Abstract
Bronchoscopy is an invasive procedure, and patient coughing during examination has been reported to cause patient distress. This study aimed to clarify the relationship between cough severity and diagnostic yield of endobronchial ultrasonography with guide sheath transbronchial biopsy (EBUS-GS-TBB). Data of patients who underwent bronchoscopy at Kyorin University Hospital between April 2019 and March 2022 were retrospectively evaluated. Bronchoscopists assessed the cough severity upon completion of the procedure using a four-point cough scale. Cough severity was included as a predictive factor along with those reportedly involved in bronchoscopic diagnosis, and their impact on diagnostic yield was evaluated. Predictors of cough severity were also examined. A total of 275 patients were enrolled in this study. In the multivariate analysis, the diagnostic group (n = 213) had significantly more ‘within’ radial endobronchial ultrasound findings (odds ratio [OR] 5.900, p < 0.001), a lower cough score (cough score per point; OR 0.455, p < 0.001), and fewer bronchial generations to target lesion(s) (OR 0.686, p < 0.001) than the non-diagnostic group (n = 62). The predictive factors for severe cough include the absence of virtual bronchoscopic navigation (VBN) and prolonged examination time. Decreased cough severity was a positive predictive factor for successful EBUS-GS-TBB, which may be controlled using VBN and awareness of the procedural duration.
1. Introduction
Flexible bronchoscopy is a safe and effective diagnostic method for patients with pulmonary diseases such as lung cancer, idiopathic or secondary interstitial pneumonia, infectious lung, and allergic diseases. However, bronchoscopy is an invasive examination, especially among those with cough sensitivity, which has been reported to cause distress [1].
In a prospective study, we conducted a questionnaire-based survey of patients who underwent bronchoscopy as a prospective study from March 2018 to July 2019 and found that a strong cough (odds ratio [OR] 1.69, p < 0.001), younger age (OR 0.96, p = 0.002), and bronchoscopists who were less experienced (OR 2.08, p = 0.047) were significant predictors of distress in patients during the examination. Furthermore, female sex (OR 2.57, p = 0.009), endobronchial ultrasonography (EBUS)-transbronchial needle aspiration (TBNA) (OR 2.95, p = 0.004), and prolonged examination time (>36 min) (OR 2.32, p = 0.022) were identified as predictive factors for strong cough in all bronchoscopy procedures [2].
However, it remains unknown whether a strong or persistent cough itself affects the diagnostic accuracy of bronchoscopy. Therefore, we focused on EBUS with guide sheath (GS) transbronchial biopsy (EBUS-GS-TBB). EBUS-GS-TBB, devised by Kurimoto et al. [3], enables clinicians to perform repeated tissue sampling within a target lesion using a guide sheath and radial EBUS (R-EBUS). This highly reliable method has been widely applied for the treatment of peripheral lung cancer. However, EBUS-GS-TBB is also associated with challenges, with diagnostic yield dependent on lesion size, computed tomography (CT) bronchus sign, and R-EBUS findings [4,5].
From this perspective, we studied the impact of cough severity and known diagnostic predictors on the diagnostic yield of EBUS-GS-TBB to improve the diagnostic yield of EBUS-GS-TBB. Additionally, the predictors of severe cough on EBUS-GS-TBB were examined.
2. Materials and Methods
2.1. Patients
Data from consecutive patients who underwent EBUS-GS-TBB at the Respiratory Department of Kyorin University Hospital (a 1100-bed tertiary center in Tokyo, Japan) between April 2019 and March 2022 were retrospectively reviewed. Patients who underwent bronchoalveolar lavage (BAL) or other biopsy procedures such as EBUS-TBNA and endobronchial biopsy at the time of EBUS-GS-TBB were excluded.
2.2. Bronchoscopy Procedure
All bronchoscopy procedures were performed in an inpatient setting using flexible bronchoscopes selected according to the lesion size and location (BF-P290F or BF-1TQ290 (Olympus, Tokyo, Japan).
After endotracheal anesthesia and observation of the airway to the subsegmental bronchi, the scope was inserted as far as possible into the bronchus toward the target lesion, according to the route on virtual bronchoscopic navigation (VBN) (SYNAPSE VINCENT, Fujifilm, Tokyo, Japan). The R-EBUS (UM-S20-17S, Olympus, Tokyo, Japan), in combination with a guide sheath (K-201 or K-203, Olympus, Tokyo, Japan), was inserted through the bronchoscopy channel under real-time X-ray fluoroscopy. When the target bronchus was difficult to settle, a curette-type inductor (CC-6DR-1, Olympus, Tokyo, Japan) was used with the GS before R-EBUS insertion. After confirming the target lesion using R-EBUS, forceps were inserted into the GS, and repeated biopsies were performed. Specimens were obtained from at least six biopsies, if possible, as well as brush and bronchial lavages used for histology, cytology, and bacterial culture.
2.3. Anesthesia Method
Patients who underwent the procedure between April 2019 and July 2020 were anesthetized by instilling lidocaine (5 mL [2%]) into the throat using a Jackson-type spray (face-to-face application) before bronchoscope insertion. Subsequently, an additional instillation of 2% lidocaine was administered when the bronchoscope passed through the larynx and trachea. However, owing to the coronavirus disease 2019 (COVID-19) epidemic, pharyngeal and laryngeal anesthesia with a Jackson-type spray was omitted for an 18-month period from August 2020 to March 2022 to prevent the risk of medical staff being exposed to to severe acute respiratory coronavirus 2 (SARS-CoV-2). Alternatively, pharyngolaryngeal and endotracheal anesthesia was administered using a spray catheter (PW-6C-1; Olympus, Tokyo, Japan) after inserting the scope into the mouth.
Midazolam (1–3 mg) and pethidine (35 mg) were administered intravenously at doses that are commonly used in Japanese clinical practice to provide sedation before the start of the examination. This was followed by an additional 1 mg of midazolam to maintain moderate sedation during the examination [6,7,8]. When coughing occurred during the procedure, 2% lidocaine was repeatedly administered through bronchoscopic channels.
2.4. Cough Severity Score on Bronchoscopy
Soon after completion of bronchoscopy, the bronchoscopist evaluated the severity of cough during the procedure and divided it into four grades, ranging from 0 to 3, which was defined as: score, 0 (no cough); 1 (slight cough); 2 (moderate cough not requiring interruption of the procedure); and 3 (severe cough requiring interruption of the procedure) (Table 1), similar to the authors’ previous report [2].

Table 1.
Cough score.
The predictors of severe cough on EBUS-GS-TBB were evaluated by dividing the patients into two groups, with a cough score of 0 or 1 as the weak cough group and a cough score of 2 or 3 as the severe cough group. The effect of the cough score on diagnostic accuracy was also examined.
2.5. Collection of Associated Data
The following data were retrieved from the patients: age, sex, smoking status (smoker or never smoker), smoking index (pack/years), final diagnosis (malignancy or benign), lesion size (mm), lobar position (bilateral upper lobes or other), location area (outer or inner/intermediate), “ground-glass” lesion (positive or negative), visibility on chest X-ray (visible or invisible), bronchus sign on CT (positive or negative), the bronchial generation order of target lesions, GS size (small or large), use of virtual VBN (yes or none), rapid onsite cytology evaluation (i.e., “ROSE”) (use or not), visibility on X-ray fluoroscopy (visible or invisible), the bronchial generation order permitting insertion of the scope, R-EBUS findings (within or adjacent to or invisible), examination time for bronchoscopy (min), number of obtained tissue samples, cough score, method of pharyngolaryngeal anesthesia (Jackson spray or spray catheter), and bronchoscopist experience (≥5 or <5 years).
2.6. Diagnostic Criteria in Bronchoscopy
After bronchoscopy, subsequent surgery or other procedures for tissue sampling (e.g., CT-guided biopsy) were considered definitive final diagnosis. When the final diagnosis matched that of bronchoscopy, the patient was considered to have had a successful diagnosis. If the results of bronchoscopy and subsequent procedures did not match, the case was considered nondiagnostic.
When no additional procedures for tissue sampling were performed, the final diagnosis was determined based solely on bronchoscopy findings. If the bronchoscopic samples were cytology class IV or V, or compatible with malignant diseases on histological examination, they were diagnosed with malignancy. Benign disease was defined as follows: bronchoscopy samples exhibiting non-malignant findings (e.g., granuloma, fibrotic change, and inflammation, irrespective of the presence of a pathogen) or when subsequent clinical outcomes were good after 1 year. Patients lost to follow-up were excluded from the analysis.
The non-diagnostic group included cases with insufficient samples for diagnosis (e.g., peripheral lung tissue and peribranchial tissue).
2.7. Statistical Analysis
Continuous variables are expressed as median and interquartile range (IQR), unless otherwise indicated, and were compared using the Mann–Whitney U test. Categorical variables were compared using Fisher’s exact test. Multivariate analyses were performed using multiple logistic regression. Differences with p < 0.05 were considered to be statistically significant. All statistical analyses were performed using EZR version 1.40 (Saitama Medical Center, Jichi Medical University, Tochigi, Japan) [9]. This study was approved by the ethics committee of Kyorin University Hospital (approval number: 2273).
3. Results
During the study period, 867 patients underwent planned bronchoscopy, and 309 underwent EBUS-GS-TBB alone, without any other biopsy procedures or BAL.
Twenty-eight patients lacking cough data and six patients without a definitive diagnosis were excluded. Finally, 275 patients were enrolled in this study.
3.1. Diagnostic Yield
A total of 275 patients (153 male and 122 female; age range, 30–89 years) were enrolled. The diagnostic and nondiagnostic groups comprised 213 and 62 patients, respectively, and the overall diagnostic yield of bronchoscopy with EBUS-GS-TBB was 77.5% (Table 2). The final diagnoses were malignancy (n = 199), infectious disease (n = 51), and inflammatory disease (n = 25) (Table 3). According to the severity of the cough score, the diagnostic yields of cough scores of 0, 1, 2, and 3 were 84.9% (106/122), 74.2% (124/156), 72.5% (40/51), and 40.0% (5/8), respectively, and appeared to decline as the cough score increased (Figure 1).

Table 2.
Characteristics of patients and findings during examination (n = 275).

Table 3.
Final diagnosis of target lesions (n = 275).
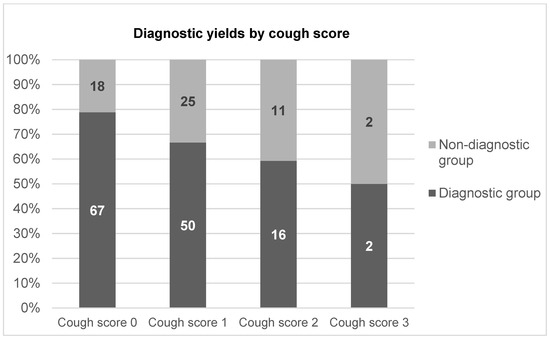
Figure 1.
Diagnostic yields by cough score.
3.2. Comparison of Diagnostic and Non-Diagnostic Groups
Comparison of diagnostic and non-diagnostic groups was as follows: the diagnostic group exhibited a significantly larger size of lesion (median 35.20 mm [IQR 25.80–47.57 mm] versus [vs.] 26.48 mm [IQR 19.78–40.67 mm]; p = 0.001); fewer number of bronchial generations to target lesion (median 4.00 [IQR 3.00–6.00] vs. 6.00 [IQR 5.00–7.00]; p < 0.001); more common use of large GS (n = 42 [93.3%] vs. n = 171 [74.3%]; p = 0.003) and ROSE (n = 54 [87.1%] vs. 159 [74.6]; p = 0.040); visible on X-ray fluoroscopy (n = 196 [80.3%] vs. n = 17 [54.8%]; p = 0.003); and within R-EBUS findings (n = 172 [88.7%] vs. n = 41 [50.6%]; p < 0.001), shorter examination time (median 38.00 min [IQR 31.00–47.00 min] vs. 42.00 min [IQR 37.00– 50.50 min]; p = 0.001), greater number of samples (median 6.00 [IQR 6.00–8.00] vs. 6.00 [IQR 4.00–7.00]; p = 0.030), and decreased cough score (median 1.00 [IQR 0.00–1.00] vs. median 1.00 [IQR 0.25–1.00]; p = 0.013) (Table 4).

Table 4.
Univariate analysis of factors on diagnostic yield of TBB using EBUS-GS.
3.3. Multivariant Analysis between Diagnostic and Non-Diagnostic Groups
Based on logistic regression analysis, three factors, including R-EBUS findings within (OR 5.900 [95% CI 2.990–11.600]; p < 0.001), decrease in cough score per 1 point (OR 0.455 [95% CI 0.289–0.718]; p < 0.001), and fewer number of bronchial generations to target lesion (OR 0.686; [95% CI 0.550–0.855]; p < 0.001), were identified as significant factors for definitive diagnosis (Table 5).

Table 5.
Multivariate analysis for success of diagnosis.
3.4. Predictive Factors for Severe Cough
The weak and severe cough groups comprised 230 and 45 patients, respectively (Table 6). The severe cough group exhibited a significantly larger size of target lesion (median 41.80 mm [IQR 25.24–62.39 mm] vs. 33.65 mm [IQR 23.53–44.00 mm ]; p = 0.047), more use of large GS (n = 12 [26.7%] vs. n = 33 [14.3%]; p = 0.049), high proportion of no use of virtual bronchoscopy (n = 26 [22.4%] vs. 19 [11.9%]; p = 0.031), and ratio of bronchoscopists with < 5 years’ experience (n = 24 [22.0%] vs. 21 [12.7%]; p = 0.046) than those in the weak cough group (Table 6).

Table 6.
Univariate analysis for severe cough.
Multivariate analysis revealed that use of VBN (OR 0.449 [95% CI 0.233–0.865]; p = 0.017) and a prolonged examination time (OR 1.030 [95% CI 1.000–1.060]; p = 0.045) were the only factors predicting severe score (Table 7). A schematic representation of the relationship between the diagnostic yield of EBUS-GS-TBB and cough factors is shown in Figure 2.

Table 7.
Multivariate analysis for severe cough.
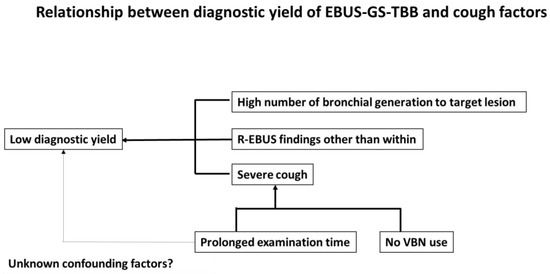
Figure 2.
Schema of the relationship between diagnostic yield of EBUS-GS-TBB and cough factors.
4. Discussion
The results of the present study demonstrated that the overall definitive diagnostic yield of EBUS-GS-TBB was 77.5%, as in previous reports [10], and a novel positive predictive factor for successful diagnosis was associated with decreased cough severity, which may be controlled using VBN and awareness of the examination time.
Previous studies have reported that coughing during bronchoscopy increases patient distress and decreases tolerance to repeat examinations [1,2,11]; however, no studies have examined its impact on the diagnostic yield(s) of bronchoscopy. Therefore, this is the first study to demonstrate that cough severity affects the diagnostic yield, even in EBUS-GS-TBB.
We found three factors for definitive diagnosis, including R-EBUS findings “within”, fewer number of bronchial generations to target lesion(s), and decrease in cough score. Numerous studies have reported that R-EBUS findings are strong diagnostic predictive factors [3,4,10,12,13,14,15] for EBUS-GS-TBB, as in this study, whereas few reports have described the number of bronchial generations to the target lesion(s) for a definitive diagnosis using EBUS-GS-TBB. Katsurada et al. reported that the diagnostic yield in large GS is high when the bronchial generation order of the target lesion is ≤3 [16], which is similar to the trend observed in our study (≤4). Furthermore, severe cough can lead to a low diagnostic yield for EBUS-GS-TBB, probably because of impaired visibility of the bronchial lumen and difficulties in holding the guide sheath in the same position and obtaining adequate specimens with forceps.
Previous studies have shown that lesion size, guide sheath size, use of ROSE, visibility on X-ray fluoroscopy, and the total number of biopsied samples contributed to the diagnosis [14,17,18,19]. However, in the multivariate analysis in our study, the results were not statistically significant. The combined use of fluoroscopy with ROSE can correct misalignment for sufficient tissue sampling due to coughing; however, this might not outweigh the influence of coughing. In fact, our previous study included all procedures, except EBUS-GS-TBB, and determined that crucial factors for a strong cough included female sex, EBUS-TBNA, and prolonged examination time (>36 min) [2].
Notably, the present study demonstrated that the absence of VBN and prolonged examination time were two significant risk factors for a strong cough during the EBUS-GS-TBB procedure. Previous reports have demonstrated that VBN can improve the diagnostic yield of EBUS-GS-TBB and reduce the examination time [20,21]; however, in our study, VBN did not contribute to the diagnostic yield. In contrast, VBN may reduce coughing by decreasing the risk of contact with the bronchial wall and reducing the examination time.
The relationship between the examination time and cough severity requires careful consideration. First, in the general concept of bronchoscopy, a prolonged examination time is a predictor of severe cough [2], similar to the present study. It is unclear whether prolonged examination time in technically difficult cases with severe cough, presumably emerging from anesthesia or severe cough itself, decreases the diagnostic yield of EBUS-GS-TBB; however, our study supported the latter hypothesis based on multivariate analysis.
The present study had some limitations, the first of which was its single-center, retrospective design and relatively small number of patients. Second, the evaluation of cough severity depended only on the physician’s subjective assessment and not on quantitative methods such as counting the absolute number of coughs. However, a positive correlation was noted for coughing sensation among bronchoscopists’ and patients’ or bronchoscopists’ and nurses’ VAS scores and the reproducibility of bronchoscopists’ evaluations [22,23]. As coughing may involve both frequency and intensity, a subjective and simple assessment using the VAS score may be more useful than an objective index (i.e., the absolute number of coughs).
Third, pethidine was administered as an opioid along with midazolam. Pethidine is widely used as an alternative to fentanyl in Japan; however, guidelines from the British Thoracic Society and a statement from the American College of Chest Physicians recommend fentanyl with midazolam because of its superiority in decreasing coughing during bronchoscopy [24,25]; as such, the results of the present study should be interpreted with caution. Last, we did not collect data on disease profiles such as COPD and asthma, which increase airway secretions. Furthermore, 11.6% of the enrolled patients were current smokers and their smoking status on the day of bronchoscopy was unknown. This might represent real-world data collection for consecutive cases. Nevertheless, we found that severe cough, a novel predictive factor for successful diagnosis using EBUS-GS-TBB, could be correlated with prolonged examination time and the use of VBN.
5. Conclusions
R-EBUS findings, low cough score, and low number of bronchial generations to the target lesions were associated with successful EBUS-GS-TBB. Using VBN and shortening the examination time may improve the accuracy of EBUS-GS-TBB by controlling coughing during the procedure.
Author Contributions
Conceptualization, F.K.; methodology, F.K.; software, F.K.; investigation, T.A. (Takatora Akizawa), T.A. (Taro Abe), E.I., R.T., N.I., N.K., J.A., H.N., Y.N., M.I., M.S., K.N. and S.T.; data curation, F.K. and T.S.; writing—original draft preparation, F.K. and T.S.; writing—review and editing, T.S.; supervision, T.S. and H.I.; project administration, T.A. (Takatora Akizawa), T.A. (Taro Abe), E.I., R.T., N.I., N.K., J.A., H.N., Y.N., M.I., M.S., K.N. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethical Committee of Kyorin University in December 2023 (approval number: 2273).
Informed Consent Statement
Written informed consent was obtained from all patients.
Data Availability Statement
Data are fully available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hirose, T.; Okuda, K.; Ishida, H.; Sugiyama, T.; Kusumoto, S.; Nakashima, M.; Yamaoka, T.; Adachi, M. Patient satisfaction with sedation for flexible bronchoscopy. Respirology 2008, 13, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.; Saraya, T.; Oda, M.; Sakuma, S.; Watanabe, M.; Takata, S.; Tamura, M.; Takakura, H.; Nakamoto, K.; Honda, K.; et al. Novel predictive factors for patient discomfort and severe cough during bronchoscopy: A prospective questionnaire analysis. PLoS ONE 2020, 15, e0240485. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, N.; Miyazawa, T.; Okimasa, S.; Maeda, A.; Oiwa, H.; Miyazu, Y.; Murayama, M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004, 126, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Yamazaki, K.; Kurimoto, N.; Asahina, H.; Kikuchi, E.; Shinagawa, N.; Oizumi, S.; Nishimura, M. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007, 132, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Minezawa, T.; Okamura, T.; Yatsuya, H.; Yamamoto, N.; Morikawa, S.; Yamaguchi, T.; Morishita, M.; Niwa, Y.; Takeyama, T.; Mieno, Y.; et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: A retrospective observational study. BMC Med. Imaging 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, M.; Tachihara, M.; Katsurada, N.; Takata, N.; Sato, H.; Mimura, C.; Yoshioka, J.; Furukawa, K.; Yumura, M.; Otoshi, T.; et al. Randomized single-blind comparative study of the midazolam/pethidine combination and midazolam alone during bronchoscopy. BMC Cancer 2022, 22, 539. [Google Scholar] [CrossRef] [PubMed]
- Minami, D.; Takigawa, N.; Watanabe, H.; Ninomiya, T.; Kubo, T.; Ohashi, K.; Sato, A.; Hotta, K.; Tabata, M.; Tanimoto, M.; et al. Safety and discomfort during bronchoscopy performed under sedation with fentanyl and midazolam: A prospective study. Jpn J. Clin. Oncol. 2016, 46, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Otsuka, K.; Kato, R.; Shimizu, R.; Otoshi, T.; Fujimoto, D.; Kawamura, T.; Tamai, K.; Nagata, K.; Otsuka, K.; et al. Evaluation of discomfort and tolerability to bronchoscopy according to different sedation procedures with midazolam. Exp. Ther. Med. 2015, 10, 659–664. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Wang, G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc. Ultrasound 2017, 6, 292–299. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ishiwata, T.; Kasai, H.; Terada, J.; Shionoya, Y.; Ikari, J.; Kawata, N.; Tada, Y.; Tsushima, K.; Tatsumi, K. Identification of factors during bronchoscopy that affect patient reluctance to undergo repeat examination: Questionnaire analysis after initial bronchoscopy. PLoS ONE 2018, 13, e0208495. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, Y.; Tashiro, H.; Takahashi, K.; Tajiri, R.; Kuwahara, Y.; Kajiwara, K.; Komiya, N.; Ogusu, S.; Nakashima, C.; Nakamura, T.; et al. Factors related to the diagnosis of lung cancer by transbronchial biopsy with endobronchial ultrasonography and a guide sheath. Thorac. Cancer 2022, 13, 3459–3466. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Sasada, S.; Chavez, C.; Tsuchida, T. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J. Thorac. Dis. 2013, 5, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Cheng, W.C.; Wu, B.R.; Chen, C.Y.; Chen, W.C.; Hsia, T.C.; Liao, W.C.; Tu, C.Y.; Shih, C.M.; Hsu, W.H.; et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: Combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J. Thorac. Dis. 2015, 7 (Suppl. S4), S418–S425. [Google Scholar] [CrossRef] [PubMed]
- Evison, M.; Crosbie, P.A.; Morris, J.; Martin, J.; Barber, P.V.; Booton, R. Can computed tomography characteristics predict outcomes in patients undergoing radial endobronchial ultrasound-guided biopsy of peripheral lung lesions? J. Thorac. Oncol. 2014, 9, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, N.; Tachihara, M.; Jimbo, N.; Yamamoto, M.; Yoshioka, J.; Mimura, C.; Satoh, H.; Furukawa, K.; Otoshi, T.; Kiriu, T.; et al. Yield of tumor samples with a large guide-sheath in endobronchial ultrasound transbronchial biopsy for non-small cell lung cancer: A prospective study. PLoS ONE 2021, 16, e0259236. [Google Scholar] [CrossRef]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef]
- Izumo, T.; Matsumoto, Y.; Sasada, S.; Chavez, C.; Nakai, T.; Tsuchida, T. Utility of rapid on-site cytologic evaluation during endobronchial ultrasound with a guide sheath for peripheral pulmonary lesions. Jpn J. Clin. Oncol. 2017, 47, 221–225. [Google Scholar] [CrossRef]
- Ikezawa, Y.; Sukoh, N.; Shinagawa, N.; Nakano, K.; Oizumi, S.; Nishimura, M. Endobronchial ultrasonography with a guide sheath for pure or mixed ground-glass opacity lesions. Respiration 2014, 88, 137–143. [Google Scholar] [CrossRef]
- Asano, F.; Eberhardt, R.; Herth, F.J. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014, 88, 430–440. [Google Scholar] [CrossRef]
- Asano, F.; Shinagawa, N.; Ishida, T.; Shindoh, J.; Anzai, M.; Tsuzuku, A.; Oizumi, S.; Morita, S. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am. J. Respir. Crit. Care Med. 2013, 188, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hadzri, H.; Azarisman, S.; Fauzi, A.; Roslan, H.; Roslina, A.; Adina, A.; Fauzi, M. Can a bronchoscopist reliably assess a patient’s experience of bronchoscopy? JRSM Short Rep. 2010, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.; Chhajed, P.N.; Leuppi, J.D.; Brutsche, M.; Pflimlin, E.; Tamm, M. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: A randomised, double blind, placebo controlled trial. Thorax 2004, 59, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax 2013, 68 (Suppl. S1), i1–i44. [Google Scholar] [CrossRef]
- Wahidi, M.M.; Jain, P.; Jantz, M.; Lee, P.; Mackensen, G.B.; Barbour, S.Y.; Lamb, C.; Silvestri, G.A. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest 2011, 140, 1342–1350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).