Lung Involvement in Patients with Ulcerative Colitis: Relationship between Exhaled Nitric Oxide and Lung Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Study Design
2.3. Disease Activity
2.4. Pulmonary Function Tests
2.5. FeNO and CANO Measurements
2.6. CRP Collection
2.7. Endpoints and Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Pulmonary Function Tests
3.3. FeNO Measurements
3.4. Lung Function, FeNO, CRP, and Correlation with Clinical Disease Activity
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schreiber, S. Diagnostics of Inflammatory Bowel Disease. Gastroenterology 2007, 133, 1670–1689. [Google Scholar] [CrossRef] [PubMed]
- Rothfuss, K.S.; Stange, E.F.; Herrlinger, K.R. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4819–4831. [Google Scholar] [CrossRef]
- Georgakopoulou, V.E.; Tarantinos, K.; Papalexis, P.; Spandidos, D.A.; Damaskos, C.; Gkoufa, A.; Chlapoutakis, S.; Sklapani, P.; Trakas, N.; Mermigkis, D. Role of pulmonary function testing in inflammatory bowel diseases (Review). Med. Int. 2022, 2, 25. [Google Scholar] [CrossRef]
- Herrlinger, K.R.; Noftz, M.K.; Dalhoff, K.; Ludwig, D.; Stange, E.F.; Fellermann, K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am. J. Gastroenterol. 2002, 97, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ragnoli, B.; Radaeli, A.; Pochetti, P.; Kette, S.; Morjaria, J.; Malerba, M. Fractional nitric oxide measurement in exhaled air (FeNO): Perspectives in the management of respiratory diseases. Ther. Adv. Chronic. Dis. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- American Thoracic Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. Available online: https://www.atsjournals.org/doi/10.1164/rccm.200406-710ST (accessed on 29 October 2023). [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A european respiratory society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef]
- Högman, M.; Malinovschi, A.; Norbäck, D.; Janson, C. Added value with extended NO analysis in atopy and asthma. Clin. Physiol. Funct. Imaging 2011, 31, 294–299. [Google Scholar] [CrossRef]
- George, S.C.; Hogman, M.; Permutt, S.; Silkoff, P.E. Modeling pulmonary nitric oxide exchange. J. Appl. Physiol. 2004, 96, 831–839. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the mayo score to assess clinical response in Ulcerative Colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Graham, B.L.; Cooper, B.G.; Thompson, B.R.; Carter, K.W.; Francis, R.W.; Hall, G.L. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur. Respir. J. 2017, 50, 1700010. [Google Scholar] [CrossRef]
- Hall, G.L.; Filipow, N.; Ruppel, G.; Okitika, T.; Thompson, B.; Kirkby, J.; Steenbruggen, I.; Cooper, B.G.; Stanojevic, S. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur. Respir. J. 2020, 57, 2000289. [Google Scholar] [CrossRef]
- Tsoukias, N.M.; George, S.C. A two-compartment model of pulmonary nitric oxide exchange dynamics. Appl. Physiol. 1998, 85, 65366. [Google Scholar] [CrossRef]
- Olivieri, M.; Talamini, G.; Corradi, M.; Perbellini, L.; Mutti, A.; Tantucci, C.; Malerba, M. Reference values for exhaled nitric oxide (reveno) study. Respir. Res. 2006, 7, 94. [Google Scholar] [CrossRef]
- Olin, A.-C.; Bake, B.; Torén, K. Fraction of exhaled nitric oxide at 50 mL/s: Reference values for adult lifelong never-smokers. Chest 2007, 131, 1852–1856. [Google Scholar] [CrossRef]
- Kovesi, T.; Kulka, R.; Dales, R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest 2008, 133, 169–175. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Malerba, M.; Damiani, G.; Carpagnano, G.E.; Olivini, A.; Radaeli, A.; Ragnoli, B.; Foschino, M.P.; Olivieri, M. Values in elderly people for exhaled nitric oxide study. Rejuvenation Res. 2016, 19, 233–238. [Google Scholar] [CrossRef] [PubMed]
- See, K.C.; Christiani, D.C. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: Results from the national health and nutrition examination survey 2007–2010. Chest 2013, 143, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; Bagnasco, D.; Carriero, V.; Bertolini, F.; Ricciardolo, F.L.M.; Nicola, S.; Brussino, L.; Nappi, E.; Paoletti, G.; Canonica, G.W.; et al. Critical evaluation of asthma biomarkers in clinical practice. Front. Med. 2022, 9, 969243. [Google Scholar] [CrossRef] [PubMed]
- Storch, I.; Sachar, D.; Katz, S. Pulmonary manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Songür, N.; Songür, Y.; Tüzün, M.; Dogan, I.; Tüzün, D.; Ensari, A.; Hekimoglu, B. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J. Clin. Gastroenterol. 2003, 37, 292–298. [Google Scholar] [CrossRef]
- Black, H.; Mendoza, M.; Murin, S. Thoracic manifestations of inflammatory bowel disease. Chest 2007, 131, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.J.; Gupta, V.L.; Kothari, H.G.; Samarth, A.R.; Gaikwad, N.R.; Parmar, S.M. Assessment of occult pulmonary involvement in ulcerative colitis. Inflamm. Intestig. Dis. 2020, 5, 144–150. [Google Scholar] [CrossRef]
- Godet, P.G.; Cowie, R.; Woodman, R.C.; Sutherland, L.R. Pulmonary function abnormalities in patients with ulcerative colitis. Am. J. Gastroenterol. 1997, 92, 1154–1156. [Google Scholar]
- Dierkes-Globisch, A.; Mohr, H. Pulmonary function abnormalities in respiratory asymptomatic patients with inflammatory bowel disease. Eur. J. Intern. Med. 2002, 13, 385. [Google Scholar] [CrossRef]
- Mohamed-Hussein, A.A.; Mohamed, N.A.; Mohamed-Eltaher, A.R.I. Changes in pulmonary function in patients with ulcerative colitis. Respir. Med. 2007, 101, 977–982. [Google Scholar] [CrossRef]
- Ji, X.Q.; Wang, L.X.; Lu, D.-G. Pulmonary manifestations of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 13501–13511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Liu, Z.; Lin, H.; Shi, Y.; Sun, X. Pulmonary dysfunction in 114 patients with inflammatory bowel disease. Medicine 2017, 96, e6808. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yilmaz Demirci, N.; Hoşgün, D.; Uner, E.; Erdoğan, Y.; Gökçek, A.; Cağlar, A. A Pulmonary involvement in inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 4952–4957. [Google Scholar] [CrossRef]
- Ellrichmann, M.; Bethge, J.; Boesenkoetter, J.; Conrad, C.; Noth, R.; Bahmer, T.; Nikolaus, S.; Aden, K.; Zeissig, S.; Schreiber, S. Subclinical pulmonary involvement in active IBD responds to biologic therapy. J. Crohn’s Colitis 2021, 15, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Tunc, B.; Filik, L.; Arda, K.; Ulker, A. Pulmonary function tests, high-resolution computed tomography findings and inflammatory bowel disease. Acta Gastroenterol. Belg. 2006, 69, 255–260. [Google Scholar]
- Wallaert, B.; Colombel, J.F.; Tonnel, A.; Bonniere, P.; Cortot, A.; Paris, J.; Voisin, C. Evidence of lymphocyte alveolitis in Crohn’s disease. Chest 1985, 87, 363–367. [Google Scholar] [CrossRef]
- Protopapas, A.; Vradelis, S.; Karampitsakos, T.; Steiropoulos, P.; Chatzimichael, A.; Paraskakis, E. Elevated levels of alveolar nitric oxide may indicate presence of small airway inflammation in patients with inflammatory bowel disease. Lung 2019, 197, 663–670. [Google Scholar] [CrossRef]
- Christie, P.M.; Hill, G.L. Effect of intravenous nutrition on nutrition and function in acute attacks of inflammatory bowel disease. Gastroenterology 1990, 90, 730–736. [Google Scholar] [CrossRef]
- Tzanakis, N.E.; Tsiligianni, I.G.; Siafakas, N.M. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 299–305. [Google Scholar] [CrossRef]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, N.; Vigliarolo, R.; Sanguinetti, C.M. Respiratory involvement in inflammatory bowel diseases. Multidiscip. Respir. Med. 2010, 5, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Fireman, E.; Masarwy, F.; Groisman, G.; Shtark, M.; Kopelman, Y.; Kivity, S.; Fireman, Z. Induced sputum eosinophilia in ulcerative colitis patients: The lung as a mirror image of intestine? Respir. Med. 2009, 103, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Marvisi, M.; Borrello, P.D.; Brianti, M.; Fornarsari, G.; Marani, G.; Guariglia, A. Changes in the carbon monoxide diffusing capacity of the lung in ulcerative colitis. Eur. Respir. J. 2000, 16, 965–968. [Google Scholar] [CrossRef]
- Sethy, P.K.; Dutta, U.; Aggrawal, A.N.; Das, R.; Gulati, M.; Sinha, S.K.; Singh, K. Pulmonary and hematological alterations in idiopathic ulcerative colitis. Indian J. Gastroenterol. 2003, 22, 176–179. [Google Scholar]
- Ateş, F.; Karincaoğlu, M.; Hacievlıyagıl, S.S.; Yalniz, M.; Seçkın, Y. Alterations in the pulmonary function tests of inflammatory bowel diseases. Turk. J. Gastroenterol. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Crawford, M.s.S.; Nordgren, T.M.; McCole, D.F. Every breath you take: Impacts of environmental dust exposure on intestinal barrier function-from the gut-lung axis to COVID-19. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G586–G600. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. Mechanisms of disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Salzman, N.H.; Bevins, C.L. Negative interactions with the microbiota: IBD. Adv. Exp. Med. Biol. 2008, 635, 67–78. [Google Scholar]
- Salmi, M.; Jalkanen, S. Lymphocyte homing to the gut: Attraction, adhesion, and commitment. Immunol. Rev. 2005, 206, 100–113. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Yildirim, B.; Aydogdu, M.; Dincel, A.S.; Elmas, C.; Oguzulgen, K.; Tuncer, C. Is there any link between oxidative stress and lung involvement due to inflammatory bowel disease: An experimental study. Hepatogastroenterology 2011, 58, 1898–1903. [Google Scholar] [PubMed]
- Parrón-Ballesteros, J.; Gordo, R.G.; López-Rodríguez, J.C.; Olmo, N.; Villalba, M.; Batanero, E.; Turnay, J. Beyond allergic progression: From molecules to microbes as barrier modulators in the gut-lung axis functionality. Front. Allergy 2023, 30, 1093800. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Hellstrom, P.M.; Lundberg, J.M.; Alving, K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet 1994, 344, 1673–1674. [Google Scholar] [CrossRef] [PubMed]
- Koek, G.H.; Verleden, G.M.; Evenepoel, P.; Rutgeerts, P. Activity related increase of exhaled nitric oxide in Crohn’s disease and ulcerative colitis: A manifestation of systemic involvement? Respir. Med. 2002, 96, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Ragnoli, B. Exhaled nitric oxide as a marker of lung involvement in Crohn’s disease. Int. J. Immunol. Immunopathol. 2011, 24, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Quenon, L.; Hindryckx, P.; De Vos, M.; De Looze, D.; Joos, G.; Brusselle, G.; Peeters, H. Hand-held fractional exhaled nitric oxide measurements as a non-invasive indicator of systemic inflammation in Crohn’s disease. J. Crohn’s Colitis 2013, 7, 644–648. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Yildirim, B.; Erbas, G.; Akten, S.; Oguzulgen, K.I.; Tunc, B.; Tuncer, C.; Turktas, H. Value of fractional exhaled nitric oxide (FE NO) for the diagnosis of pulmonary involvement due to inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 670–676. [Google Scholar] [CrossRef]
- Ikonomi, E.; Rothstein, R.D.; Ehrlich, A.C.; Friedenberg, F.K. Measurement of fractional exhaled nitric oxide as a marker of disease activity in inflammatory bowel disease. J. Gastroenterol. Pancreatol. Liver Disord. 2016, 3, 10. [Google Scholar] [CrossRef]
- Paraskakis, E.; Brindicci, C.; Fleming, L.; Krol, R.; Kharitonov, S.A.; Wilson, N.M.; Barnes, P.J.; Bush, A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 260–267. [Google Scholar] [CrossRef]
- Lázár, Z.; Horváth, P.; Puskás, R.; Gálffy, G.; Losonczy, G.; Horváth, I.; Bikov, A. A suitable protocol for measuring alveolar nitric oxide in asthma with differing severity to assess peripheral airways inflammation. J. Asthma 2019, 56, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Hargadon, B.; Morgan, A.; Shelley, M.; Richter, J.; Shaw, D.; Green, R.H.; Brightling, C.; Wardlaw, A.J.; Pavord, I.D. Alveolar nitric oxide in adults with asthma: Evidence of distal lung inflammation in refractory asthma. Eur. Respir. J. 2005, 25, 986–991. [Google Scholar] [CrossRef] [PubMed]
- van Veen, I.H.; Sterk, P.J.; Schot, R.; Gauw, S.A.; Rabe, K.F.; Bel, E.H. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. Eur. Respir. J. 2006, 27, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.A.; Clearie, K.; Menzies, D.; Vaidyanathan, S.; Lipworth, B.J. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung 2011, 189, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.; Paraskakis, E.; Bush, A. Alveolar, but not bronchial nitric oxide production is elevated in cystic fibrosis. Pediatr. Pulmonol. 2007, 42, 1215–1221. [Google Scholar] [CrossRef]
- Brindicci, C.; Ito, K.; Resta, O.; Pride, N.B.; Barnes, P.J.; Kharitonov, S.A. Exhaled nitric oxide from lung periphery is increased in COPD. Eur. Respir. J. 2005, 26, 52–59. [Google Scholar] [CrossRef]
- Lázár, Z.; Kelemen Gálffy, G.; Gálffy, G.; Losonczy, G.; Horváth, I.; Bikov, A. Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J. Breath Res. 2018, 12, 036017. [Google Scholar] [CrossRef] [PubMed]
- Cameli, P.; Bargagli, E.; Bergantini, L.; d’Alessandro, M.; Pieroni, M.; Fontana, G.A.; Sestini, P.; Refini, R.M. Extended exhaled nitric oxide analysis in interstitial lung diseases: A systematic review. Int. J. Mol. Sci. 2020, 21, 6187. [Google Scholar] [CrossRef]
- Malerba, M.; Ragnoli, B.; Radaeli, A. Exhaled nitric oxide levels in alpha-1-antitrypsin PiMZ subjects. J. Intern. Med. 2009, 265, 382–387. [Google Scholar] [CrossRef]
- Vincken, S.; Verbanck, S.; Schuermans, D.; Evenepoel, T.; Vanderhelst, E. The role of FeNO in stable COPD patients with eosinophilic airway inflammation. Respir. Med. 2021, 181, 106377. [Google Scholar] [CrossRef]
- Yamaji, Y.; Oishi, K.; Hamada, K.; Ohteru, Y.; Chikumoto, A.; Murakawa, K.; Matsuda, K.; Suetake, R.; Murata, Y.; Kosuke, I. Detection of type 2 biomarkers for response in COPD. J. Breath Res. 2020, 14, 026007. [Google Scholar] [CrossRef]
- Malerba, M.; Radaeli, A.; Olivini, A.; Damiani, G.; Ragnoli, B.; Montuschi, P.; Ricciardolo, F.M.L. Exhaled Nitric Oxide as a Biomarker in COPD and Related Comorbidities. BioMed Res. Int. 2014, 2014, 271918. [Google Scholar] [CrossRef] [PubMed]

| Variable | All, n = 83 | UC, n = 42 | Controls, n = 41 | p-Value |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 32 (28–37) | 36 (31–43) | 29 (27–32) | <0.0001 |
| Mean ± std | 34.3 ± 10.9 | 39.0 ± 13.3 | 29.5 ± 3.8 | |
| Male sex, | ||||
| n (%) | 39 (46.9) | 22 (52.4) | 17 (41.4) | 0.32 |
| Disease activity, n (%) | 42 (100) | |||
| Mild (1) | 3 (7.1) | |||
| Intermediate (2) | 13 (31) | |||
| Severe (3) | 26 (61.9) |
| Variable | All, n = 83 Median (IQR) Mean ± Std | UC, n = 42 Median (IQR) Mean ± Std | Controls, n = 41 Median (IQR) Mean ± Std | t-Test p-Value | Multivariate Linear Regression Model | |
|---|---|---|---|---|---|---|
| Beta Estimated (CI 95%) | p-Value | |||||
| Lung function | ||||||
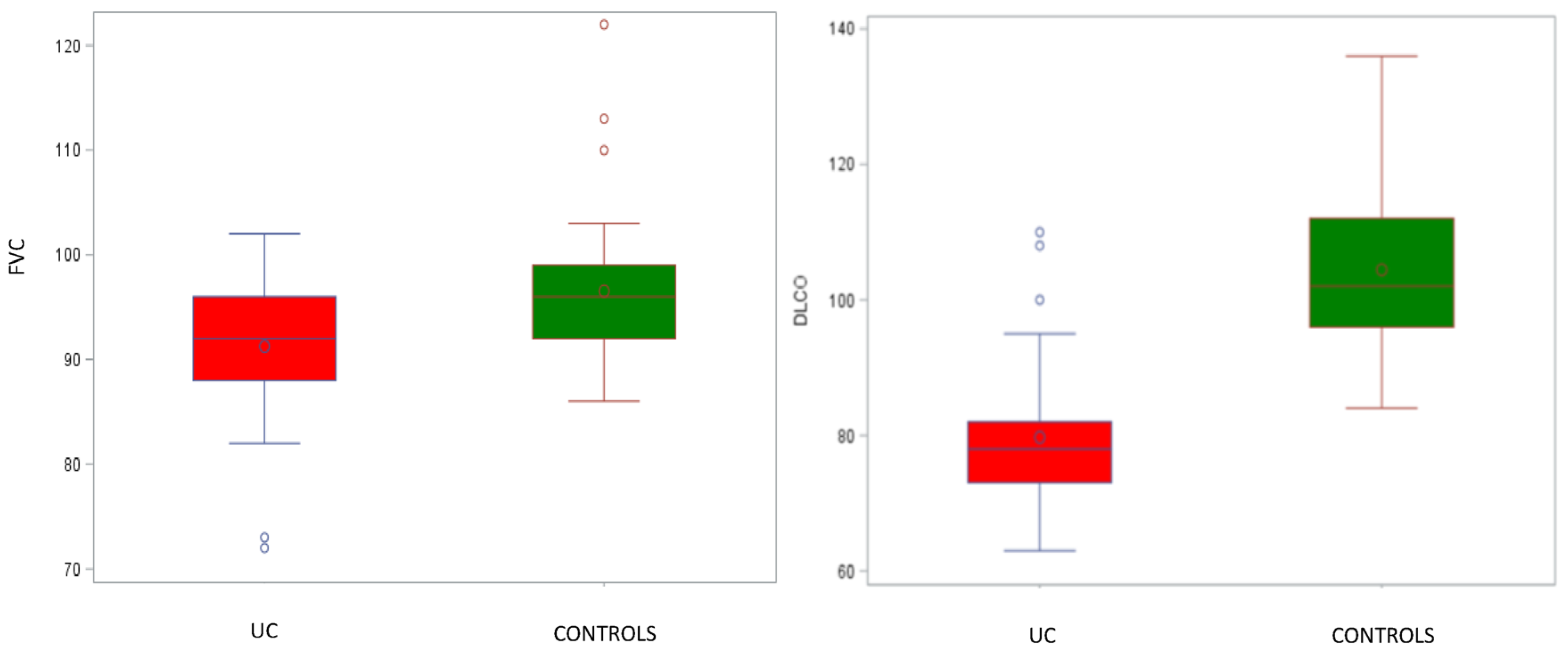
| FVC, % | 94 (90–98) 93.9 ± 7.4 | 92 (88–96) 91.3 ± 6.7 | 96 (92–99) 96.5 ± 7.3 | 0.0009 | −3.3 (−6.60; −0.00038) | 0.0500 |
| FEV1/FVC, % | 84 (81–87) 83.3 ± 4.9 | 84 (78–88) 82.0 ± 5.9 | 84 (82–87) 84.5 ± 3.1 | 0.019 | −0.67 (−2.81; 1.47) | 0.5300 |
| FEV1, % | 97 (93–103) 98.0 ± 9.6 | 95 (92–99) 95.8 ± 9.6 | 97 (94–104) 100.2 ± 9.2 | 0.04 | −1.42 (−5.79; 2.96) | 0.5200 |
| TLC, % | 96 (86–104) 95.4 ± 12.1 | 90 (82–98) 92.0 ± 11.1 | 98 (90–106) 98.8 ± 12.2 | 0.01 | −1.45 (−6.55; 3.64) | 0.5700 |
| DLCO, % | 90 (78–103) 91.9 ± 16.8 | 78 (73–82) 79.7 ± 10.6 | 102 (96–112) 104.4 ± 12.1 | <0.0001 | −21.46 (−26.79; −16.14) | <0.0001 |
| Inflammatory markers | ||||||
| CRP, mg/dL | NA | 12.9 (6.1–35.5) 24.1 ± 23.3 | NA | |||
| FeNO, ppb | 10.8 (7.9–18.4) 15.4 ± 11.8 | 18.3 (6.7–34.1) 20.4 ± 14.7 | 10.1 (8.2–12) 10.3 ± 3.4 | <0.0001 | 9.17 (3.92; 14.41) | 0.0008 |
| CANO, ppb | 2.4 (1.8–6.1) 4.3 ± 3.9 | 5.9 (2.1–10) 6.3 ± 4.5 | 2.1(1.5–2.5) 2.2 ± 1.0 | <0.0001 | 4.01 (2.40; 5.62) | <0.0001 |
| * | ||||||
| Variable | Mild (1–2), n = 16 Median (IQR) Mean ± Std | Severe (3), n = 26 Median (IQR) Mean ± Std | t-Test | Multivariate Linear Regression Model | |
|---|---|---|---|---|---|
| p-Value | Beta Estimated (CI 95%) | p-Value | |||
| Age, years | 35.0 (32.5–38.0) 39.6 ± 16.3 | 37.0 (30.0–43.0) 38.7 ± 11.5 | 0.8400 | ||
| BMI, ratio | 21.5 (19.3–25.9) 22.1 ± 3.6 | 21.5 (19.3–25.9) 22.9 ± 2.2 | 0.3900 | ||
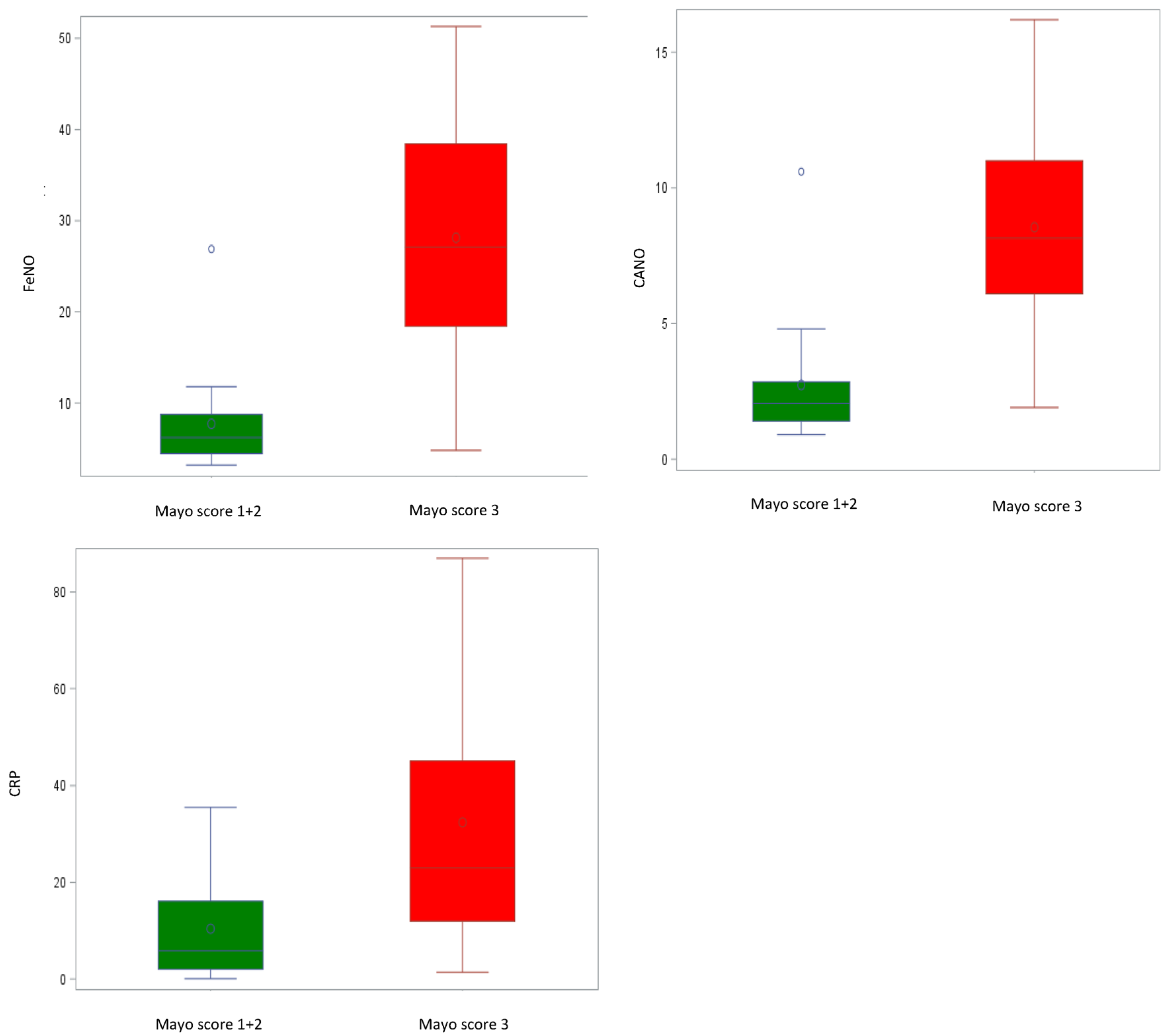
| FeNO, ppb | 6.2 (4.5–8.8) 7.7 ± 5.7 | 27.1 (18.4–38.4) 28.1 ± 12.9 | <0.0001 | 20.43 (13.40; 27.47) | <0.0001 |
| CANO, ppb | 2.1 (1.4–2.9) 2.7 ± 2.3 | 8.2 (6.1–11.0) 8.6 ± 4.1 | <0.0001 | 5.80 (3.49; 8.11) | <0.0001 |
| CRP, mg/dL | 5.9 (2.0–16.1) 10.4 ± 11.4 | 23.0 (12.0–45.0) 32.5 ± 24.9 | 0.0020 | 22.68 (9.24; 36.12) | 0.0020 |
| TLC, % | 98.0 (89.5–102.0) 95.9 ± 12.01 | 88.0 (80.0–98.0) 89.6 ± 9.9 | 0.0700 | −6.81 (−11.99; −1.63) | 0.0100 |
| DLCO, % | 84.5 (76.0–93.5) 84.8 ± 14.5 | 77.5 (72.0–80.0) 76.6 ± 5.7 | 0.0100 | −8.71 (−14.28; −3.15) | 0.0030 |
| * | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragnoli, B.; Cena, T.; Pochetti, P.; Pignatti, P.; Malerba, M. Lung Involvement in Patients with Ulcerative Colitis: Relationship between Exhaled Nitric Oxide and Lung Function. J. Clin. Med. 2024, 13, 354. https://doi.org/10.3390/jcm13020354
Ragnoli B, Cena T, Pochetti P, Pignatti P, Malerba M. Lung Involvement in Patients with Ulcerative Colitis: Relationship between Exhaled Nitric Oxide and Lung Function. Journal of Clinical Medicine. 2024; 13(2):354. https://doi.org/10.3390/jcm13020354
Chicago/Turabian StyleRagnoli, Beatrice, Tiziana Cena, Patrizia Pochetti, Patrizia Pignatti, and Mario Malerba. 2024. "Lung Involvement in Patients with Ulcerative Colitis: Relationship between Exhaled Nitric Oxide and Lung Function" Journal of Clinical Medicine 13, no. 2: 354. https://doi.org/10.3390/jcm13020354
APA StyleRagnoli, B., Cena, T., Pochetti, P., Pignatti, P., & Malerba, M. (2024). Lung Involvement in Patients with Ulcerative Colitis: Relationship between Exhaled Nitric Oxide and Lung Function. Journal of Clinical Medicine, 13(2), 354. https://doi.org/10.3390/jcm13020354






