Transcatheter Aortic Valve Replacement: Current Status and Future Indications
Abstract
1. Introduction
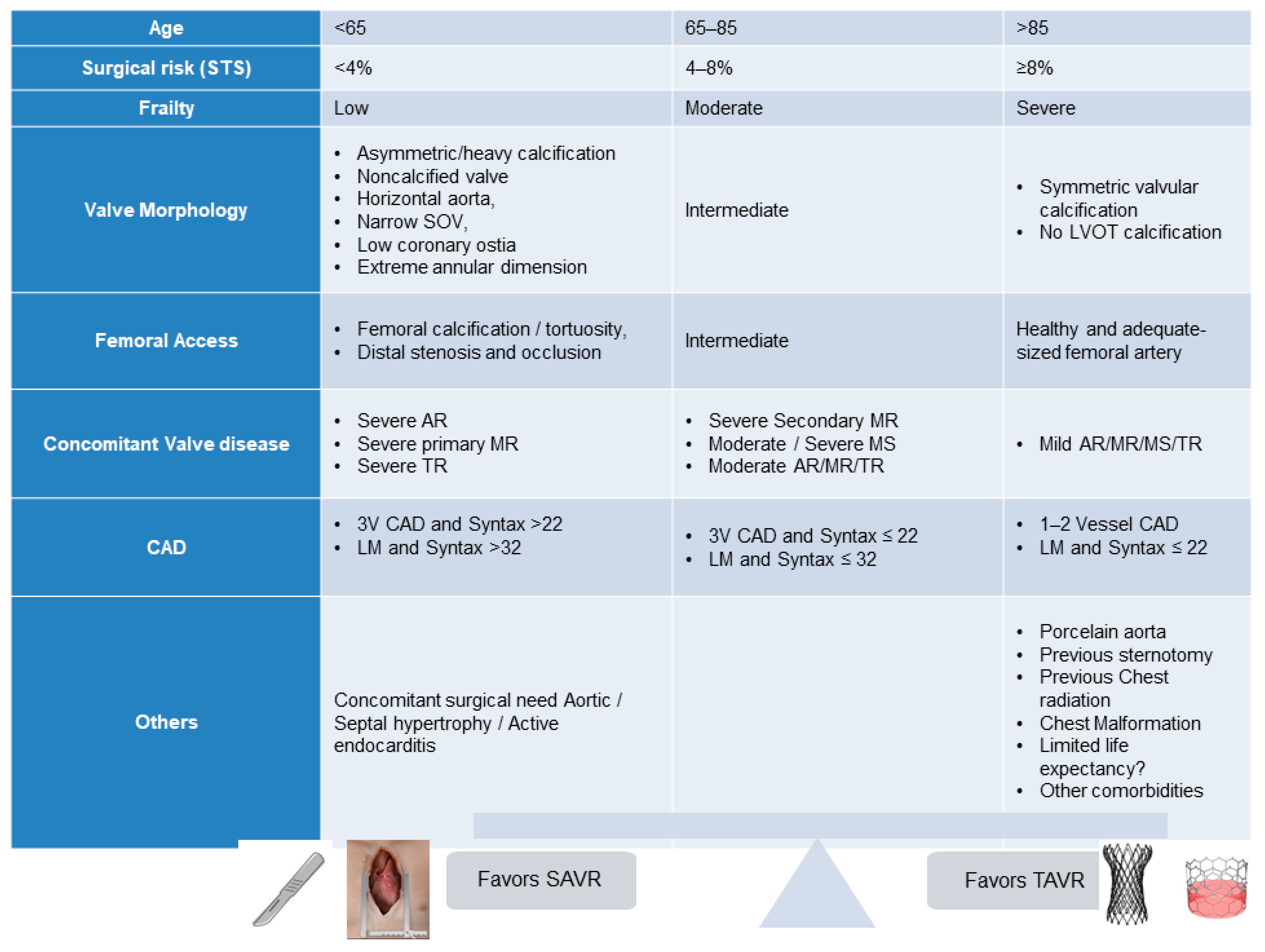
2. Current TAVR Indications
3. Current TAVR Devices
3.1. Self-Expanding Valves
3.2. Balloon-Expandable Valves
3.3. Mechanically Expandable Valves
4. Valve Durability
4.1. RESILIA Tissue
4.2. DurAVR THV System (Anteris Technologies)
5. TAVR in Bicuspid Valve Anatomy
6. Valve-in-Valve
6.1. Surgical Bioprosthetic Aortic Valves
6.2. Preprocedural and Procedural Aspects
6.3. Valve Sizing
6.4. Bioprosthetic Valve Fracture or Remodeling
6.5. Clinical Outcomes after ViV
7. Challenges Associated with TAVR
7.1. Stroke
7.2. Coronary Access
7.3. Coronary Obstruction Risk Evaluation and Management during Valve-in-Valve Procedures
8. Future Indications
8.1. Asymptomatic AS
8.2. Moderate AS
8.3. Aortic Regurgitation
9. Conclusions (VD)
Author Contributions
Funding
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Colombo, A.; Leone, P.P. Adoption of TAVR in Europe vs the United States: Is it Deja-Vù? J. Am. Coll. Cardiol. 2023, 82, 1903–1905. [Google Scholar] [CrossRef]
- Gupta, T.; Kolte, D.; Khera, S.; Goel, K.; Villablanca, P.A.; Kalra, A.; Abbott, J.D.; Elmariah, S.; Fonarow, G.C.; Rihal, C.S.; et al. The changing landscape of aortic valve replacement in the USA. EuroIntervention 2019, 15, e968–e974. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Windecker, S.; Okuno, T.; Unbehaun, A.; Mack, M.; Kapadia, S.; Falk, V. Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation? Eur. Heart J. 2022, 43, 2729–2750. [Google Scholar] [CrossRef]
- Zaid, S.; Attizzani, G.F.; Krishnamoorthy, P.; Yoon, S.-H.; Dallan, L.A.P.; Chetcuti, S.; Fukuhara, S.; Grossman, P.M.; Goel, S.S.; Atkins, M.D.; et al. First-in-Human Multicenter Experience of the Newest Generation Supra-Annular Self-Expanding Evolut FX TAVR System. JACC Cardiovasc. Interv. 2023, 16, 1626–1635. [Google Scholar] [CrossRef]
- Leone, P.P.; Regazzoli, D.; Pagnesi, M.; Cannata, F.; Mangieri, A.; Hokken, T.W.; Costa, G.; Barbanti, M.; Teles, R.C.; Adamo, M.; et al. Implantation of contemporary transcatheter aortic valves in small aortic annuli: The international multicentre TAVI-SMALL 2 registry. EuroIntervention 2023, 19, 256–266. [Google Scholar] [CrossRef]
- Leone, P.P.; Regazzoli, D.; Pagnesi, M.; Sanz-Sanchez, J.; Chiarito, M.; Cannata, F.; Van Mieghem, N.M.; Barbanti, M.; Tamburino, C.; Teles, R.; et al. Predictors and Clinical Impact of Prosthesis-Patient Mismatch after Self-Expandable TAVR in Small Annuli. JACC Cardiovasc. Interv. 2021, 14, 1218–1228. [Google Scholar] [CrossRef]
- Gooley, R.; Murdoch, D.; Ng, M.K.; Modolo, R.; Allocco, D.J. First results from the ACURATE Prime XL human feasibility study. Cardiovasc. Revascularization Med. 2023, 57, 1–5. [Google Scholar] [CrossRef]
- Bajoras, V.; Nuyens, P.; Vanhaverbeke, M.; Wang, X.; Bieliauskas, G.; De Backer, O.; Sondergaard, L. TAVR with the Novel Navitor TitanTM Transcatheter Heart Valve to Treat Aortic Stenosis Patients with Large Aortic Annuli. Cardiovasc. Revascularization Med. 2022, 40, 120–122. [Google Scholar] [CrossRef]
- Okuno, T.; Terauchi, K.; Kai, T.; Sato, Y.; Kuwata, S.; Koga, M.; Izumo, M.; Akashi, Y.J. Enhanced Hemodynamic Performance of a New-Generation 23-mm Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc. Interv. 2023, in press. [CrossRef]
- Zaid, S.; Atkins, M.D.; Kleiman, N.S.; Reardon, M.J.; Tang, G.H.L. What’s New with TAVR? An Update on Device Technology. Methodist DeBakey Cardiovasc. J. 2023, 19, 4–14. [Google Scholar] [CrossRef]
- Revaiah, P.C.; Jose, J.; Gunasekaran, S.; Mandalay, A.; Garg, S.; Bhatt, S.; Seth, A.; Soliman, O.; Onuma, Y.; Serruys, P.W. Neocommissural/Coronary Alignment with a Novel Balloon Expandable Transcatheter Aortic Valve: First-in-Human Experience. JACC Cardiovasc. Interv. 2023, 16, 2581–2583. [Google Scholar] [CrossRef]
- Kodali, S.K.; Sorajja, P.; Meduri, C.U.; Feldt, K.; Cavalcante, J.L.; Garg, P.; Hamid, N.; Poon, K.K.; Settergren, M.R.; Burns, M.R.; et al. Early safety and feasibility of a first-in-class biomimetic transcatheter aortic valve—DurAVR. EuroIntervention 2023, 19, e352–e362. [Google Scholar] [CrossRef]
- Daubert, M.A.; Weissman, N.J.; Hahn, R.T.; Pibarot, P.; Parvataneni, R.; Mack, M.J.; Svensson, L.G.; Gopal, D.; Kapadia, S.; Siegel, R.J.; et al. Long-Term Valve Performance of TAVR and SAVR: A Report from the PARTNER I Trial. JACC Cardiovasc. Imaging 2017, 10, 15–25. [Google Scholar] [CrossRef]
- Barbanti, M.; Petronio, A.S.; Ettori, F.; Latib, A.; Bedogni, F.; De Marco, F.; Poli, A.; Boschetti, C.; De Carlo, M.; Fiorina, C.; et al. 5-Year Outcomes after Transcatheter Aortic Valve Implantation with CoreValve Prosthesis. JACC Cardiovasc. Interv. 2015, 8, 1084–1091. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes from the Nordic Aortic Valve Intervention (NOTION) Randomized Clinical Trial in Lower Surgical Risk Patients. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years after Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; Tchétché, D.; Huang, J.; et al. 4-Year Outcomes of Patients with Aortic Stenosis in the Evolut Low Risk Trial. J. Am. Coll. Cardiol. 2023, 82, 2163–2165. [Google Scholar] [CrossRef]
- Jørgensen, T.H. Ten-Year Clinical and Echocardiographic Outcomes from the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. In Proceedings of the ESC Congress, Amsterdam, The Netherlands, 25–29 August 2023. [Google Scholar]
- Sathananthan, J.; Hensey, M.; Landes, U.; Alkhodair, A.; Saiduddin, A.; Sellers, S.; Cheung, A.; Lauck, S.; Blanke, P.; Leipsic, J.; et al. Long-Term Durability of Transcatheter Heart Valves: Insights From Bench Testing to 25 Years. JACC Cardiovasc. Interv. 2020, 13, 235–249. [Google Scholar] [CrossRef]
- Sathananthan, J.; Landes, U.; Flaction, L.; Humair, A.; Delaloye, S.; Toggweiler, S.; Søndergaard, L.; Wood, D.A.; Webb, J.G. Long-Term Durability of the Next-Generation Acurate neo 2 Transcatheter Heart Valve: Insights from Bench Testing to 25 Years. JACC Cardiovasc. Interv. 2021, 14, 586–588. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Griffith, B.; Heimansohn, D.A.; Rozanski, J.; Johnston, D.R.; Bartus, K.; Girardi, L.N.; Beaver, T.; Takayama, H.; Mumtaz, M.A.; et al. Five-year Outcomes of the COMMENCE Trial Investigating Aortic Valve Replacement With RESILIA Tissue. Ann. Thorac. Surg. 2023, 115, 1429–1436. [Google Scholar] [CrossRef]
- Roberts, W.C. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am. J. Cardiol. 1970, 26, 72–83. [Google Scholar] [CrossRef]
- Williams, M.R.; Jilaihawi, H.; Makkar, R.; O’neill, W.W.; Guyton, R.; Malaisrie, S.C.; Brown, D.L.; Blanke, P.; Leipsic, J.A.; Pibarot, P.; et al. The PARTNER 3 Bicuspid Registry for Transcatheter Aortic Valve Replacement in Low-Surgical-Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 523–532. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, W.-K.; Dhoble, A.; Pio, S.M.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Tarantini, G.; Dvir, D.; Tang, G.H. Transcatheter aortic valve implantation in degenerated surgical aortic valves. EuroIntervention 2021, 17, 709–719. [Google Scholar] [CrossRef]
- Ziccardi, M.R.; Groves, E.M. Bioprosthetic Valve Fracture for Valve-in-Valve Transcatheter Aortic Valve Replacement: Rationale, Patient Selection, Technique, and Outcomes. Interv. Cardiol. Clin. 2019, 8, 373–382. [Google Scholar]
- Bapat, V. Valve-in-valve apps: Why and how they were developed and how to use them. EuroIntervention 2014, 10, U44–U51. [Google Scholar] [CrossRef]
- Vinayak, M.; Tang, G.H.; Anastasius, M.; Moreno, P.; Dangas, G.D.; Kini, A.S.; Sharma, S.K.; Khera, S. Palliative Balloon Valvuloplasty for Late Stenosis of a Degenerated Transcatheter Heart Valve: Proof of Concept. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 101188. [Google Scholar] [CrossRef]
- Majmundar, M.; Doshi, R.; Kumar, A.; Johnston, D.; Brockett, J.; Kanaa’N, A.; Lahorra, J.L.; Svensson, L.S.; Krishnaswamy, A.; Reed, G.R.; et al. Valve-in-valve transcatheter aortic valve implantation versus repeat surgical aortic valve replacement in patients with a failed aortic bioprosthesis. EuroIntervention 2022, 17, 1227–1237. [Google Scholar] [CrossRef]
- Percy, E.D.; Harloff, M.T.; Hirji, S.; McGurk, S.; Yazdchi, F.; Newell, P.; Malarczyk, A.; Sabe, A.; Landes, U.; Webb, J.; et al. Nationally Representative Repeat Transcatheter Aortic Valve Replacement Outcomes: Report from the Centers for Medicare and Medicaid Services. JACC Cardiovasc. Interv. 2021, 14, 1717–1726. [Google Scholar] [CrossRef]
- Makkar, R.R.; Kapadia, S.; Chakravarty, T.; Cubeddu, R.J.; Kaneko, T.; Mahoney, P.; Patel, D.; Gupta, A.; Cheng, W.; Kodali, S.; et al. Outcomes of repeat transcatheter aortic valve replacement with balloon-expandable valves: A registry study. Lancet 2023, 402, 1529–1540. [Google Scholar] [CrossRef]
- Mendiz, O.A.; Noč, M.; Fava, C.M.; Jaikel, L.A.G.; Sztejfman, M.; Pleskovič, A.; Gamboa, P.; Valdivieso, L.R.; Gada, H.; Tang, G.H.L. Impact of Cusp-Overlap View for TAVR with Self-Expandable Valves on 30-Day Conduction Disturbances. J. Interv. Cardiol. 2021, 2021, 9991528. [Google Scholar] [CrossRef]
- Sammour, Y.; Banerjee, K.; Kumar, A.; Lak, H.; Chawla, S.; Incognito, C.; Patel, J.; Kaur, M.; Abdelfattah, O.; Svensson, L.G.; et al. Systematic Approach to High Implantation of SAPIEN-3 Valve Achieves a Lower Rate of Conduction Abnormalities Including Pacemaker Implantation. Circ. Cardiovasc. Interv. 2021, 14, 57–69. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Nazir, S.; Butala, N.M.; Bhatt, D.L.; Elmariah, S. Trends in Cerebral Embolic Protection Device Use and Association with Stroke following Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 152, 106–112. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; van Gils, L.; Ahmad, H.; van Kesteren, F.; van der Werf, H.W.; Brueren, G.; Storm, M.; Lenzen, M.; Daemen, J.; Heuvel, A.F.v.D.; et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: The randomised MISTRAL-C trial. EuroIntervention 2016, 12, 499–507. [Google Scholar] [CrossRef]
- Haussig, S.; Mangner, N.; Dwyer, M.G.; Lehmkuhl, L.; Lücke, C.; Woitek, F.; Holzhey, D.M.; Mohr, F.W.; Gutberlet, M.; Zivadinov, R.; et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: The CLEAN-TAVI randomized clinical trial. JAMA 2016, 316, 592–601. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef]
- Diaz, V.A.; Kapadia, S.R.; Linke, A.; Mylotte, D.; Lansky, A.J.; Grube, E.; Settergren, M.; Puri, R. Cerebral embolic protection during transcatheter heart interventions. EuroIntervention 2023, 19, 549–570. [Google Scholar] [CrossRef]
- Jagielak, D.; Targonski, R.; Frerker, C.; Abdel-Wahab, M.; Wilde, J.; Werner, N.; Lauterbach, M.; Leick, J.; Grygier, M.; Misterski, M.; et al. Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: The PROTEMBO C Trial. EuroIntervention 2022, 18, 590–597. [Google Scholar] [CrossRef]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.; Kini, A. Coronary Angiography and Percutaneous Coronary Intervention after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef]
- Tarantini, G.; Fovino, L.N.; Scotti, A.; Massussi, M.; Cardaioli, F.; Rodinò, G.; Benedetti, A.; Boiago, M.; Matsuda, Y.; Continisio, S.; et al. Coronary Access after Transcatheter Aortic Valve Replacement with Commissural Alignment: The ALIGN-ACCESS Study. Circ. Cardiovasc. Interv. 2022, 15, 206–217. [Google Scholar] [CrossRef]
- Tang, G.H.; Komatsu, I.; Tzemach, L.; Simonato, M.; Wolak, A.; Blanke, P.; Dvir, D. Risk of coronary obstruction and the need to perform BASILICA: The VIVID classification. EuroIntervention 2020, 16, e757–e759. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Kamioka, N.; Vavalle, J.P.; Lisko, J.C.; Gleason, P.T.; Paone, G.; Grubb, K.J.; Bruce, C.G.; Lederman, R.J.; Babaliaros, V.C. Balloon-Assisted BASILICA to Facilitate Redo TAVR. JACC Cardiovasc. Interv. 2021, 14, 578–580. [Google Scholar] [CrossRef]
- Prandi, F.R.; Granot, Y.N.; Margonato, D.; Belli, M.; Illuminato, F.; Vinayak, M.; Barillà, F.; Romeo, F.; Tang, G.H.L.; Sharma, S.; et al. Coronary Obstruction during Valve-in-Valve Transcatheter Aortic Valve Replacement: Pre-Procedural Risk Evaluation, Intra-Procedural Monitoring, and Follow-Up. J. Cardiovasc. Dev. Dis. 2023, 10, 187. [Google Scholar] [CrossRef]
- Banovic, M.; Putnik, S.; Penicka, M.; Doros, G.; Deja, M.A.; Kockova, R.; Kotrc, M.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: The AVATAR Trial. Circulation 2022, 145, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.J.; Lee, S.A.; Lee, S.; Kim, D.H.; Kim, H.K.; Yun, S.C.; Hong, G.R.; Song, J.M.; Chung, C.H.; et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Montaigne, D.; Aghezzaf, S.; Ridon, H.; Mouton, S.; Richardson, M.; Polge, A.S.; Lancellotti, P.; Bauters, C.; Abramovici, L.; et al. Association of Mortality with Aortic Stenosis Severity in Outpatients: Results from the VALVENOR Study. JAMA Cardiol. 2021, 6, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D. Poor Long-Term Survival in Patients with Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Gohlke-Bärwolf, C.; Minners, J.; Jander, N.; Gerdts, E.; Wachtell, K.; Ray, S.; Pedersen, T.R. Natural History of Mild and of Moderate Aortic Stenosis—New Insights From a Large Prospective European Study. Curr. Probl. Cardiol. 2013, 38, 365–409. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, E.; Frittitta, V.; Motta, S.; Strazzieri, O.; Valvo, R.; Reddavid, C.; Costa, G.; Tamburino, C. Treatment in patients with severe asymptomatic aortic stenosis: Is it best not to wait? Eur. Heart J. Suppl. 2022, 24, I170–I174. [Google Scholar] [CrossRef]
- Abdelfattah, O.M.; Krishnaswamy, A.; Kapadia, S.R. Cautious Optimism Regarding Early Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2022, 11, e026010. [Google Scholar] [CrossRef]
- Sawaya, F.J.; Deutsch, M.A.; Seiffert, M.; Yoon, S.H.; Codner, P.; Wickramarachchi, U.; Latib, A.; Petronio, A.S.; Rodés-Cabau, J.; Taramasso, M.; et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc. Interv. 2017, 10, 1048–1056. [Google Scholar] [CrossRef]
- Hira, R.S.; Vemulapalli, S.; Li, Z.; McCabe, J.M.; Rumsfeld, J.S.; Kapadia, S.R.; Alam, M.; Jneid, H.; Don, C.; Reisman, M.; et al. Trends and outcomes of off-label use of transcatheter aortic valve replacement: Insights from the NCDR STS/ACC TVT registry. JAMA Cardiol. 2017, 2, 846–854. [Google Scholar] [CrossRef]
- Vahl, T.; Makkar, R.; Kodali, S.; Baldus, S.; Treede, H.; Daniels, D.; Khalique, O.; Kempfert, J.; Waksman, R.; McCabe, J.; et al. 30-day outcomes of transfemoral transcatheter aortic valve replacement for aortic regurgitation with a novel self-expanding prosthesis. J. Am. Coll. Cardiol. 2021, 77, 919. [Google Scholar] [CrossRef]
- Shi, J.; Wei, L.; Chen, Y.; Wang, X.; Ye, J.; Qin, C.; Liu, L.; Qian, H.; Wang, C.; Guo, Y. Transcatheter Aortic Valve Implantation with J-Valve: 2-Year Outcomes from a Multicenter Study. Ann. Thorac. Surg. 2021, 111, 1530–1536. [Google Scholar] [CrossRef] [PubMed]


| 2020 ACC/AHA Guidelines [4] | 2021 ESC Guidelines [5] |
|---|---|
| Transfemoral TAVR is recommended over SAVR in patients aged >80 years or in younger patients with a life expectancy <10 years and no anatomic contraindication to transfemoral TAVR. (Class IA) | TAVR is recommended in older patients with age >75 years or those at high risk (STS PROM/EuroSCORE II > 8%) or unsuitable for surgery. (Class IA) |
| TAVR is recommended for patients with symptomatic severe AS aged 65–80 years and no anatomic contraindication to transfemoral TAVR. (Class IA) | SAVR or TAVR is recommended for remaining patients after taking into account of patient’s clinical, anatomic, and procedural characteristics. (Class IB) |
| Expansion Mechanism | Prosthesis | Valve Height (mm) | Leaflet Position | Frame Cell Size | Outer Seal | Access | Sheath | Delivery System | Repositionable | Retrievable | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID/OD (French) | Integrated | Expandable | OD (French) | Flexibility | Steerability | |||||||||
| SEV | CoreValve | 53–55 | Supra-annular | + | - | TV, TAo | Variable | - | Variable | 18 | - | - | + | + |
| Evolut R | 45–46 | Supra-annular | + | - | TV/TAo | 14/18, 16/20 (34 mm) | + | - | 14, 16 | - | - | + | + | |
| Evolut PRO | 45 | Supra-annular | + | + | TV/TAo | 16/20 | + | - | 16 | - | - | + | + | |
| Evolut PRO+ | 45–46 | Supra-annular | + | ++ | TV | 14/18, 16/20 (34 mm) | + | - | 14, 16 | - | - | + | + | |
| Acurate TA | 44–46 | Intra-annular | +++ | - | TA | - | - | - | 28 | - | - | - | - | |
| Acurate Neo | 48–51 | Supra-annular | +++ | + | TV, TA | 14/23 | - | + | 18 | + | - | - | - | |
| Acurate Neo 2 | 48–51 | Supra-annular | +++ | ++ | TV, TA | 14/23 | - | + | 14 | + | - | - | - | |
| Portico | 47–51 | Intra-annular | ++ | + | TV, TAo | 14/18, 15/19 (27, 29 mm) | + | - | 18, 19 | ++ | - | + | + | |
| Navitor | 47–48 | Intra-annular | ++ | ++ | TV | 14/18, 15/19 (27, 29, 35 mm) | + | - | 14, 15 | ++ | - | + | + | |
| Hydra | 51–55 | Supra-annular | +++ | + | TV | 18/NA | - | - | 18 | + | - | + | + | |
| Allegra | 37–43 | Supra-annular | + | - | TV | 18/20.4 | - | - | 18 | + | - | + | + | |
| JenaValve | NA | Supra-annular | +++ | - | TA | - | - | - | 32 | - | - | + | - | |
| Trilogy | NA | Supra-annular | ++ | + | TV | 18 | - | - | 18 | + | + | + | - | |
| J-Valve | NA | Intra-annular | NA | - | TV/TA | NA | - | - | 18 | - | + | - | - | |
| Venus-A | NA | Supra-annular | + | + | TV | NA | NA | NA | 19 | NA | NA | + | - | |
| VitaFlow | NA | Supra-annular | ++ | ++ | TV | NA | NA | NA | 16/18 | NA | NA | + | - | |
| TaurusOne | NA | Supra-annular | ++ | + | TV | NA | NA | NA | 18 | NA | NA | + | - | |
| BEV | Sapien | 14–16 | Intra-annular | + | - | TV/TA/TAo | 22/26, 24/28 (26 mm) | - | + | 22, 24 | - | - | - | - |
| Sapien XT | 14–19 | Intra-annular | + | - | TV/TA/TAo | 16/20, 18/22 (26 mm), 20/24 (29 mm) | - | + | 16, 18, 20 | - | - | - | - | |
| Sapien 3 | 15–22 | Intra-annular | ++ | + | TV/TA/TAo | 14/17.4, 16/20 (29 mm) | - | + | 18, 21 | - | + | - | - | |
| Sapien 3 Ultra | 15–20 | Intra-annular | ++ | ++ | TV | 14/17.4 | - | + | 18 | - | + | - | - | |
| Sapien 3 Ultra Resilia | 15–20 | Intra-annular | ++ | ++ | TV | TV | - | - | - | - | - | - | - | |
| Sapien X4 | 15–20 | Intra-annular | ++ | ++ | TV | TV | - | + | 18 | - | + | - | - | |
| MyVal | 17–21 | Intra-annular | ++ | ++ | TV | 14/17.4 | - | + | 14 | - | + | - | + | |
| MyVal | 17–21 | Intra-annular | ++ | ++ | TV | 14/17.4 | - | + | 14 | - | + | - | + | |
| MyVal Octacor | 17–21 | Intra-annular | ++ | ++ | TV | 14/17.4 | - | + | 14 | - | + | - | + | |
| MEV | Lotus | 19 | Intra-annular | + | ++ | TV, TAo | 18/22, 20/24 (25, 27 mm) | - | - | 18, 20 | - | - | ++ | ++ |
| Lotus Edge | 19 | Intra-annular | + | ++ | TV, TAo | 15/23.7 | - | + | 22 | + | - | ++ | ++ | |
 |  |  |  |  |  |  |  |  | |
|---|---|---|---|---|---|---|---|---|---|
| SENTINEL (Boston Scientific, Marlborough, MA, USA) | ProtEmbo (Protembis, Aachen, Germany) | TriGUARD 3 (Keystone Heart, Tampa, FL, USA) | Embrella (Edwards Lifesciences, Irvine, CA, USA) | Emblok (Innovative Cardiovascular Solutions, Grand Rapids, MI, USA) | Emboliner (Emboline, Santa Cruz, CA, USA) | Point-Guard (Transverse Medical Inc., Denver, CO, USA) | CAPTIS (Filterlex Medical, Caesarea, Israel) | FLOWer (Aortic Lab, Turin, Italy) | |
| Vessels covered | 2 vessels | 3 vessels | 3 vessels | 2 vessels | 3 vessels | 3 vessels | 3 vessels | 3 vessels | 3 vessels |
| Access site | Radial | Left Radial | Femoral | Radial | Femoral | Femoral | Femoral | Femoral | Femoral |
| Sheath size | 6 French | 6 French | 8 French | 6 French | 11 French | 10 French | 10 French | 16 French * | 12 French |
| Pore size (µm) | 140 | 60 | 145 | 100 | 100 | 150 | 105 | 115 × 145 | 70 |
| Mechanism | Capture | Deflector | Deflector | Deflector | Capture and removal | Capture and removal | Deflector | Capture and removal | Capture and removal |
| FDA approval/CE marked | FDA/CE | CE | CE | CE | SIH | CE | SIH | FIH | FIH |
| Anatomy | |
| STJ diameter | Narrow |
| SoV height | Short |
| SoV width | Narrow |
| Coronary height | Low |
| VTC | <4 mm |
| VTSTJ | <2.5 mm |
| Device/procedure | |
| THV type | Supra-annular |
| THV implantation depth | High |
| THV commissural alignment | Misalignment |
| Previous THV | |
| THV type | Tall stent frame, supra-annular |
| Index THV commissural alignment | Misalignment |
| Previous surgical valve | |
| Surgical valve type | Supra-annular |
| Surgical valve leaflet length | Long leaflets |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinayak, M.; Leone, P.P.; Tanner, R.; Dhulipala, V.; Camaj, A.; Makhija, R.R.K.; Hooda, A.; Kini, A.S.; Sharma, S.K.; Khera, S. Transcatheter Aortic Valve Replacement: Current Status and Future Indications. J. Clin. Med. 2024, 13, 373. https://doi.org/10.3390/jcm13020373
Vinayak M, Leone PP, Tanner R, Dhulipala V, Camaj A, Makhija RRK, Hooda A, Kini AS, Sharma SK, Khera S. Transcatheter Aortic Valve Replacement: Current Status and Future Indications. Journal of Clinical Medicine. 2024; 13(2):373. https://doi.org/10.3390/jcm13020373
Chicago/Turabian StyleVinayak, Manish, Pier Pasquale Leone, Richard Tanner, Vishal Dhulipala, Anton Camaj, Rakhee Rajendra Kumar Makhija, Amit Hooda, Annapoorna S. Kini, Samin K. Sharma, and Sahil Khera. 2024. "Transcatheter Aortic Valve Replacement: Current Status and Future Indications" Journal of Clinical Medicine 13, no. 2: 373. https://doi.org/10.3390/jcm13020373
APA StyleVinayak, M., Leone, P. P., Tanner, R., Dhulipala, V., Camaj, A., Makhija, R. R. K., Hooda, A., Kini, A. S., Sharma, S. K., & Khera, S. (2024). Transcatheter Aortic Valve Replacement: Current Status and Future Indications. Journal of Clinical Medicine, 13(2), 373. https://doi.org/10.3390/jcm13020373







