orthostatic hypotension, hyponatremia, thirst, anemia, hypotension, pyrexia, nausea, confusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilgeri, V.; Spitaler, P.; Puelacher, C.; Messner, M.; Adukauskaite, A.; Barbieri, F.; Bauer, A.; Senoner, T.; Dichtl, W. Decongestion in Acute Heart Failure—Time to Rethink and Standardize Current Clinical Practice? J. Clin. Med. 2024, 13, 311. https://doi.org/10.3390/jcm13020311
Bilgeri V, Spitaler P, Puelacher C, Messner M, Adukauskaite A, Barbieri F, Bauer A, Senoner T, Dichtl W. Decongestion in Acute Heart Failure—Time to Rethink and Standardize Current Clinical Practice? Journal of Clinical Medicine. 2024; 13(2):311. https://doi.org/10.3390/jcm13020311
Chicago/Turabian StyleBilgeri, Valentin, Philipp Spitaler, Christian Puelacher, Moritz Messner, Agne Adukauskaite, Fabian Barbieri, Axel Bauer, Thomas Senoner, and Wolfgang Dichtl. 2024. "Decongestion in Acute Heart Failure—Time to Rethink and Standardize Current Clinical Practice?" Journal of Clinical Medicine 13, no. 2: 311. https://doi.org/10.3390/jcm13020311
APA StyleBilgeri, V., Spitaler, P., Puelacher, C., Messner, M., Adukauskaite, A., Barbieri, F., Bauer, A., Senoner, T., & Dichtl, W. (2024). Decongestion in Acute Heart Failure—Time to Rethink and Standardize Current Clinical Practice? Journal of Clinical Medicine, 13(2), 311. https://doi.org/10.3390/jcm13020311






