Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases
Abstract
:1. Introduction
2. Alzheimer’s Disease and Dementia
3. Rheumatoid Arthritis
4. Cancer
5. Chronic Pain
6. Depression and Anxiety
7. Epilepsy
8. Other Indications and Applications
9. “Patent Cliff” as an Incentive for Developing Drug + Digital Combination Therapies
10. Limitations of This Review
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef]
- Watanabe, J.H.; McInnis, T.; Hirsch, J.D. Cost of Prescription Drug-Related Morbidity and Mortality. Ann. Pharmacother. 2018, 52, 829–837. [Google Scholar] [CrossRef]
- Wang, C.; Lee, C.; Shin, H. Digital therapeutics from bench to bedside. NPJ Digit. Med. 2023, 6, 38. [Google Scholar] [CrossRef]
- Patel, N.A.; Butte, A.J. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit. Med. 2020, 3, 159. [Google Scholar] [CrossRef]
- Shuren, J.; Patel, B.; Gottlieb, S. FDA Regulation of Mobile Medical Apps. JAMA 2018, 320, 337–338. [Google Scholar] [CrossRef]
- Shafai, G.; Aungst, T.D. Prescription digital therapeutics: A new frontier for pharmacists and the future of treatment. J. Am. Pharm. Assoc. 2023, 63, 1030–1034. [Google Scholar] [CrossRef]
- Ribba, B.; Peck, R.; Hutchinson, L.; Bousnina, I.; Motti, D. Digital Therapeutics as a New Therapeutic Modality: A Review from the Perspective of Clinical Pharmacology. Clin. Pharmacol. Ther. 2023, 114, 578–590. [Google Scholar] [CrossRef]
- Abbadessa, G.; Brigo, F.; Clerico, M.; De Mercanti, S.; Trojsi, F.; Tedeschi, G.; Bonavita, S.; Lavorgna, L. Digital therapeutics in neurology. J. Neurol. 2022, 269, 1209–1224. [Google Scholar] [CrossRef]
- FDA. Combination Products. Available online: https://www.fda.gov/combination-products/about-combination-products/combination-product-definition-combination-product-types (accessed on 5 October 2023).
- FDA. Regulatory Considerations for Prescription Drug Use-Related Software. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-prescription-drug-use-related-software (accessed on 1 October 2023).
- Sverdlov, O.; van Dam, J.; Hannesdottir, K.; Thornton-Wells, T. Digital Therapeutics: An Integral Component of Digital Innovation in Drug Development. Clin. Pharmacol. Ther. 2018, 104, 72–80. [Google Scholar] [CrossRef]
- Bulaj, G. Combining non-pharmacological treatments with pharmacotherapies for neurological disorders: A unique interface of the brain, drug-device, and intellectual property. Front. Neurol. 2014, 5, 126. [Google Scholar] [CrossRef]
- Bulaj, G.; Clark, J.; Ebrahimi, M.; Bald, E. From Precision Metapharmacology to Patient Empowerment: Delivery of Self-Care Practices for Epilepsy, Pain, Depression and Cancer Using Digital Health Technologies. Front. Pharmacol. 2021, 12, 612602. [Google Scholar] [CrossRef]
- Rafiei, R.; Williams, C.; Jiang, J.; Aungst, T.D.; Durrer, M.; Tran, D.; Howald, R. Digital Health Integration Assessment and Maturity of the United States Biopharmaceutical Industry: Forces Driving the Next Generation of Connected Autoinjectable Devices. JMIR mHealth uHealth 2021, 9, e25406. [Google Scholar] [CrossRef]
- Maricich, Y.A.; Gerwien, R.; Kuo, A.; Malone, D.C.; Velez, F.F. Real-world use and clinical outcomes after 24 weeks of treatment with a prescription digital therapeutic for opioid use disorder. Hosp. Pract. 2021, 49, 348–355. [Google Scholar] [CrossRef]
- Kawasaki, S.; Mills-Huffnagle, S.; Aydinoglo, N.; Maxin, H.; Nunes, E. Patient-and provider-reported experiences of a Mobile Novel Digital Therapeutic in People with Opioid Use Disorder (reSET-O): Feasibility and acceptability study. JMIR Form. Res. 2022, 6, e33073. [Google Scholar] [CrossRef]
- Maricich, Y.A.; Bickel, W.K.; Marsch, L.A.; Gatchalian, K.; Botbyl, J.; Luderer, H.F. Safety and efficacy of a prescription digital therapeutic as an adjunct to buprenorphine for treatment of opioid use disorder. Curr. Med. Res. Opin. 2021, 37, 167–173. [Google Scholar] [CrossRef]
- Giravi, H.Y.; Biskupiak, Z.; Tyler, L.S.; Bulaj, G. Adjunct Digital Interventions Improve Opioid-Based Pain Management: Impact of Virtual Reality and Mobile Applications on Patient-Centered Pharmacy Care. Front. Digit. Health 2022, 4, 884047. [Google Scholar] [CrossRef]
- Al-Arkee, S.; Mason, J.; Lane, D.A.; Fabritz, L.; Chua, W.; Haque, M.S.; Jalal, Z. Mobile Apps to Improve Medication Adherence in Cardiovascular Disease: Systematic Review and Meta-analysis. J. Med. Internet Res. 2021, 23, e24190. [Google Scholar] [CrossRef]
- Cazeau, N. Mobile Health Interventions: Examining Medication Adherence Outcomes Among Patients With Cancer. Clin. J. Oncol. Nurs. 2021, 25, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Afra, P.; Bruggers, C.S.; Sweney, M.; Fagatele, L.; Alavi, F.; Greenwald, M.; Huntsman, M.; Nguyen, K.; Jones, J.K.; Shantz, D.; et al. Mobile Software as a Medical Device (SaMD) for the Treatment of Epilepsy: Development of Digital Therapeutics Comprising Behavioral and Music-Based Interventions for Neurological Disorders. Front. Hum. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Metcalf, C.S.; Huntsman, M.; Garcia, G.; Kochanski, A.K.; Chikinda, M.; Watanabe, E.; Underwood, T.; Vanegas, F.; Smith, M.D.; White, H.S.; et al. Music-Enhanced Analgesia and Antiseizure Activities in Animal Models of Pain and Epilepsy: Toward Preclinical Studies Supporting Development of Digital Therapeutics and Their Combinations with Pharmaceutical Drugs. Front. Neurol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Rohaj, A.; Bulaj, G. Digital Therapeutics (DTx) Expand Multimodal Treatment Options for Chronic Low Back Pain: The Nexus of Precision Medicine, Patient Education, and Public Health. Healthcare 2023, 11, 1469. [Google Scholar] [CrossRef]
- McGowan, R. Digital Combination Products and Software. In The Combination Products Handbook; CRC Press: Boca Raton, FL, USA, 2023; pp. 425–440. [Google Scholar]
- Bodner, K.A.; Goldberg, T.E.; Devanand, D.P.; Doraiswamy, P.M. Advancing Computerized Cognitive Training for MCI and Alzheimer’s Disease in a Pandemic and Post-pandemic World. Front. Psychiatry 2020, 11, 557571. [Google Scholar] [CrossRef]
- Leisher, S.; Bohorquez, A.; Gay, M.; Garcia, V.; Jones, R.; Baldaranov, D.; Rafii, M.S. Amyloid-Lowering Monoclonal Antibodies for the Treatment of Early Alzheimer’s Disease. CNS Drugs 2023, 37, 671–677. [Google Scholar] [CrossRef]
- Dickson, S.P.; Hennessey, S.; Nicodemus Johnson, J.; Knowlton, N.; Hendrix, S.B. Avoiding future controversies in the Alzheimer’s disease space through understanding the aducanumab data and FDA review. Alzheimer’s Res. Ther. 2023, 15, 98. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Rashad, A.; Rasool, A.; Shaheryar, M.; Sarfraz, A.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare 2022, 11, 32. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Tijms, B.M. Treatments for AD: Towards the right target at the right time. Nat. Rev. Neurol. 2023, 19, 581–582. [Google Scholar] [CrossRef]
- Loeffler, D.A. Antibody-Mediated Clearance of Brain Amyloid-β: Mechanisms of Action, Effects of Natural and Monoclonal Anti-Aβ Antibodies, and Downstream Effects. J. Alzheimer’s Dis. Rep. 2023, 7, 873–899. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Peng, Y.; Jin, H.; Xue, Y.H.; Chen, Q.; Yao, S.Y.; Du, M.Q.; Liu, S. Current and future therapeutic strategies for Alzheimer’s disease: An overview of drug development bottlenecks. Front. Aging Neurosci. 2023, 15, 1206572. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, A.O.; Alotaibi, M. The role of M-health applications in the fight against Alzheimer’s: Current and future directions. Mhealth 2018, 4, 32. [Google Scholar] [CrossRef]
- Al-Salah, R.; Salam, A.; Alzamil, M.; Alaskr, R.; Alyemni, M.; Alahmdi, M.; Alqahtani, B. Thakirni Application: An Assistive Application for Alzheimer Patients. Int. J. Online Biomed. Eng. 2020, 16, 121–131. [Google Scholar] [CrossRef]
- Øksnebjerg, L.; Woods, B.; Ruth, K.; Lauridsen, A.; Kristiansen, S.; Holst, H.D.; Waldemar, G. A tablet app supporting self-management for people with dementia: Explorative study of adoption and use patterns. JMIR mHealth uHealth 2020, 8, e14694. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S.; Wang, J. Digital health for aging populations. Nat. Med. 2023, 29, 1623–1630. [Google Scholar] [CrossRef]
- Shuren, J.; Doraiswamy, P. Digital therapeutics for MCI and Alzheimer’s disease: A regulatory perspective—Highlights From The Clinical Trials on Alzheimer’s Disease conference (CTAD). J. Prev. Alzheimer’s Dis. 2022, 9, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Sposaro, F.; Danielson, J.; Tyson, G. iWander: An Android application for dementia patients. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3875–3878. [Google Scholar]
- Sakamoto, M.; Guo, Y.P.; Wong, K.L.Y.; Mann, J.; Berndt, A.; Boger, J.; Currie, L.; Raber, C.; Egeberg, E.; Burke, C. Co-design of a digital app “WhatMatters” to support person-centred care: A critical reflection. Int. J. Geriatr. Psychiatry 2023, 38, e6014. [Google Scholar] [CrossRef]
- Anthony Berauk, V.L.; Murugiah, M.K.; Soh, Y.C.; Chuan Sheng, Y.; Wong, T.W.; Ming, L.C. Mobile health applications for caring of older people: Review and comparison. Ther. Innov. Regul. Sci. 2018, 52, 374–382. [Google Scholar] [CrossRef]
- Tak, S.H. In Quest of Tablet Apps for Elders With Alzheimer’s Disease: A Descriptive Review. Comput. Inform. Nurs. 2021, 39, 347–354. [Google Scholar] [CrossRef]
- Kuo, H.L.; Chang, C.H.; Ma, W.F. A Survey of Mobile Apps for the Care Management of Patients with Dementia. Healthcare 2022, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Clay, F.; Howett, D.; FitzGerald, J.; Fletcher, P.; Chan, D.; Price, A. Use of Immersive Virtual Reality in the Assessment and Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2020, 75, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Ambegaonkar, A.; Ritchie, C.; de la Fuente Garcia, S. The Use of Mobile Applications as Communication Aids for People with Dementia: Opportunities and Limitations. J. Alzheimer’s Dis. Rep. 2021, 5, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Cammisuli, D.M.; Cipriani, G.; Castelnuovo, G. Technological Solutions for Diagnosis, Management and Treatment of Alzheimer’s Disease-Related Symptoms: A Structured Review of the Recent Scientific Literature. Int. J. Environ. Res. Public Health 2022, 19, 3122. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Gamito, P.; Souto, T.; Conde, R.; Ferreira, M.; Corotnean, T.; Fernandes, A.; Silva, H.; Neto, T. Virtual Reality-Based Cognitive Stimulation on People with Mild to Moderate Dementia due to Alzheimer’s Disease: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 5290. [Google Scholar] [CrossRef] [PubMed]
- Son, C.; Park, J.H. Ecological Effects of VR-Based Cognitive Training on ADL and IADL in MCI and AD patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 5875. [Google Scholar] [CrossRef]
- Marin, A.; DeCaro, R.; Schiloski, K.; Elshaar, A.; Dwyer, B.; Vives-Rodriguez, A.; Palumbo, R.; Turk, K.; Budson, A. Home-Based Electronic Cognitive Therapy in Patients With Alzheimer Disease: Feasibility Randomized Controlled Trial. JMIR Form. Res. 2022, 6, e34450. [Google Scholar] [CrossRef]
- Rai, H.K.; Kernaghan, D.; Schoonmade, L.; Egan, K.J.; Pot, A.M. Digital Technologies to Prevent Social Isolation and Loneliness in Dementia: A Systematic Review. J. Alzheimer’s Dis. 2022, 90, 513–528. [Google Scholar] [CrossRef]
- Iso-Markku, P.; Kujala, U.M.; Knittle, K.; Polet, J.; Vuoksimaa, E.; Waller, K. Physical activity as a protective factor for dementia and Alzheimer’s disease: Systematic review, meta-analysis and quality assessment of cohort and case-control studies. Br. J. Sports Med. 2022, 56, 701–709. [Google Scholar] [CrossRef]
- Papatsimpas, V.; Vrouva, S.; Papathanasiou, G.; Papadopoulou, M.; Bouzineki, C.; Kanellopoulou, S.; Moutafi, D.; Bakalidou, D. Does Therapeutic Exercise Support Improvement in Cognitive Function and Instrumental Activities of Daily Living in Patients with Mild Alzheimer’s Disease? A Randomized Controlled Trial. Brain Sci. 2023, 13, 1112. [Google Scholar] [CrossRef]
- Bleibel, M.; El Cheikh, A.; Sadier, N.S.; Abou-Abbas, L. The effect of music therapy on cognitive functions in patients with Alzheimer’s disease: A systematic review of randomized controlled trials. Alzheimer’s Res. Ther. 2023, 15, 65. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cui, C.; Zhang, H.; Jia, L.; Li, R.; Hu, H.Y. Exploration of combined physical activity and music for patients with Alzheimer’s disease: A systematic review. Front. Aging Neurosci. 2022, 14, 962475. [Google Scholar] [CrossRef] [PubMed]
- Prinz, A.; Schumacher, A.; Witte, K. Changes in Selected Cognitive and Motor Skills as Well as the Quality of Life After a 24-Week Multidimensional Music-Based Exercise Program in People With Dementia. Am. J. Alzheimer’s Dis. Other Demen 2023, 38, 15333175231191022. [Google Scholar] [CrossRef]
- Jung, Y.H.; Park, S.C.; Lee, J.H.; Kim, M.J.; Lee, S.; Chung, S.J.; Moon, J.Y.; Choi, Y.H.; Ju, J.; Han, H.J.; et al. Effect of internet-based vs. in-person multimodal interventions on patients with mild to moderate Alzheimer’s disease: A randomized, cross-over, open-label trial. Front. Public Health 2023, 11, 1203201. [Google Scholar] [CrossRef]
- Shepherd, A.; Zhang, T.D.; Zeleznikow-Johnston, A.M.; Hannan, A.J.; Burrows, E.L. Transgenic Mouse Models as Tools for Understanding How Increased Cognitive and Physical Stimulation Can Improve Cognition in Alzheimer’s Disease. Brain Plast. 2018, 4, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Stazi, M.; Wirths, O. Physical activity and cognitive stimulation ameliorate learning and motor deficits in a transgenic mouse model of Alzheimer’s disease. Behav. Brain Res. 2021, 397, 112951. [Google Scholar] [CrossRef]
- Jin, X.; Li, T.; Zhang, L.; Ma, J.; Yu, L.; Li, C.; Niu, L. Environmental Enrichment Improves Spatial Learning and Memory in Vascular Dementia Rats with Activation of Wnt/β-Catenin Signal Pathway. Med. Sci. Monit. 2017, 23, 207–215. [Google Scholar] [CrossRef]
- Zhou, T.; Lin, L.; Hao, C.; Liao, W. Environmental enrichment rescues cognitive impairment with suppression of TLR4-p38MAPK signaling pathway in vascular dementia rats. Neurosci. Lett. 2020, 737, 135318. [Google Scholar] [CrossRef]
- Cao, M.; Hu, P.P.; Zhang, Y.L.; Yan, Y.X.; Shields, C.B.; Zhang, Y.P.; Hu, G.; Xiao, M. Enriched physical environment reverses spatial cognitive impairment of socially isolated APPswe/PS1dE9 transgenic mice before amyloidosis onset. CNS Neurosci. Ther. 2018, 24, 202–211. [Google Scholar] [CrossRef]
- Zhang, S.S.; Zhu, L.; Peng, Y.; Zhang, L.; Chao, F.L.; Jiang, L.; Xiao, Q.; Liang, X.; Tang, J.; Yang, H.; et al. Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice. J. Neuroinflammation 2022, 19, 34. [Google Scholar] [CrossRef]
- Gholami, J.S.N.S.; Rajabian, A.; Saburi, E.; Hajali, V. The effect of combination pretreatment of donepezil and environmental enrichment on memory deficits in amyloid-beta-induced Alzheimer-like rat model. Biochem. Biophys. Rep. 2022, 32, 101392. [Google Scholar] [CrossRef]
- Nakai, T.; Yamada, K.; Mizoguchi, H. Alzheimer’s Disease Animal Models: Elucidation of Biomarkers and Therapeutic Approaches for Cognitive Impairment. Int. J. Mol. Sci. 2021, 22, 5549. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, F. Does Experience Enhance Cognitive Flexibility? An Overview of the Evidence Provided by the Environmental Enrichment Studies. Front. Behav. Neurosci. 2019, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Liew, A.K.Y.; Teo, C.H.; Soga, T. The Molecular Effects of Environmental Enrichment on Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 7095–7118. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Sánchez-Flórez, J.C.; Seija-Butnaru, D.; Valero, E.G.; Acosta, C.; Amaya, S. Pain Management Strategies in Rheumatoid Arthritis: A Narrative Review. J. Pain Palliat. Care Pharmacother. 2021, 35, 291–299. [Google Scholar] [CrossRef]
- Albert, D.A. Are All Biologics the Same? Optimal Treatment Strategies for Patients With Early Rheumatoid Arthritis: Systematic Review and Indirect Pairwise Meta-Analysis. J. Clin. Rheumatol. 2015, 21, 398–404. [Google Scholar] [CrossRef]
- Findeisen, K.E.; Sewell, J.; Ostor, A.J.K. Biological Therapies for Rheumatoid Arthritis: An Overview for the Clinician. Biologics 2021, 15, 343–352. [Google Scholar] [CrossRef]
- Adas, M.A.; Allen, V.B.; Yates, M.; Bechman, K.; Clarke, B.D.; Russell, M.D.; Rutherford, A.I.; Cope, A.P.; Norton, S.; Galloway, J.B. A systematic review and network meta-analysis of the safety of early interventional treatments in rheumatoid arthritis. Rheumatology 2021, 60, 4450–4462. [Google Scholar] [CrossRef]
- Lorig, K.; Ritter, P.L.; Plant, K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis Rheum. 2005, 53, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Mollard, E.; Michaud, K. Mobile Apps for Rheumatoid Arthritis: Opportunities and Challenges. Rheum. Dis. Clin. N. Am. 2019, 45, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Mollard, E.; Michaud, K. Self-Management of Rheumatoid Arthritis: Mobile Applications. Curr. Rheumatol. Rep. 2020, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Gossec, L.; Weill, C.; Benoist, D.; Berenbaum, F.; Nikiphorou, E. Mobile Health Apps for Self-Management of Rheumatic and Musculoskeletal Diseases: Systematic Literature Review. JMIR mHealth uHealth 2019, 7, e14730. [Google Scholar] [CrossRef] [PubMed]
- Fedkov, D.; Berghofen, A.; Weiss, C.; Peine, C.; Lang, F.; Knitza, J.; Kuhn, S.; Krämer, B.K.; Leipe, J. Efficacy and safety of a mobile app intervention in patients with inflammatory arthritis: A prospective pilot study. Rheumatol. Int. 2022, 42, 2177–2190. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Sanchez-Laulhe, P.; Luque-Romero, L.G.; Barrero-Garcia, F.J.; Biscarri-Carbonero, A.; Blanquero, J.; Suero-Pineda, A.; Heredia-Rizo, A.M. An Exercise and Educational and Self-management Program Delivered With a Smartphone App (CareHand) in Adults With Rheumatoid Arthritis of the Hands: Randomized Controlled Trial. JMIR mHealth uHealth 2022, 10, e35462. [Google Scholar] [CrossRef]
- Elnady, B.; Abd-Elmaksoud, S.; El Miedany, O.; El Miedany, Y. AB0325 Development of a Mobile App for Disease-Specific Cognitive Behavior Therapy Management in Individuals with Rheumatoid Arthritis. Ann. Rheum. Dis. 2023, 82, 1346. [Google Scholar]
- Luo, D.; Wang, P.; Lu, F.; Elias, J.; Sparks, J.A.; Lee, Y.C. Mobile Apps for Individuals With Rheumatoid Arthritis: A Systematic Review. J. Clin. Rheumatol. 2019, 25, 133–141. [Google Scholar] [CrossRef]
- Grainger, R.; Townsley, H.; White, B.; Langlotz, T.; Taylor, W.J. Apps for People With Rheumatoid Arthritis to Monitor Their Disease Activity: A Review of Apps for Best Practice and Quality. JMIR mHealth uHealth 2017, 5, e7. [Google Scholar] [CrossRef]
- Bearne, L.M.; Sekhon, M.; Grainger, R.; La, A.; Shamali, M.; Amirova, A.; Godfrey, E.L.; White, C.M. Smartphone Apps Targeting Physical Activity in People With Rheumatoid Arthritis: Systematic Quality Appraisal and Content Analysis. JMIR mHealth uHealth 2020, 8, e18495. [Google Scholar] [CrossRef]
- Cozad, M.J.; Crum, M.; Tyson, H.; Fleming, P.R.; Stratton, J.; Kennedy, A.B.; Lindley, L.C.; Horner, R.D. Mobile Health Apps for Patient-Centered Care: Review of United States Rheumatoid Arthritis Apps for Engagement and Activation. JMIR mHealth uHealth 2022, 10, e39881. [Google Scholar] [CrossRef] [PubMed]
- Mollard, E.; Michaud, K. A Mobile App With Optical Imaging for the Self-Management of Hand Rheumatoid Arthritis: Pilot Study. JMIR mHealth uHealth 2018, 6, e12221. [Google Scholar] [CrossRef] [PubMed]
- Labinsky, H.; Gupta, L.; Raimondo, M.G.; Schett, G.; Knitza, J. Real-world usage of digital health applications (DiGA) in rheumatology: Results from a German patient survey. Rheumatol. Int. 2023, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.G.; Chehab, G.; Stachwitz, P.; Hagen, J.; Larsen, D.; Knitza, J.; Schneider, M.; Voormann, A.; Specker, C. One year of digital health applications (DiGA) in Germany—Rheumatologists’ perspectives. Front. Med. 2022, 9, 1000668. [Google Scholar] [CrossRef]
- Roodenrijs, N.M.T.; Hamar, A.; Kedves, M.; Nagy, G.; van Laar, J.M.; van der Heijde, D.; Welsing, P.M.J. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: A systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open 2021, 7, e001512. [Google Scholar] [CrossRef]
- Taylor, P.C.; Van de Laar, M.; Laster, A.; Fakhouri, W.; Quebe, A.; de la Torre, I.; Jain, S. Call for action: Incorporating wellness practices into a holistic management plan for rheumatoid arthritis-going beyond treat to target. RMD Open 2021, 7, e001959. [Google Scholar] [CrossRef]
- Majnik, J.; Császár-Nagy, N.; Böcskei, G.; Bender, T.; Nagy, G. Non-pharmacological treatment in difficult-to-treat rheumatoid arthritis. Front. Med. 2022, 9, 991677. [Google Scholar] [CrossRef]
- Thorlund, J.B.; Simic, M.; Pihl, K.; Berthelsen, D.B.; Day, R.; Koes, B.; Juhl, C.B. Similar Effects of Exercise Therapy, Nonsteroidal Anti-inflammatory Drugs, and Opioids for Knee Osteoarthritis Pain: A Systematic Review with Network Meta-analysis. J. Orthop. Sports Phys. Ther. 2022, 52, 207–216. [Google Scholar] [CrossRef]
- Derue, H.; Ribeiro-da-Silva, A. Therapeutic exercise interventions in rat models of arthritis. Neurobiol. Pain 2023, 13, 100130. [Google Scholar] [CrossRef]
- Kito, T.; Teranishi, T.; Nishii, K.; Sakai, K.; Matsubara, M.; Yamada, K. Effectiveness of exercise-induced cytokines in alleviating arthritis symptoms in arthritis model mice. Okajimas Folia Anat. Jpn. 2016, 93, 81–88. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; López-Loeza, S.M.; Acosta-Jiménez, S.; Cuevas-Martínez, R.; Pacheco-Silva, C.; Chaparro-Barrera, E.; Pacheco-Tena, C. Low-Intensity Physical Exercise Decreases Inflammation and Joint Damage in the Preclinical Phase of a Rheumatoid Arthritis Murine Model. Biomolecules 2023, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Andonian, B.J.; Abraham, D.M.; Bareja, A.; Lee, D.E.; Katz, L.H.; Huebner, J.L.; Kraus, W.E.; White, J.P. Exercise protects against cardiac and skeletal muscle dysfunction in a mouse model of inflammatory arthritis. J. Appl. Physiol. 2021, 130, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Estrázulas, M.; Freitas, R.D.S.; Käfer, E.T.; Dagino, A.P.A.; Campos, M.M. Central and peripheral effects of environmental enrichment in a mouse model of arthritis. Int. Immunopharmacol. 2022, 102, 108386. [Google Scholar] [CrossRef] [PubMed]
- Bruggers, C.S.; Altizer, R.A.; Kessler, R.R.; Caldwell, C.B.; Coppersmith, K.; Warner, L.; Davies, B.; Paterson, W.; Wilcken, J.; D’Ambrosio, T.A.; et al. Patient-empowerment interactive technologies. Sci. Transl. Med. 2012, 4, 152ps116. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.; Bowen, R.C.; German, M.L.; Bulaj, G.; Bruggers, C.S. Clinical and Neurobiological Perspectives of Empowering Pediatric Cancer Patients Using Videogames. Games Health J. 2015, 4, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.H.; Go, K.; Go, K.; McKinley, N.J.; Dougherty, K.R.; You, K.L.; Lee, Y.J. Empowerment through technology: A systematic evaluation of the content and quality of mobile applications to empower individuals with cancer. Int. J. Med. Inform. 2022, 163, 104782. [Google Scholar] [CrossRef] [PubMed]
- Graetz, I.; Hu, X.; Curry, A.N.; Robles, A.; Vidal, G.A.; Schwartzberg, L.S. Mobile application to support oncology patients during treatment on patient outcomes: Evidence from a randomized controlled trial. Cancer Med. 2023, 12, 6190–6199. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, S.; Kim, S.H.; Yoo, S.H.; Sung, J.H.; Oh, E.G.; Kim, N.; Lee, J. Digital Health Interventions for Adult Patients With Cancer Evaluated in Randomized Controlled Trials: Scoping Review. J. Med. Internet Res. 2023, 25, e38333. [Google Scholar] [CrossRef]
- Wasserman, S.; Ould Brahim, L.; Attiya, A.; Belzile, E.; Lambert, S.D. An Evaluation of Interactive mHealth Applications for Adults Living with Cancer. Curr. Oncol. 2023, 30, 7151–7166. [Google Scholar] [CrossRef]
- Katsaros, D.; Hawthorne, J.; Patel, J.; Pothier, K.; Aungst, T.; Franzese, C. Optimizing Social Support in Oncology with Digital Platforms. JMIR Cancer 2022, 8, e36258. [Google Scholar] [CrossRef]
- Beale, I.L.; Kato, P.M.; Marin-Bowling, V.M.; Guthrie, N.; Cole, S.W. Improvement in cancer-related knowledge following use of a psychoeducational video game for adolescents and young adults with cancer. J. Adolesc. Health 2007, 41, 263–270. [Google Scholar] [CrossRef]
- Kato, P.M.; Cole, S.W.; Bradlyn, A.S.; Pollock, B.H. A video game improves behavioral outcomes in adolescents and young adults with cancer: A randomized trial. Pediatrics 2008, 122, e305–e317. [Google Scholar] [CrossRef]
- Bruggers, C.S.; Baranowski, S.; Beseris, M.; Leonard, R.; Long, D.; Schulte, E.; Shorter, A.; Stigner, R.; Mason, C.C.; Bedrov, A.; et al. A Prototype Exercise-Empowerment Mobile Video Game for Children With Cancer, and Its Usability Assessment: Developing Digital Empowerment Interventions for Pediatric Diseases. Front. Pediatr. 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Iivanainen, S.; Alanko, T.; Vihinen, P.; Konkola, T.; Ekstrom, J.; Virtanen, H.; Koivunen, J. Follow-Up of Cancer Patients Receiving Anti-PD-(L)1 Therapy Using an Electronic Patient-Reported Outcomes Tool (KISS): Prospective Feasibility Cohort Study. JMIR Form. Res. 2020, 4, e17898. [Google Scholar] [CrossRef] [PubMed]
- Lijnsvelt, J.; Addeo, A.; Vitale, M.; Mohr, P.; Queirolo, P.; Ekström, J.; Vainio, J.; Kataja, V.; Calado, F.; Fagan, A. 840P Electronic patient-reported outcomes (ePROs) of adults with BRAF V600–mutant stage III-IV melanoma treated with dabrafenib+ trametinib (D+ T) collected using the Kaiku Health digital patient (pt) monitoring platform. Ann. Oncol. 2022, 33, S933–S934. [Google Scholar] [CrossRef]
- Schmalz, O.; Jacob, C.; Ammann, J.; Liss, B.; Iivanainen, S.; Kammermann, M.; Koivunen, J.; Klein, A.; Popescu, R.A. Digital Monitoring and Management of Patients With Advanced or Metastatic Non-Small Cell Lung Cancer Treated With Cancer Immunotherapy and Its Impact on Quality of Clinical Care: Interview and Survey Study Among Health Care Professionals and Patients. J. Med. Internet Res. 2020, 22, e18655. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Yossi, S.; Septans, A.L.; Charron, A.; Voog, E.; Dupuis, O.; Ganem, G.; Pointreau, Y.; Letellier, C. Improving Survival in Patients Treated for a Lung Cancer Using Self-Evaluated Symptoms Reported Through a Web Application. Am. J. Clin. Oncol. 2017, 40, 464–469. [Google Scholar] [CrossRef]
- Gussoni, G.; Ravot, E.; Zecchina, M.; Recchia, G.; Santoro, E.; Ascione, R.; Perrone, F. Digital therapeutics in oncology: Findings, barriers and prospects. A narrative review. Ann. Res. Oncol. 2022, 2, 55–69. [Google Scholar] [CrossRef]
- Shaffer, K.M.; Turner, K.L.; Siwik, C.; Gonzalez, B.D.; Upasani, R.; Glazer, J.V.; Ferguson, R.J.; Joshua, C.; Low, C.A. Digital health and telehealth in cancer care: A scoping review of reviews. Lancet Digit. Health 2023, 5, e316–e327. [Google Scholar] [CrossRef]
- Graetz, I.; McKillop, C.N.; Stepanski, E.; Vidal, G.A.; Anderson, J.N.; Schwartzberg, L.S. Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: A randomized controlled feasibility trial. J. Cancer Surviv. 2018, 12, 431–440. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, X.; Weng, L.; Guo, L.; Xu, H.; Lin, M.; Xue, Y.; Lin, X.; Yang, A.; Yu, L.; et al. Benefits of Mobile Apps for Cancer Pain Management: Systematic Review. JMIR mHealth uHealth 2020, 8, e17055. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, F.; Gu, J.J.; Wang, Y.K.; Hua, H.; Li, J.; Cheng, Z.; Liao, Z.; Huang, Q.; Hu, W.; et al. Development and Testing of an Intelligent Pain Management System (IPMS) on Mobile Phones Through a Randomized Trial Among Chinese Cancer Patients: A New Approach in Cancer Pain Management. JMIR mHealth uHealth 2017, 5, e108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weng, L.; Chen, Z.; Cai, H.; Lin, X.; Hu, Z.; Li, N.; Lin, B.; Zheng, B.; Zhuang, Q.; et al. Development and Testing of a Mobile App for Pain Management Among Cancer Patients Discharged From Hospital Treatment: Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e12542. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, J.E.; Cheng, A.S.K.; Cheng, H.; Wefel, J.S. Meta-Analysis of the Efficacy of Virtual Reality-Based Interventions in Cancer-Related Symptom Management. Integr. Cancer Ther. 2019, 18, 1534735419871108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Zhang, Z.X.; Zhang, Q. Efficacy of virtual reality-based interventions for patients with breast cancer symptom and rehabilitation management: A systematic review and meta-analysis. BMJ Open 2022, 12, e051808. [Google Scholar] [CrossRef] [PubMed]
- Gautama, M.S.N.; Huang, T.-W.; Haryani, H. A systematic review and meta-analysis of randomized controlled trials on the effectiveness of immersive virtual reality in cancer patients receiving chemotherapy. Eur. J. Oncol. Nurs. 2023, 67, 102424. [Google Scholar] [CrossRef]
- Ester, M.; Eisele, M.; Wurz, A.; McDonough, M.H.; McNeely, M.; Culos-Reed, S.N. Current Evidence and Directions for Future Research in eHealth Physical Activity Interventions for Adults Affected by Cancer: Systematic Review. JMIR Cancer 2021, 7, e28852. [Google Scholar] [CrossRef]
- Purdy, G.M.; Venner, C.P.; Tandon, P.; McNeely, M.L. Feasibility of a tailored and virtually supported home exercise program for people with multiple myeloma using a novel eHealth application. Digit. Health 2022, 8, 20552076221129066. [Google Scholar] [CrossRef]
- Purdy, G.M.; Sobierajski, F.M.; Al Onazi, M.M.; Effa, C.J.; Venner, C.P.; Tandon, P.; McNeely, M.L. Exploring participant perceptions of a virtually supported home exercise program for people with multiple myeloma using a novel eHealth application: A qualitative study. Support. Care Cancer 2023, 31, 298. [Google Scholar] [CrossRef]
- Robertson, M.C.; Tsai, E.; Lyons, E.J.; Srinivasan, S.; Swartz, M.C.; Baum, M.L.; Basen-Engquist, K.M. Mobile Health Physical Activity Intervention Preferences in Cancer Survivors: A Qualitative Study. JMIR mHealth uHealth 2017, 5, e3. [Google Scholar] [CrossRef]
- Hong, Y.; Dahlke, D.V.; Ory, M.; Hochhalter, A.; Reynolds, J.; Purcell, N.P.; Talwar, D.; Eugene, N. Designing iCanFit: A Mobile-Enabled Web Application to Promote Physical Activity for Older Cancer Survivors. JMIR Res. Protoc. 2013, 2, e12. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Goldberg, D.; Ory, M.G.; Towne, S.D., Jr.; Forjuoh, S.N.; Kellstedt, D.; Wang, S. Efficacy of a Mobile-Enabled Web App (iCanFit) in Promoting Physical Activity Among Older Cancer Survivors: A Pilot Study. JMIR Cancer 2015, 1, e7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ryu, S.; Zhou, W.; Adams, K.; Hassan, M.; Zhang, R.; Blaes, A.; Wolfson, J.; Sun, J. Effects of personalized exercise prescriptions and social media delivered through mobile health on cancer survivors’ physical activity and quality of life. J. Sport. Health Sci. 2023, 12, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, S.; Lin, S.; Han, H. Effect of music therapy on pain behaviors in rats with bone cancer pain. J. BUON 2016, 21, 466–472. [Google Scholar] [PubMed]
- Bice, B.D.; Stephens, M.R.; Georges, S.J.; Venancio, A.R.; Bermant, P.C.; Warncke, A.V.; Affolter, K.E.; Hidalgo, J.R.; Angus-Hill, M.L. Environmental Enrichment Induces Pericyte and IgA-Dependent Wound Repair and Lifespan Extension in a Colon Tumor Model. Cell Rep. 2017, 19, 760–773. [Google Scholar] [CrossRef]
- Foglesong, G.D.; Queen, N.J.; Huang, W.; Widstrom, K.J.; Cao, L. Enriched environment inhibits breast cancer progression in obese models with intact leptin signaling. Endocr. Relat. Cancer 2019, 26, 483–495. [Google Scholar] [CrossRef]
- Takai, T.; Abe, A.; Miura, H.; Tanaka, S.; Komura, J. Minimum environmental enrichment is effective in activating antitumor immunity to transplanted tumor cells in mice. Exp. Anim. 2019, 68, 569–576. [Google Scholar] [CrossRef]
- Li, G.; Gan, Y.; Fan, Y.; Wu, Y.; Lin, H.; Song, Y.; Cai, X.; Yu, X.; Pan, W.; Yao, M.; et al. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci. Rep. 2015, 5, 7856. [Google Scholar] [CrossRef]
- Yang, L.; Morielli, A.R.; Heer, E.; Kirkham, A.A.; Cheung, W.Y.; Usmani, N.; Friedenreich, C.M.; Courneya, K.S. Effects of Exercise on Cancer Treatment Efficacy: A Systematic Review of Preclinical and Clinical Studies. Cancer Res. 2021, 81, 4889–4895. [Google Scholar] [CrossRef]
- Eschke, R.K.; Lampit, A.; Schenk, A.; Javelle, F.; Steindorf, K.; Diel, P.; Bloch, W.; Zimmer, P. Impact of Physical Exercise on Growth and Progression of Cancer in Rodents-A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 35. [Google Scholar] [CrossRef]
- Martín-Ruiz, A.; Fiuza-Luces, C.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Gálvez, B.G.; Martínez-Martínez, E.; Martín-Acosta, P.; Coronado, M.J.; Franco-Luzón, L.; González-Murillo, Á. Benefits of exercise and immunotherapy in a murine model of human non-small-cell lung carcinoma. Exerc. Immunol. Rev. 2020, 26, 100–115. [Google Scholar]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 2022, 40, 720–737.e725. [Google Scholar] [CrossRef] [PubMed]
- Brummer, C.; Pukrop, T.; Wiskemann, J.; Bruss, C.; Ugele, I.; Renner, K. Can Exercise Enhance the Efficacy of Checkpoint Inhibition by Modulating Anti-Tumor Immunity? Cancers 2023, 15, 4668. [Google Scholar] [CrossRef] [PubMed]
- Jeyabal, P.; Bhagat, A.; Wang, F.; Roth, M.; Livingston, J.A.; Gilchrist, S.C.; Banchs, J.; Hildebrandt, M.A.T.; Chandra, J.; Deswal, A.; et al. Circulating microRNAs and cytokines as prognostic biomarkers for doxorubicin-induced cardiac injury and for evaluating the effectiveness of an exercise intervention. Clin. Cancer Res. 2023, 29, 4430–4440. [Google Scholar] [CrossRef] [PubMed]
- Berrueta, L.; Bergholz, J.; Munoz, D.; Muskaj, I.; Badger, G.J.; Shukla, A.; Kim, H.J.; Zhao, J.J.; Langevin, H.M. Stretching Reduces Tumor Growth in a Mouse Breast Cancer Model. Sci. Rep. 2018, 8, 7864. [Google Scholar] [CrossRef]
- Canali, M.M.; Guyot, M.; Simon, T.; Daoudlarian, D.; Chabry, J.; Panzolini, C.; Petit-Paitel, A.; Hypolite, N.; Nicolas, S.; Bourdely, P.; et al. Environmental signals perceived by the brain abate pro-metastatic monocytes by dampening glucocorticoids receptor signaling. Cancer Cell Int. 2023, 23, 15. [Google Scholar] [CrossRef]
- Nunez, M.J.; Mana, P.; Linares, D.; Riveiro, M.P.; Balboa, J.; Suarez-Quintanilla, J.; Maracchi, M.; Mendez, M.R.; Lopez, J.M.; Freire-Garabal, M. Music, immunity and cancer. Life Sci. 2002, 71, 1047–1057. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Lerman, S.F.; Rudich, Z.; Brill, S.; Shalev, H.; Shahar, G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom. Med. 2015, 77, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Vowles, K.E.; McEntee, M.L.; Julnes, P.S.; Frohe, T.; Ney, J.P.; Van Der Goes, D.N. Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 2015, 156, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Viswanath, O.; Peck, J.; Kaye, A.D.; Gill, J.S.; Simopoulos, T.T. A Brief History of the Opioid Epidemic and Strategies for Pain Medicine. Pain Ther. 2018, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, W. Multimodal non-invasive non-pharmacological therapies for chronic pain: Mechanisms and progress. BMC Med. 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Garza-Villarreal, E.A.; Pando, V.; Vuust, P.; Parsons, C. Music-Induced Analgesia in Chronic Pain Conditions: A Systematic Review and Meta-Analysis. Pain Physician 2017, 20, 597–610. [Google Scholar] [CrossRef]
- Sihvonen, A.J.; Pitkäniemi, A.; Särkämö, T.; Soinila, S. Isn’t There Room for Music in Chronic Pain Management? J. Pain Off. J. Am. Pain Soc. 2022, 23, 1143–1150. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Stewart, S.A.; Bagg, M.K.; Stanojevic, S.; Yamato, T.P.; Saragiotto, B.T. Some types of exercise are more effective than others in people with chronic low back pain: A network meta-analysis. J. Physiother. 2021, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.M. Chronic Musculoskeletal Pain: Nonpharmacologic, Noninvasive Treatments. Am. Fam. Physician 2020, 102, 465–477. [Google Scholar]
- Hochheim, M.; Ramm, P.; Amelung, V. The effectiveness of low-dosed outpatient biopsychosocial interventions compared to active physical interventions on pain and disability in adults with nonspecific chronic low back pain: A systematic review with meta-analysis. Pain Pract. 2023, 23, 409–436. [Google Scholar] [CrossRef]
- Bilika, P.; Karampatsou, N.; Stavrakakis, G.; Paliouras, A.; Theodorakis, Y.; Strimpakos, N.; Kapreli, E. Virtual Reality-Based Exercise Therapy for Patients with Chronic Musculoskeletal Pain: A Scoping Review. Healthcare 2023, 11, 2412. [Google Scholar] [CrossRef]
- Bulaj, G.; Ahern, M.M.; Kuhn, A.; Judkins, Z.S.; Bowen, R.C.; Chen, Y. Incorporating Natural Products, Pharmaceutical Drugs, Self-care and Digital/Mobile Health Technologies into Molecular-Behavioral Combination Therapies for Chronic Diseases. Curr. Clin. Pharmacol. 2016, 11, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.G.; Doctor, J.N.; Patterson, D.R.; Carrougher, G.J.; Furness, T.A., 3rd. Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain 2000, 85, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.G.; Seibel, E.J.; Richards, T.L.; Furness, T.A.; Patterson, D.R.; Sharar, S.R. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J. Pain 2006, 7, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.G.; Patterson, D.R.; Seibel, E.; Soltani, M.; Jewett-Leahy, L.; Sharar, S.R. Virtual reality pain control during burn wound debridement in the hydrotank. Clin. J. Pain 2008, 24, 299–304. [Google Scholar] [CrossRef]
- Priebe, J.A.; Haas, K.K.; Moreno Sanchez, L.F.; Schoefmann, K.; Utpadel-Fischler, D.A.; Stockert, P.; Thoma, R.; Schiessl, C.; Kerkemeyer, L.; Amelung, V.; et al. Digital Treatment of Back Pain versus Standard of Care: The Cluster-Randomized Controlled Trial, Rise-uP. J. Pain Res. 2020, 13, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.M.; Birckhead, B.J.; Krishnamurthy, P.; Sackman, J.; Mackey, I.G.; Louis, R.G.; Salmasi, V.; Maddox, T.; Darnall, B.D. An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19. J. Med. Internet Res. 2021, 23, e26292. [Google Scholar] [CrossRef]
- Maddox, T.; Oldstone, L.; Sparks, C.Y.; Sackman, J.; Oyao, A.; Garcia, L.; Maddox, R.U.; Ffrench, K.; Garcia, H.; Adair, T.; et al. In-Home Virtual Reality Program for Chronic Lower Back Pain: A Randomized Sham-Controlled Effectiveness Trial in a Clinically Severe and Diverse Sample. Mayo Clin. Proc. Digit. Health 2023, 1, 563–573. [Google Scholar] [CrossRef]
- Toelle, T.R.; Utpadel-Fischler, D.A.; Haas, K.K.; Priebe, J.A. App-based multidisciplinary back pain treatment versus combined physiotherapy plus online education: A randomized controlled trial. npj Digit. Med. 2019, 2, 34. [Google Scholar] [CrossRef]
- Priebe, J.A.; Utpadel-Fischler, D.; Toelle, T.R. Less pain, better sleep? The effect of a multidisciplinary back pain app on sleep quality in individuals suffering from back pain–a secondary analysis of app user data. J. Pain Res. 2020, 13, 1121–1128. [Google Scholar] [CrossRef]
- Chuan, A.; Zhou, J.J.; Hou, R.M.; Stevens, C.J.; Bogdanovych, A. Virtual reality for acute and chronic pain management in adult patients: A narrative review. Anaesthesia 2021, 76, 695–704. [Google Scholar] [CrossRef]
- Nagpal, A.S.; Raghunandan, A.; Tata, F.; Kibler, D.; McGeary, D. Virtual Reality in the Management of Chronic Low Back Pain: A Scoping Review. Front. Pain Res. 2022, 3, 856935. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.; Mayne, A.; Hood, B. Virtual Reality-Based Mindfulness for Chronic Pain Management: A Scoping Review. Pain Manag. Nurs. 2022, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Won, A.S.; Bailey, J.; Bailenson, J.; Tataru, C.; Yoon, I.A.; Golianu, B. Immersive Virtual Reality for Pediatric Pain. Children 2017, 4, 52. [Google Scholar] [CrossRef]
- Pfeifer, A.C.; Uddin, R.; Schröder-Pfeifer, P.; Holl, F.; Swoboda, W.; Schiltenwolf, M. Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness. J. Clin. Med. 2020, 9, 3557. [Google Scholar] [CrossRef]
- Shetty, A.; Delanerolle, G.; Zeng, Y.; Shi, J.Q.; Ebrahim, R.; Pang, J.; Hapangama, D.; Sillem, M.; Shetty, S.; Shetty, B.; et al. A systematic review and meta-analysis of digital application use in clinical research in pain medicine. Front. Digit. Health 2022, 4, 850601. [Google Scholar] [CrossRef]
- Darnall, B.D.; Edwards, K.A.; Courtney, R.E.; Ziadni, M.S.; Simons, L.E.; Harrison, L.E. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front. Pain Res. 2023, 4, 1223172. [Google Scholar] [CrossRef]
- Jain, D.; Norman, K.; Werner, Z.; Makovoz, B.; Baker, T.; Huber, S. Using postmarket surveillance to assess safety-related events in a digital rehabilitation app (Kaia App): Observational study. JMIR Hum. Factors 2021, 8, e25453. [Google Scholar] [CrossRef]
- Ma, K.P.K.; Stephens, K.A.; Geyer, R.E.; Prado, M.G.; Mollis, B.L.; Zbikowski, S.M.; Waters, D.; Masterson, J.; Zhang, Y. Developing Digital Therapeutics for Chronic Pain in Primary Care: A Qualitative Human-Centered Design Study of Providers’ Motivations and Challenges. JMIR Form. Res. 2023, 7, e41788. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.; Gholamrezaei, A.; McNeilage, A.G.; Dwyer, L.; Sim, A.; Ferreira, M.; Darnall, B.; Glare, P.; Ashton-James, C. Evaluating acceptability and feasibility of a mobile health intervention to improve self-efficacy in prescription opioid tapering in patients with chronic pain: Protocol for a pilot randomised, single-blind, controlled trial. BMJ Open 2022, 12, e057174. [Google Scholar] [CrossRef]
- Magee, M.R.; McNeilage, A.G.; Avery, N.; Glare, P.; Ashton-James, C.E. mHealth Interventions to Support Prescription Opioid Tapering in Patients With Chronic Pain: Qualitative Study of Patients’ Perspectives. JMIR Form. Res. 2021, 5, e25969. [Google Scholar] [CrossRef]
- White, R.; Bruggink, L.; Hayes, C.; Boyes, A.; Paul, C. Feasibility of patient-focused behavioral interventions to support adults experiencing chronic noncancer pain during opioid tapering: A systematic literature review. Transl. Behav. Med. 2021, 11, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; DiCenso-Fleming, T.; Ensign, T.; Boyd, A.J.; Monaghan, G.; Binder, D.S. Chronic pain education delivered with a virtual reality headset in outpatient physical therapy clinics: A multi-site exploratory trial. Am. J. Transl. Res. 2023, 15, 3500–3510. [Google Scholar]
- Azizoddin, D.R.; Adam, R.; Kessler, D.; Wright, A.A.; Kematick, B.; Sullivan, C.; Zhang, H.; Hassett, M.J.; Cooley, M.E.; Ehrlich, O. Leveraging mobile health technology and research methodology to optimize patient education and self-management support for advanced cancer pain. Support. Care Cancer 2021, 29, 5741–5751. [Google Scholar] [CrossRef] [PubMed]
- Howlin, C.; Rooney, B. The Cognitive Mechanisms in Music Listening Interventions for Pain: A Scoping Review. J. Music. Ther. 2020, 57, 127–167. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Chen, K.M.; Belcastro, F. The effect of music interventions on chronic pain experienced by older adults: A systematic review. J. Nurs. Scholarsh. 2022, 54, 64–71. [Google Scholar] [CrossRef]
- Lee, J.H. The Effects of Music on Pain: A Meta-Analysis. J. Music. Ther. 2016, 53, 430–477. [Google Scholar] [CrossRef]
- Lin, C.L.; Hwang, S.L.; Jiang, P.; Hsiung, N.H. Effect of Music Therapy on Pain After Orthopedic Surgery-A Systematic Review and Meta-Analysis. Pain Pract. 2020, 20, 422–436. [Google Scholar] [CrossRef]
- Lunde, S.J.; Vuust, P.; Garza-Villarreal, E.A.; Vase, L. Music-induced analgesia: How does music relieve pain? Pain 2018, 160, 989–993. [Google Scholar] [CrossRef]
- Chai, P.R.; Carreiro, S.; Ranney, M.L.; Karanam, K.; Ahtisaari, M.; Edwards, R.; Schreiber, K.L.; Ben-Ghaly, L.; Erickson, T.B.; Boyer, E.W. Music as an Adjunct to Opioid-Based Analgesia. J. Med. Toxicol. 2017, 13, 249–254. [Google Scholar] [CrossRef]
- Chai, P.R.; Schreiber, K.L.; Taylor, S.W.; Jambaulikar, G.D.; Kikut, A.; Hasdianda, M.A.; Boyer, E.W. The Feasibility and Acceptability of a Smartphone-Based Music Intervention for Acute Pain. Proc. Annu. Hawaii Int. Conf. Syst. Sci. 2019, 2019, 3917–3925. [Google Scholar]
- Chen, Q.Y.; Wan, J.; Wang, M.; Hong, S.; Zhuo, M. Sound-induced analgesia cannot always be observed in adult mice. Mol. Pain 2023, 19, 17448069231197158. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, C.; Wang, H.; Mao, Y.; Zhang, W.; Liu, A.; Yang, C.L.; Li, T.; Hayashi, L.; Zhao, W.; et al. Sound induces analgesia through corticothalamic circuits. Science 2022, 377, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, D.; Lou, W. Music alleviates pain perception in depression mouse models by promoting the release of glutamate in the hippocampus of mice to act on GRIK5. Nucleosides Nucleotides Nucleic Acids 2022, 41, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Senba, E.; Kami, K. A new aspect of chronic pain as lifestyle-related disease. Neurobiol. Pain 2017, 1, 6–15. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Patwardhan, A.; Gilbraith, K.B.; Moutal, A.; Yang, X.; Chew, L.A.; Largent-Milnes, T.; Malan, T.P.; Vanderah, T.W.; Porreca, F.; et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain 2017, 158, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Martin, L.F.; Slepian, M.J.; Patwardhan, A.M.; Ibrahim, M.M. Mechanisms and Pathways of Pain Photobiomodulation: A Narrative Review. J. Pain Off. J. Am. Pain Soc. 2021, 22, 763–777. [Google Scholar] [CrossRef]
- Martin, L.F.; Cheng, K.; Washington, S.M.; Denton, M.; Goel, V.; Khandekar, M.; Largent-Milnes, T.M.; Patwardhan, A.; Ibrahim, M.M. Green Light Exposure Elicits Anti-inflammation, Endogenous Opioid Release and Dampens Synaptic Potentiation to Relieve Post-surgical Pain. J. Pain Off. J. Am. Pain Soc. 2023, 24, 509–529. [Google Scholar] [CrossRef]
- Martin, L.F.; Moutal, A.; Cheng, K.; Washington, S.M.; Calligaro, H.; Goel, V.; Kranz, T.; Largent-Milnes, T.M.; Khanna, R.; Patwardhan, A.; et al. Green Light Antinociceptive and Reversal of Thermal and Mechanical Hypersensitivity Effects Rely on Endogenous Opioid System Stimulation. J. Pain Off. J. Am. Pain Soc. 2021, 22, 1646–1656. [Google Scholar] [CrossRef]
- Tai, L.W.; Yeung, S.C.; Cheung, C.W. Enriched Environment and Effects on Neuropathic Pain: Experimental Findings and Mechanisms. Pain Pract. 2018, 18, 1068–1082. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Jia, M.; Xie, Z.; Yang, J.; Shen, J.; Zhou, Z. Environmental enrichment improves pain sensitivity, depression-like phenotype, and memory deficit in mice with neuropathic pain: Role of NPAS4. Psychopharmacology 2019, 236, 1999–2014. [Google Scholar] [CrossRef]
- Tai, W.L.; Sun, L.; Li, H.; Gu, P.; Joosten, E.A.; Cheung, C.W. Additive Effects of Environmental Enrichment and Ketamine on Neuropathic Pain Relief by Reducing Glutamatergic Activation in Spinal Cord Injury in Rats. Front. Neurosci. 2021, 15, 635187. [Google Scholar] [CrossRef] [PubMed]
- Sadegzadeh, F.; Sakhaie, N.; Isazadehfar, K.; Saadati, H. Effects of exposure to enriched environment during adolescence on passive avoidance memory, nociception, and prefrontal BDNF level in adult male and female rats. Neurosci. Lett. 2020, 732, 135133. [Google Scholar] [CrossRef] [PubMed]
- Kimura, L.F.; Sant’Anna, M.B.; Zambelli, V.O.; Giardini, A.C.; Jared, S.G.S.; Antoniazzi, M.M.; de Moura Mattaraia, V.G.; Pagano, R.L.; Picolo, G. Early exposure to environmental enrichment protects male rats against neuropathic pain development after nerve injury. Exp. Neurol. 2020, 332, 113390. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Case, L.K.; Ceko, M.; Cotton, V.A.; Gracely, J.L.; Low, L.A.; Pitcher, M.H.; Villemure, C. Effect of environment on the long-term consequences of chronic pain. Pain 2015, 156 (Suppl. S1), S42–S49. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.N.; Xia, M. Enriched environment alleviates adolescent visceral pain, anxiety- and depression-like behaviors induced by neonatal maternal separation. Transl. Pediatr. 2022, 11, 1398–1407. [Google Scholar] [CrossRef]
- Cobos, E.J.; Ghasemlou, N.; Araldi, D.; Segal, D.; Duong, K.; Woolf, C.J. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 2012, 153, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Morgan, M.M. ‘Reinventing the wheel’ to advance the development of pain therapeutics. Behav. Pharmacol. 2021, 32, 142–152. [Google Scholar] [CrossRef]
- Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [CrossRef]
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef]
- Read, J.; Williams, J. Adverse effects of antidepressants reported by a large international cohort: Emotional blunting, suicidality, and withdrawal effects. Curr. Drug Saf. 2018, 13, 176–186. [Google Scholar] [CrossRef]
- Guina, J.; Merrill, B. Benzodiazepines I: Upping the care on downers: The evidence of risks, benefits and alternatives. J. Clin. Med. 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Chong, H.Y.; Chaiyakunapruk, N.; Tangiisuran, B.; Jacob, S.A. Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: A systematic review. J. Affect. Disord. 2016, 193, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Filteau, M.J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Quero, S.; Noma, H.; Ciharova, M.; Miguel, C.; Karyotaki, E.; Cipriani, A.; Cristea, I.A.; Furukawa, T.A. Psychotherapies for depression: A network meta-analysis covering efficacy, acceptability and long-term outcomes of all main treatment types. World Psychiatry 2021, 20, 283–293. [Google Scholar] [CrossRef]
- Wei, W.; Sambamoorthi, U.; Olfson, M.; Walkup, J.T.; Crystal, S. Use of psychotherapy for depression in older adults. Am. J. Psychiatry 2005, 162, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Bartram, M.; Stewart, J.M. Income-based inequities in access to psychotherapy and other mental health services in Canada and Australia. Health Policy 2019, 123, 45–50. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Greenleaf, W.; Bulaj, G.; Taylor, S.T.; Mitsi, G.; Saliu, D.; Czysz, A.; Silvesti, G.; Garcia, M.; Jain, R. Digital health technologies and major depressive disorder. CNS Spectr. 2023, 28, 662–673. [Google Scholar] [CrossRef]
- Wang, K.; Varma, D.S.; Prosperi, M. A systematic review of the effectiveness of mobile apps for monitoring and management of mental health symptoms or disorders. J. Psychiatr. Res. 2018, 107, 73–78. [Google Scholar] [CrossRef]
- Roepke, A.M.; Jaffee, S.R.; Riffle, O.M.; McGonigal, J.; Broome, R.; Maxwell, B. Randomized Controlled Trial of SuperBetter, a Smartphone-Based/Internet-Based Self-Help Tool to Reduce Depressive Symptoms. Games Health J. 2015, 4, 235–246. [Google Scholar] [CrossRef]
- Merry, S.N.; Stasiak, K.; Shepherd, M.; Frampton, C.; Fleming, T.; Lucassen, M.F. The effectiveness of SPARX, a computerised self help intervention for adolescents seeking help for depression: Randomised controlled non-inferiority trial. BMJ 2012, 344, e2598. [Google Scholar] [CrossRef]
- Lecomte, T.; Potvin, S.; Corbière, M.; Guay, S.; Samson, C.; Cloutier, B.; Francoeur, A.; Pennou, A.; Khazaal, Y. Mobile apps for mental health issues: Meta-review of meta-analyses. JMIR mHealth uHealth 2020, 8, e17458. [Google Scholar] [CrossRef] [PubMed]
- Litke, S.G.; Resnikoff, A.; Anil, A.; Montgomery, M.; Matta, R.; Huh-Yoo, J.; Daly, B.P. Mobile Technologies for Supporting Mental Health in Youths: Scoping Review of Effectiveness, Limitations, and Inclusivity. JMIR Ment. Health 2023, 10, e46949. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Krieger, T.; Sude, K.; Meyer, B.; Maercker, A. Evaluating an e-mental health program (“deprexis”) as adjunctive treatment tool in psychotherapy for depression: Results of a pragmatic randomized controlled trial. J. Affect. Disord. 2018, 227, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Twomey, C.; O’Reilly, G.; Bültmann, O.; Meyer, B. Effectiveness of a tailored, integrative Internet intervention (deprexis) for depression: Updated meta-analysis. PLoS ONE 2020, 15, e0228100. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Berger, T.; Caspar, F.; Beevers, C.; Andersson, G.; Weiss, M. Effectiveness of a novel integrative online treatment for depression (Deprexis): Randomized controlled trial. J. Med. Internet Res. 2009, 11, e1151. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, V.N.; Crosthwaite, P.C.; Hall, S.A.; Flannery, J.E.; Strauss, G.S.; Vierra, E.M.; Koepsell, X.L.; Lake, J.I.; Padmanabhan, A. A CBT-based mobile intervention as an adjunct treatment for adolescents with symptoms of depression: A virtual randomized controlled feasibility trial. Front. Digit. Health 2023, 5, 1062471. [Google Scholar] [CrossRef] [PubMed]
- Keller, O.C.; Budney, A.J.; Struble, C.A.; Teepe, G.W. Blending digital therapeutics within the healthcare system. In Digital Therapeutics for Mental Health and Addiction; Elsevier: Amsterdam, The Netherlands, 2023; pp. 45–64. [Google Scholar]
- Kraemer, L.V.; Gruenzig, S.-D.; Baumeister, H.; Ebert, D.D.; Bengel, J. Effectiveness of a guided web-based intervention to reduce depressive symptoms before outpatient psychotherapy: A pragmatic randomized controlled trial. Psychother. Psychosom. 2021, 90, 233–242. [Google Scholar] [CrossRef]
- Carl, J.R.; Miller, C.B.; Henry, A.L.; Davis, M.L.; Stott, R.; Smits, J.A.J.; Emsley, R.; Gu, J.; Shin, O.; Otto, M.W.; et al. Efficacy of digital cognitive behavioral therapy for moderate-to-severe symptoms of generalized anxiety disorder: A randomized controlled trial. Depress. Anxiety 2020, 37, 1168–1178. [Google Scholar] [CrossRef]
- O’Daffer, A.; Colt, S.F.; Wasil, A.R.; Lau, N. Efficacy and conflicts of interest in randomized controlled trials evaluating Headspace and calm apps: Systematic review. JMIR Ment. Health 2022, 9, e40924. [Google Scholar] [CrossRef]
- Birney, A.J.; Gunn, R.; Russell, J.K.; Ary, D.V. MoodHacker Mobile Web App With Email for Adults to Self-Manage Mild-to-Moderate Depression: Randomized Controlled Trial. JMIR mHealth uHealth 2016, 4, e8. [Google Scholar] [CrossRef]
- Twomey, C.; O’Reilly, G. Effectiveness of a freely available computerised cognitive behavioural therapy programme (MoodGYM) for depression: Meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Twomey, C.; O’Reilly, G.; Byrne, M.; Bury, M.; White, A.; Kissane, S.; McMahon, A.; Clancy, N. A randomized controlled trial of the computerized CBT programme, MoodGYM, for public mental health service users waiting for interventions. Br. J. Clin. Psychol. 2014, 53, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, N.; Chitale, V.; Hlasnik, A.; Stemmet, L.; Liang, H.-N.; Porter, R. Virtual reality for supporting the treatment of depression and anxiety: Scoping review. JMIR Ment. Health 2021, 8, e29681. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Papastavrou, E.; Avraamides, M.N.; Charalambous, A. Virtual reality and symptoms management of anxiety, depression, fatigue, and pain: A systematic review. SAGE Open Nurs. 2020, 6, 2377960820936163. [Google Scholar] [CrossRef] [PubMed]
- Riadi, I.; Kervin, L.; Dhillon, S.; Teo, K.; Churchill, R.; Card, K.G.; Sixsmith, A.; Moreno, S.; Fortuna, K.L.; Torous, J. Digital interventions for depression and anxiety in older adults: A systematic review of randomised controlled trials. Lancet Healthy Longev. 2022, 3, e558–e571. [Google Scholar] [CrossRef]
- Wang, M.; Chen, H.; Yang, F.; Li, J. Effects of digital psychotherapy for depression and anxiety: A systematic review and bayesian network meta-analysis. J. Affect. Disord. 2023, 338, 569–580. [Google Scholar] [CrossRef]
- Mamukashvili-Delau, M.; Koburger, N.; Dietrich, S.; Rummel-Kluge, C. Long-Term Efficacy of Internet-Based Cognitive Behavioral Therapy Self-Help Programs for Adults With Depression: Systematic Review and Meta-Analysis of Randomized Controlled Trials. JMIR Ment. Health 2023, 10, e46925. [Google Scholar] [CrossRef]
- Mamukashvili-Delau, M.; Koburger, N.; Dietrich, S.; Rummel-Kluge, C. Efficacy of computer- and/or internet-based cognitive-behavioral guided self-management for depression in adults: A systematic review and meta-analysis of randomized controlled trials. BMC Psychiatry 2022, 22, 730. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, L.; Han, X.; Wang, H.; Liao, Y.; Guo, L.; Shi, J.; Fan, B.; Teopiz, K.M.; Jawad, M.Y.; et al. The Effect of Internet-Based Cognitive Behavioral Therapy on Major Depressive Disorder: Randomized Controlled Trial. J. Med. Internet Res. 2023, 25, e42786. [Google Scholar] [CrossRef]
- Fundoiano-Hershcovitz, Y.; Breuer Asher, I.; Ritholz, M.D.; Feniger, E.; Manejwala, O.; Goldstein, P. Specifying the Efficacy of Digital Therapeutic Tools for Depression and Anxiety: Retrospective, 2-Cohort, Real-World Analysis. J. Med. Internet Res. 2023, 25, e47350. [Google Scholar] [CrossRef]
- Wu, M.S.; Wickham, R.E.; Chen, S.-Y.; Chen, C.; Lungu, A. Examining the impact of digital components across different phases of treatment in a blended care cognitive behavioral therapy intervention for depression and anxiety: Pragmatic retrospective study. JMIR Form. Res. 2021, 5, e33452. [Google Scholar] [CrossRef] [PubMed]
- Mantani, A.; Kato, T.; Furukawa, T.A.; Horikoshi, M.; Imai, H.; Hiroe, T.; Chino, B.; Funayama, T.; Yonemoto, N.; Zhou, Q.; et al. Smartphone Cognitive Behavioral Therapy as an Adjunct to Pharmacotherapy for Refractory Depression: Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e373. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Yamada, M.; Inagaki, M.; Watanabe, N.; Chino, B.; Mantani, A.; Furukawa, T.A. Behavioral Activation Contributed to the Total Reduction of Depression Symptoms in the Smartphone-based Cognitive Behavioral Therapy: A Secondary Analysis of a Randomized, Controlled Trial. Innov. Clin. Neurosci. 2020, 17, 21–25. [Google Scholar]
- McKennon, S.; Levitt, S.E.; Bulaj, G. Commentary: A Breathing-Based Meditation Intervention for Patients with Major Depressive Disorder Following Inadequate Response to Antidepressants: A Randomized Pilot Study. Front. Med. 2017, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Schriewer, K.; Bulaj, G. Music Streaming Services as Adjunct Therapies for Depression, Anxiety, and Bipolar Symptoms: Convergence of Digital Technologies, Mobile Apps, Emotions, and Global Mental Health. Front. Public Health 2016, 4, 217. [Google Scholar] [CrossRef]
- Gadd, S.; Tak, C.; Bulaj, G. Developing music streaming as an adjunct digital therapy for depression: A survey study to assess support from key stakeholders. J. Affect. Disord. Rep. 2020, 2, 100048. [Google Scholar] [CrossRef]
- Branchi, I.; Santarelli, S.; Capoccia, S.; Poggini, S.; D’Andrea, I.; Cirulli, F.; Alleva, E. Antidepressant Treatment Outcome Depends on the Quality of the Living Environment: A Pre-Clinical Investigation in Mice. PLoS ONE 2013, 8, e62226. [Google Scholar] [CrossRef]
- Poggini, S.; Matte Bon, G.; Golia, M.T.; Ciano Albanese, N.; Viglione, A.; Poleggi, A.; Limatola, C.; Maggi, L.; Branchi, I. Selecting antidepressants according to a drug-by-environment interaction: A comparison of fluoxetine and minocycline effects in mice living either in enriched or stressful conditions. Behav. Brain Res. 2021, 408, 113256. [Google Scholar] [CrossRef]
- Alboni, S.; van Dijk, R.M.; Poggini, S.; Milior, G.; Perrotta, M.; Drenth, T.; Brunello, N.; Wolfer, D.P.; Limatola, C.; Amrein, I.; et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatry 2017, 22, 552–561. [Google Scholar] [CrossRef]
- Tricklebank, M.D.; Robbins, T.W.; Simmons, C.; Wong, E.H.F. Time to re-engage psychiatric drug discovery by strengthening confidence in preclinical psychopharmacology. Psychopharmacology 2021, 238, 1417–1436. [Google Scholar] [CrossRef]
- Cordner, Z.A.; Marshall-Thomas, I.; Boersma, G.J.; Lee, R.S.; Potash, J.B.; Tamashiro, K.L. Fluoxetine and environmental enrichment similarly reverse chronic social stress-related depression-and anxiety-like behavior, but have differential effects on amygdala gene expression. Neurobiol. Stress. 2021, 15, 100392. [Google Scholar] [CrossRef] [PubMed]
- Coutens, B.; Lejards, C.; Bouisset, G.; Verret, L.; Rampon, C.; Guiard, B.P. Enriched environmental exposure reduces the onset of action of the serotonin norepinephrin reuptake inhibitor venlafaxine through its effect on parvalbumin interneurons plasticity in mice. Transl. Psychiatry 2023, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Sparling, J.E.; Barbeau, K.; Boileau, K.; Konkle, A.T.M. Environmental enrichment and its influence on rodent offspring and maternal behaviours, a scoping style review of indices of depression and anxiety. Pharmacol. Biochem. Behav. 2020, 197, 172997. [Google Scholar] [CrossRef] [PubMed]
- Goes, T.C.; Antunes, F.D.; Teixeira-Silva, F. Environmental enrichment for adult rats: Effects on trait and state anxiety. Neurosci. Lett. 2015, 584, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Merali, Z.; Harrison, C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav. Brain Res. 2006, 175, 1–8. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Chang, J.; Huang, Y.; Cai, M.; Zhang, M. Environmental enrichment reduces adolescent anxiety-and depression-like behaviors of rats subjected to infant nerve injury. J. Neuroinflammation 2018, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Qiu, R.; Chen, L.; Chen, Y.; Qi, W.; Cheng, Y. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl. Psychiatry 2023, 13, 317. [Google Scholar] [CrossRef]
- Mahati, K.; Bhagya, V.; Christofer, T.; Sneha, A.; Rao, B.S.S. Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2016, 134, 379–391. [Google Scholar] [CrossRef]
- Papadakakis, A.; Sidiropoulou, K.; Panagis, G. Music exposure attenuates anxiety- and depression-like behaviors and increases hippocampal spine density in male rats. Behav. Brain Res. 2019, 372, 112023. [Google Scholar] [CrossRef]
- Li, W.J.; Yu, H.; Yang, J.M.; Gao, J.; Jiang, H.; Feng, M.; Zhao, Y.X.; Chen, Z.Y. Anxiolytic effect of music exposure on BDNFMet/Met transgenic mice. Brain Res. 2010, 1347, 71–79. [Google Scholar] [CrossRef]
- Huang, G.J.; Ben-David, E.; Tort Piella, A.; Edwards, A.; Flint, J.; Shifman, S. Neurogenomic evidence for a shared mechanism of the antidepressant effects of exercise and chronic fluoxetine in mice. PLoS ONE 2012, 7, e35901. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liang, X.; Dou, X.; Qi, Y.; Yang, C.; Luo, Y.; Chao, F.; Zhang, L.; Xiao, Q.; Jiang, L.; et al. Exercise rather than fluoxetine promotes oligodendrocyte differentiation and myelination in the hippocampus in a male mouse model of depression. Transl. Psychiatry 2021, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Harden, C.; Tomson, T.; Gloss, D.; Buchhalter, J.; Cross, J.H.; Donner, E.; French, J.A.; Gil-Nagel, A.; Hesdorffer, D.C.; Smithson, W.H. Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr. 2017, 17, 180–187. [Google Scholar] [CrossRef]
- Kanner, A.M.; Bicchi, M.M. Antiseizure Medications for Adults With Epilepsy: A Review. JAMA 2022, 327, 1269–1281. [Google Scholar] [CrossRef]
- Ryvlin, P.; Rheims, S.; Hirsch, L.J.; Sokolov, A.; Jehi, L. Neuromodulation in epilepsy: State-of-the-art approved therapies. Lancet Neurol. 2021, 20, 1038–1047. [Google Scholar] [CrossRef]
- Engel, J., Jr. The current place of epilepsy surgery. Curr. Opin. Neurol. 2018, 31, 192–197. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Chen, B.; Choi, H.; Hirsch, L.J.; Katz, A.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017, 76, 24–31. [Google Scholar] [CrossRef]
- Mendorf, S.; Prell, T.; Schönenberg, A. Detecting Reasons for Nonadherence to Medication in Adults with Epilepsy: A Review of Self-Report Measures and Key Predictors. J. Clin. Med. 2022, 11, 4308. [Google Scholar] [CrossRef]
- Alexander, H.B.; Broshek, D.K.; Quigg, M. Quality of life in adults with epilepsy is associated with anticonvulsant polypharmacy independent of seizure status. Epilepsy Behav. 2018, 78, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Patel, A.A.; Trinka, E.; Mazurkiewicz-Beldzinska, M.; Cross, J.H.; Welty, T.E.; Force, I.E.T. Recommendations for treatment strategies in people with epilepsy during times of shortage of antiseizure medications. Epileptic Disord. 2022, 24, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: A 30-year longitudinal cohort study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, B.; Vieluf, S.; Dworetzky, B.A.; Reinsberger, C. The Potential of Wearable Devices and Mobile Health Applications in the Evaluation and Treatment of Epilepsy. Neurol. Clin. 2022, 40, 729–739. [Google Scholar] [CrossRef]
- Alzamanan, M.Z.; Lim, K.S.; Akmar Ismail, M.; Abdul Ghani, N. Self-Management Apps for People With Epilepsy: Systematic Analysis. JMIR mHealth uHealth 2021, 9, e22489. [Google Scholar] [CrossRef]
- Escoffery, C.; McGee, R.; Bidwell, J.; Sims, C.; Thropp, E.K.; Frazier, C.; Mynatt, E.D. A review of mobile apps for epilepsy self-management. Epilepsy Behav. 2018, 81, 62–69. [Google Scholar] [CrossRef]
- DiIorio, C.; Escoffery, C.; Yeager, K.A.; McCarty, F.; Henry, T.R.; Koganti, A.; Reisinger, E.; Robinson, E.; Kobau, R.; Price, P. WebEase: Development of a Web-based epilepsy self-management intervention. Prev. Chronic Dis. 2009, 6, A28. [Google Scholar]
- DiIorio, C.; Escoffery, C.; McCarty, F.; Yeager, K.A.; Henry, T.R.; Koganti, A.; Reisinger, E.L.; Wexler, B. Evaluation of WebEase: An epilepsy self-management Web site. Health Educ. Res. 2009, 24, 185–197. [Google Scholar] [CrossRef]
- DiIorio, C.; Bamps, Y.; Walker, E.R.; Escoffery, C. Results of a research study evaluating WebEase, an online epilepsy self-management program. Epilepsy Behav. 2011, 22, 469–474. [Google Scholar] [CrossRef]
- Shegog, R.; Bamps, Y.A.; Patel, A.; Kakacek, J.; Escoffery, C.; Johnson, E.K.; Ilozumba, U.O. Managing Epilepsy Well: Emerging e-Tools for epilepsy self-management. Epilepsy Behav. 2013, 29, 133–140. [Google Scholar] [CrossRef]
- Mirpuri, P.; Chandra, P.P.; Samala, R.; Agarwal, M.; Doddamani, R.; Kaur, K.; Ramanujan, B.; Chandra, P.S.; Tripathi, M. The development and efficacy of a mobile phone application to improve medication adherence for persons with epilepsy in limited resource settings: A preliminary study. Epilepsy Behav. 2021, 116, 107794. [Google Scholar] [CrossRef]
- Le Marne, F.A.; Butler, S.; Beavis, E.; Gill, D.; Bye, A.M.E. EpApp: Development and evaluation of a smartphone/tablet app for adolescents with epilepsy. J. Clin. Neurosci. 2018, 50, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Xiao, X.; Xia, C.; Guo, J.; Hao, Q.; Mo, Q.; Niu, Y.; Sun, H. Optimising epilepsy management with a smartphone application: A randomised controlled trial. Med. J. Aust. 2020, 212, 258–262. [Google Scholar] [CrossRef]
- Dastgheib, S.S.; Layegh, P.; Sadeghi, R.; Foroughipur, M.; Shoeibi, A.; Gorji, A. The effects of Mozart’s music on interictal activity in epileptic patients: Systematic review and meta-analysis of the literature. Curr. Neurol. Neurosci. Rep. 2014, 14, 420. [Google Scholar] [CrossRef]
- Sesso, G.; Sicca, F. Safe and sound: Meta-analyzing the Mozart effect on epilepsy. Clin. Neurophysiol. 2020, 131, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Grigg-Damberger, M.; Foldvary-Schaefer, N. Bidirectional relationships of sleep and epilepsy in adults with epilepsy. Epilepsy Behav. 2021, 116, 107735. [Google Scholar] [CrossRef] [PubMed]
- Haut, S.R.; Lipton, R.B.; Cornes, S.; Dwivedi, A.K.; Wasson, R.; Cotton, S.; Strawn, J.R.; Privitera, M. Behavioral interventions as a treatment for epilepsy: A multicenter randomized controlled trial. Neurology 2018, 90, e963–e970. [Google Scholar] [CrossRef]
- Maguire, J.; Salpekar, J.A. Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 2013, 26, 352–362. [Google Scholar] [CrossRef]
- May, T.W.; Pfafflin, M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): Results of a controlled, randomized study. Modular Service Package Epilepsy. Epilepsia 2002, 43, 539–549. [Google Scholar] [CrossRef]
- Akyuz, E.; Eroglu, E. Envisioning the crosstalk between environmental enrichment and epilepsy: A novel perspective. Epilepsy Behav. 2021, 115, 107660. [Google Scholar] [CrossRef] [PubMed]
- Kotloski, R.J.; Sutula, T.P. Environmental enrichment: Evidence for an unexpected therapeutic influence. Exp. Neurol. 2015, 264, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, G.; Ozturk, E.; Salzberg, M.R.; Morris, M.; O’Brien, T.J.; Jones, N.C. Environmental enrichment imparts disease-modifying and transgenerational effects on genetically-determined epilepsy and anxiety. Neurobiol. Dis. 2016, 93, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Manno, I.; Macchi, F.; Caleo, M.; Bozzi, Y. Environmental enrichment reduces spontaneous seizures in the Q54 transgenic mouse model of temporal lobe epilepsy. Epilepsia 2011, 52, e113–e117. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ozturk, E.; Salzberg, M.R.; Rees, S.; Morris, M.; O’Brien, T.J.; Jones, N.C. Environmental enrichment delays limbic epileptogenesis and restricts pathologic synaptic plasticity. Epilepsia 2016, 57, 484–494. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhou, Z.; Mu, X.; Song, C.; Xiao, T.; Zhao, M.; Zhao, C. Enriched Environment Altered Aberrant Hippocampal Neurogenesis and Improved Long-Term Consequences After Temporal Lobe Epilepsy in Adult Rats. J. Mol. Neurosci. 2015, 56, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Vrinda, M.; Sasidharan, A.; Aparna, S.; Srikumar, B.N.; Kutty, B.M.; Shankaranarayana Rao, B.S. Enriched environment attenuates behavioral seizures and depression in chronic temporal lobe epilepsy. Epilepsia 2017, 58, 1148–1158. [Google Scholar] [CrossRef]
- Nair, K.P.; Salaka, R.J.; Srikumar, B.N.; Kutty, B.M.; Shankaranarayana Rao, B.S. Enriched Environment Rescues Impaired Sleep-Wake Architecture and Abnormal Neural Dynamics in Chronic Epileptic Rats. Neuroscience 2022, 495, 97–114. [Google Scholar] [CrossRef]
- Suemaru, K.; Yoshikawa, M.; Aso, H.; Watanabe, M. Environmental enrichment alleviates cognitive and behavioral impairments in EL mice. Epilepsy Behav. 2018, 85, 227–233. [Google Scholar] [CrossRef]
- Xing, Y.; Qin, Y.; Jing, W.; Zhang, Y.; Wang, Y.; Guo, D.; Xia, Y.; Yao, D. Exposure to Mozart music reduces cognitive impairment in pilocarpine-induced status epilepticus rats. Cogn. Neurodyn 2016, 10, 23–30. [Google Scholar] [CrossRef]
- Bodner, M.; Turner, R.P.; Schwacke, J.; Bowers, C.; Norment, C. Reduction of seizure occurrence from exposure to auditory stimulation in individuals with neurological handicaps: A randomized controlled trial. PLoS ONE 2012, 7, e45303. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Patel, K.; Groppe, D.M.; Andrade, D.M.; Bercovici, E.; Bui, E.; Carlen, P.L.; Reid, A.; Tai, P.; Weaver, D.; et al. Daily listening to Mozart reduces seizures in individuals with epilepsy: A randomized control study. Epilepsia Open 2020, 5, 285–294. [Google Scholar] [CrossRef]
- Paprad, T.; Veeravigrom, M.; Desudchit, T. Effect of Mozart K.448 on interictal epileptiform discharges in children with epilepsy: A randomized controlled pilot study. Epilepsy Behav. 2021, 114, 107177. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Tang, H.; Liu, Y.; Yin, Y.; Yan, B.; Jiang, Y.; Toussaint, P.J.; Xia, Y.; Evans, A.C.; Zhou, D.; et al. Therapeutic effect of tempo in Mozart’s “Sonata for two pianos” (K. 448) in patients with epilepsy: An electroencephalographic study. Epilepsy Behav. 2023, 145, 109323. [Google Scholar] [CrossRef] [PubMed]
- Quon, R.J.; Casey, M.A.; Camp, E.J.; Meisenhelter, S.; Steimel, S.A.; Song, Y.; Testorf, M.E.; Leslie, G.A.; Bujarski, K.A.; Ettinger, A.B.; et al. Musical components important for the Mozart K448 effect in epilepsy. Sci. Rep. 2021, 11, 16490. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R. The Mozart Effect. Epilepsy Behav. 2001, 2, 396–417. [Google Scholar] [CrossRef]
- Lin, L.C.; Juan, C.T.; Chang, H.W.; Chiang, C.T.; Wei, R.C.; Lee, M.W.; Mok, H.K.; Yang, R.C. Mozart K.448 attenuates spontaneous absence seizure and related high-voltage rhythmic spike discharges in Long Evans rats. Epilepsy Res. 2013, 104, 234–240. [Google Scholar] [CrossRef]
- Xu, C.L.; Nao, J.Z.; Shen, Y.J.; Gong, Y.W.; Tan, B.; Zhang, S.; Shen, K.X.; Sun, C.R.; Wang, Y.; Chen, Z. Long-term music adjuvant therapy enhances the efficacy of sub-dose antiepileptic drugs in temporal lobe epilepsy. CNS Neurosci. Ther. 2022, 28, 206–217. [Google Scholar] [CrossRef]
- Johnson, E.C.; Helen Cross, J.; Reilly, C. Physical activity in people with epilepsy: A systematic review. Epilepsia 2020, 61, 1062–1081. [Google Scholar] [CrossRef]
- Arida, R.M.; Cavalheiro, E.A.; Da Silva, A.C.; Scorza, F.A. Physical activity and epilepsy: Proven and predicted benefits. Sports Med. 2008, 38, 607–615. [Google Scholar] [CrossRef]
- Häfele, C.A.; Freitas, M.P.; da Silva, M.C.; Rombaldi, A.J. Are physical activity levels associated with better health outcomes in people with epilepsy? Epilepsy Behav. 2017, 72, 28–34. [Google Scholar] [CrossRef]
- Häfele, C.A.; Rombaldi, A.J.; Feter, N.; Häfele, V.; Gervini, B.L.; Domingues, M.R.; da Silva, M.C. Effects of an exercise program on health of people with epilepsy: A randomized clinical trial. Epilepsy Behav. 2021, 117, 107904. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-Y.; Cui, Y.; Wang, L.; Chen, W. Chronic exercise buffers the cognitive dysfunction and decreases the susceptibility to seizures in PTZ-treated rats. Epilepsy Behav. 2019, 98, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Arida, R.M.; Scorza, F.A.; dos Santos, N.F.; Peres, C.A.; Cavalheiro, E.A. Effect of physical exercise on seizure occurrence in a model of temporal lobe epilepsy in rats. Epilepsy Res. 1999, 37, 45–52. [Google Scholar] [CrossRef]
- Peixinho-Pena, L.F.; Fernandes, J.; de Almeida, A.A.; Gomes, F.G.N.; Cassilhas, R.; Venancio, D.P.; de Mello, M.T.; Scorza, F.A.; Cavalheiro, E.A.; Arida, R.M. A strength exercise program in rats with epilepsy is protective against seizures. Epilepsy Behav. 2012, 25, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Barzroodi Pour, M.; Bayat, M.; Navazesh, A.; Soleimani, M.; Karimzadeh, F. Exercise improved the anti-epileptic effect of carbamazepine through gaba enhancement in epileptic rats. Neurochem. Res. 2021, 46, 2112–2130. [Google Scholar] [CrossRef]
- Acar, S.; Kapucu, A.; Akgün-Dar, K. The effects of regular swimming exercise during sodium valproate treatment on seizure behaviors and EEG recordings in pentylenetetrazole-kindled rats. Epilepsy Res. 2022, 179, 106830. [Google Scholar] [CrossRef]
- Arida, R.M.; de Almeida, A.-C.G.; Cavalheiro, E.A.; Scorza, F.A. Experimental and clinical findings from physical exercise as complementary therapy for epilepsy. Epilepsy Behav. 2013, 26, 273–278. [Google Scholar] [CrossRef]
- Cavalcante, B.R.R.; Improta-Caria, A.C.; de Melo, V.H.; De Sousa, R.A.L. Exercise-linked consequences on epilepsy. Epilepsy Behav. 2021, 121, 108079. [Google Scholar] [CrossRef]
- Agarwal, P.; Mukerji, G.; Desveaux, L.; Ivers, N.M.; Bhattacharyya, O.; Hensel, J.M.; Shaw, J.; Bouck, Z.; Jamieson, T.; Onabajo, N. Mobile app for improved self-management of type 2 diabetes: Multicenter pragmatic randomized controlled trial. JMIR mHealth uHealth 2019, 7, e10321. [Google Scholar] [CrossRef]
- Quinn, C.C.; Clough, S.S.; Minor, J.M.; Lender, D.; Okafor, M.C.; Gruber-Baldini, A. WellDoc mobile diabetes management randomized controlled trial: Change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol. Ther. 2008, 10, 160–168. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, X.; Vespasiani, G.; Nicolucci, A.; Dong, Y.; Kwong, J.; Li, L.; Sun, X.; Tian, H.; Li, S. Mobile App-Based Interventions to Support Diabetes Self-Management: A Systematic Review of Randomized Controlled Trials to Identify Functions Associated with Glycemic Efficacy. JMIR mHealth uHealth 2017, 5, e35. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Carter, B.; Hewitt, J.; Francisa, T.; Mayor, S. Do Mobile Phone Applications Improve Glycemic Control (HbA1c) in the Self-management of Diabetes? A Systematic Review, Meta-analysis, and GRADE of 14 Randomized Trials. Diabetes Care 2016, 39, 2089–2095. [Google Scholar] [CrossRef]
- Fleming, G.A.; Petrie, J.R.; Bergenstal, R.M.; Holl, R.W.; Peters, A.L.; Heinemann, L. Diabetes Digital App Technology: Benefits, Challenges, and Recommendations. A Consensus Report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetes Care 2020, 43, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Doyle-Delgado, K.; Chamberlain, J.J. Use of diabetes-related applications and digital health tools by people with diabetes and their health care providers. Clin. Diabetes 2020, 38, 449–461. [Google Scholar] [CrossRef]
- Daly, A.B.; Boughton, C.K.; Nwokolo, M.; Hartnell, S.; Wilinska, M.E.; Cezar, A.; Evans, M.L.; Hovorka, R. Fully automated closed-loop insulin delivery in adults with type 2 diabetes: An open-label, single-center, randomized crossover trial. Nat. Med. 2023, 29, 203–208. [Google Scholar] [CrossRef]
- Kario, K.; Harada, N.; Okura, A. Digital therapeutics in hypertension: Evidence and perspectives. Hypertension 2022, 79, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, A.; Hosseini, H.; Eftekhar, H.; Majdzadeh, R.; Yoonessi, A.; Ramezankhani, A.; Mansouri, M.; Ashoorkhani, M. The effect of the mobile “blood pressure management application” on hypertension self-management enhancement: A randomized controlled trial. Trials 2021, 22, 413. [Google Scholar] [CrossRef]
- Moravcová, K.; Karbanová, M.; Bretschneider, M.P.; Sovová, M.; Ožana, J.; Sovová, E. Comparing digital therapeutic intervention with an intensive obesity management program: Randomized controlled trial. Nutrients 2022, 14, 2005. [Google Scholar] [CrossRef]
- Schaaf, J.; Weber, T.; von Wagner, M.; Stephan, C.; Carney, J.; Köhler, S.M.; Voigt, A.; Noll, R.; Storf, H.; Müller, A. Interviews with HIV Experts for Development of a Mobile Health Application in HIV Care-A Qualitative Study. Healthcare 2023, 11, 2180. [Google Scholar] [CrossRef]
- Crowley, T.; Petinger, C.; Nchendia, A.I.; van Wyk, B. Effectiveness, Acceptability and Feasibility of Technology-Enabled Health Interventions for Adolescents Living with HIV in Low- and Middle-Income Countries: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 2464. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, C.A.; du Toit, S.; Ameyan, W.; Melendez-Torres, G.J.; Kara, T.; Brand, A.; Chideya, Y.; Abrahams, N.; Bradshaw, M.; Page, D.T.; et al. Psychosocial interventions for improving engagement in care and health and behavioural outcomes for adolescents and young people living with HIV: A systematic review and meta-analysis. J. Int. AIDS Soc. 2021, 24, e25741. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Fernandes, M.S.; Santos, G.C.J.; Filgueira, T.O.; Gomes, D.A.; Barbosa, E.A.S.; Dos Santos, T.M.; Câmara, N.O.S.; Castoldi, A.; Souto, F.O. Cytokines and immune cells profile in different tissues of rodents induced by environmental enrichment: Systematic review. Int. J. Mol. Sci. 2022, 23, 11986. [Google Scholar] [CrossRef]
- Lee, M.-H.; Wang, T.; Jang, M.-H.; Steiner, J.; Haughey, N.; Ming, G.-L.; Song, H.; Nath, A.; Venkatesan, A. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol. Dis. 2011, 41, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Amin, N.D.; Venkatesan, A.; Wang, T.; Tyagi, R.; Pant, H.C.; Nath, A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J. Neurovirology 2013, 19, 418–431. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.C.; Johnson, T.P. Chronic inflammation mediates brain injury in HIV infection: Relevance for cure strategies. Curr. Opin. Neurol. 2020, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- van Roon-Mom, W.; Ferguson, C.; Aartsma-Rus, A. From Failure to Meet the Clinical Endpoint to US Food and Drug Administration Approval: 15th Antisense Oligonucleotide Therapy Approved Qalsody (Tofersen) for Treatment of SOD1 Mutated Amyotrophic Lateral Sclerosis. Nucleic Acid Ther. 2023, 33, 234–237. [Google Scholar] [CrossRef]
- Stevens, D.; Claborn, M.K.; Gildon, B.L.; Kessler, T.L.; Walker, C. Onasemnogene abeparvovec-xioi: Gene therapy for spinal muscular atrophy. Ann. Pharmacother. 2020, 54, 1001–1009. [Google Scholar] [CrossRef]
- Johnson, S.A.; Karas, M.; Burke, K.M.; Straczkiewicz, M.; Scheier, Z.A.; Clark, A.P.; Iwasaki, S.; Lahav, A.; Iyer, A.S.; Onnela, J.-P. Wearable device and smartphone data quantify ALS progression and may provide novel outcome measures. npj Digit. Med. 2023, 6, 34. [Google Scholar] [CrossRef]
- Ortega-Hombrados, L.; Molina-Torres, G.; Galán-Mercant, A.; Sánchez-Guerrero, E.; González-Sánchez, M.; Ruiz-Muñoz, M. Systematic Review of Therapeutic Physical Exercise in Patients with Amyotrophic Lateral Sclerosis over Time. Int. J. Environ. Res. Public Health 2021, 18, 1074. [Google Scholar] [CrossRef]
- Gerhalter, T.; Müller, C.; Maron, E.; Thielen, M.; Schätzl, T.; Mähler, A.; Schütte, T.; Boschmann, M.; Herzer, R.; Spuler, S.; et al. “suMus”, a novel digital system for arm movement metrics and muscle energy expenditure. Front. Physiol. 2023, 14, 1057592. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.; Renly, S.; Maiorano, A.; Young, J.; Medina, E.; Neinstein, A.; Odisho, A.Y. Digital Health at Enterprise Scale: Evaluation Framework for Selecting Patient-Facing Software in a Digital-First Health System. JMIR Form. Res. 2023, 7, e43009. [Google Scholar] [CrossRef]
- Cafazzo, J.A. A digital-first model of diabetes care. Diabetes Technol. Ther. 2019, 21, S2-25–S2-58. [Google Scholar] [CrossRef] [PubMed]
- Ekman, B.; Nero, H.; Lohmander, L.; Dahlberg, L. Costing analysis of a digital first-line treatment platform for patients with knee and hip osteoarthritis in Sweden. PLoS ONE 2020, 15, e0236342. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Nero, H.; Dahlberg, L.E.; Ignjatovic, M.M.; Lohmander, L.S.; Cronström, A.; Kiadaliri, A. Within-person change in patient-reported outcomes and their association with the wish to undergo joint surgery during a digital first-line intervention for osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1257–1264. [Google Scholar] [CrossRef]
- Kerschbaumer, A.; Sepriano, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.J.; Burmester, G.R.; de Wit, M.; et al. Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 744–759. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2018, 175, 168–180. [Google Scholar] [CrossRef]
- Oprea, T.I.; Bauman, J.E.; Bologa, C.G.; Buranda, T.; Chigaev, A.; Edwards, B.S.; Jarvik, J.W.; Gresham, H.D.; Haynes, M.K.; Hjelle, B.; et al. Drug Repurposing from an Academic Perspective. Drug Discov. Today Ther. Strateg. 2011, 8, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chaffey, L.; Roberti, A.; Greaves, D.R. Drug repurposing in cardiovascular inflammation: Successes, failures, and future opportunities. Front. Pharmacol. 2022, 13, 1046406. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bang, M. Anti-inflammatory strategies for schizophrenia: A review of evidence for therapeutic applications and drug repurposing. Clin. Psychopharmacol. Neurosci. 2020, 18, 10. [Google Scholar] [CrossRef]
- Sisignano, M.; Gribbon, P.; Geisslinger, G. Drug repurposing to target neuroinflammation and sensory neuron-dependent pain. Drugs 2022, 82, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, A.; Blaess, J.; Sibilia, J.; Felten, R.; Arnaud, L. Shared development of targeted therapies among autoimmune and inflammatory diseases: A systematic repurposing analysis. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20969261. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.; Laber, M.; Bennett, C. Humira: The First $20 Billion Drug. Am. J. Manag. Care 2023, 29, 78–80. [Google Scholar] [CrossRef]
- Cisek, S.; Choi, D.; Stubbings, J.; Bhat, S. Preparing for the market entry of adalimumab biosimilars in the US in 2023: A primer for specialty pharmacists. Am. J. Health Syst. Pharm. 2023, 80, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Kelly, J.P. The impact of environmental enrichment in laboratory rats—Behavioural and neurochemical aspects. Behav. Brain Res. 2011, 222, 246–264. [Google Scholar] [CrossRef]
- Alarcón, T.A.; Presti-Silva, S.M.; Simões, A.P.T.; Ribeiro, F.M.; Pires, R.G.W. Molecular mechanisms underlying the neuroprotection of environmental enrichment in Parkinson’s disease. Neural Regen. Res. 2023, 18, 1450–1456. [Google Scholar]
- Balietti, M.; Conti, F. Environmental enrichment and the aging brain: Is it time for standardization? Neurosci. Biobehav. Rev. 2022, 139, 104728. [Google Scholar] [CrossRef]
- Ellis, T.D.; Earhart, G.M. Digital therapeutics in Parkinson’s disease: Practical applications and future potential. J. Park. Dis. 2021, 11, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Murray, G. What Would Digital Early Intervention for Bipolar Disorder Look Like? Theoretical and Translational Considerations for Future Therapies. Front. Psychiatry 2019, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Lagan, S.; Ramakrishnan, A.; Lamont, E.; Ramakrishnan, A.; Frye, M.; Torous, J. Digital health developments and drawbacks: A review and analysis of top-returned apps for bipolar disorder. Int. J. Bipolar Disord. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Sverdlov, O.; van Dam, J. Digital Therapeutics: Strategic, Scientific, Developmental, and Regulatory Aspects; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Velez, F.F.; Malone, D.C. Cost-Effectiveness Analysis of a Prescription Digital Therapeutic for the Treatment of Opioid Use Disorder. J. Mark. Access Health Policy 2021, 9, 1966187. [Google Scholar] [CrossRef]
- Lewkowicz, D.; Wohlbrandt, A.M.; Bottinger, E. Digital therapeutic care apps with decision-support interventions for people with low back pain in germany: Cost-effectiveness analysis. JMIR mHealth uHealth 2022, 10, e35042. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Tanigawa, T.; Kario, K.; Igarashi, A. Cost-effectiveness of digital therapeutics for essential hypertension. Hypertens. Res. 2022, 45, 1538–1548. [Google Scholar] [CrossRef]
- van Kessel, R.; Roman-Urrestarazu, A.; Anderson, M.; Kyriopoulos, I.; Field, S.; Monti, G.; Reed, S.D.; Pavlova, M.; Wharton, G.; Mossialos, E. Mapping factors that affect the uptake of digital therapeutics within health systems: Scoping review. J. Med. Internet Res. 2023, 25, e48000. [Google Scholar] [CrossRef]
- Dahlhausen, F.; Zinner, M.; Bieske, L.; Ehlers, J.P.; Boehme, P.; Fehring, L. There’s an app for that, but nobody’s using it: Insights on improving patient access and adherence to digital therapeutics in Germany. Digit. Health 2022, 8, 20552076221104672. [Google Scholar] [CrossRef]
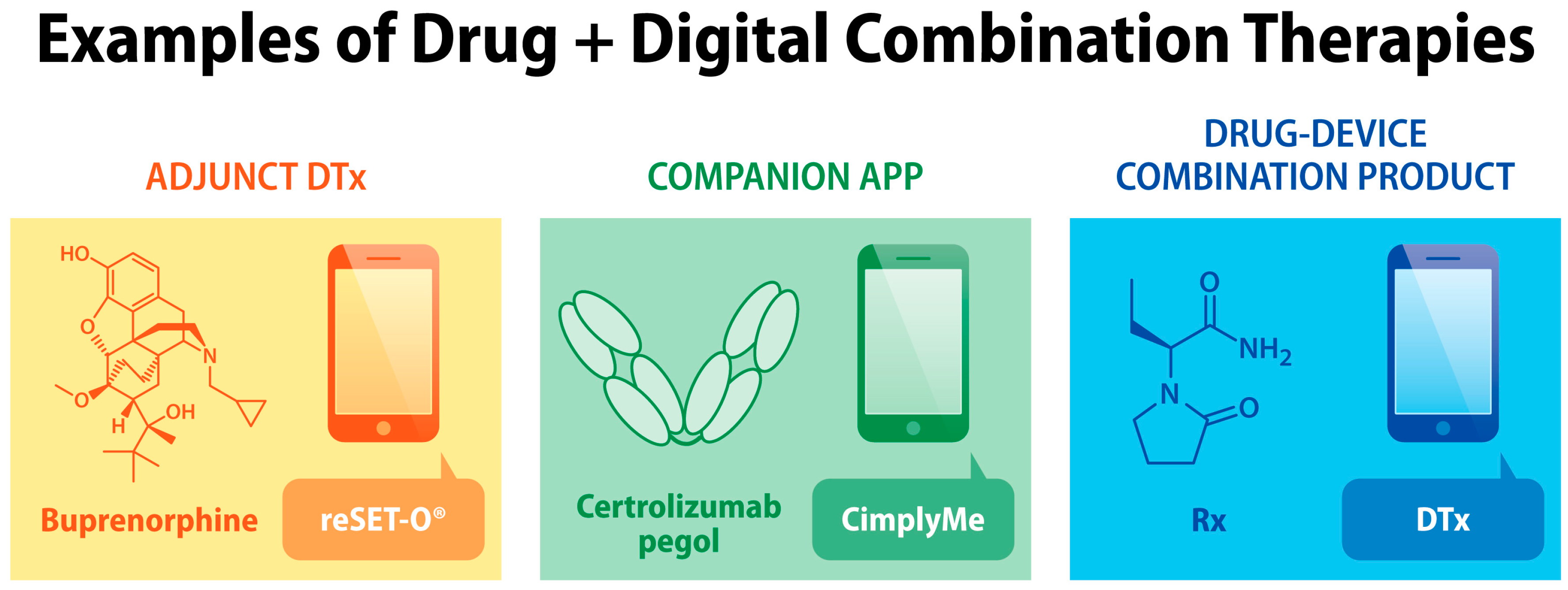
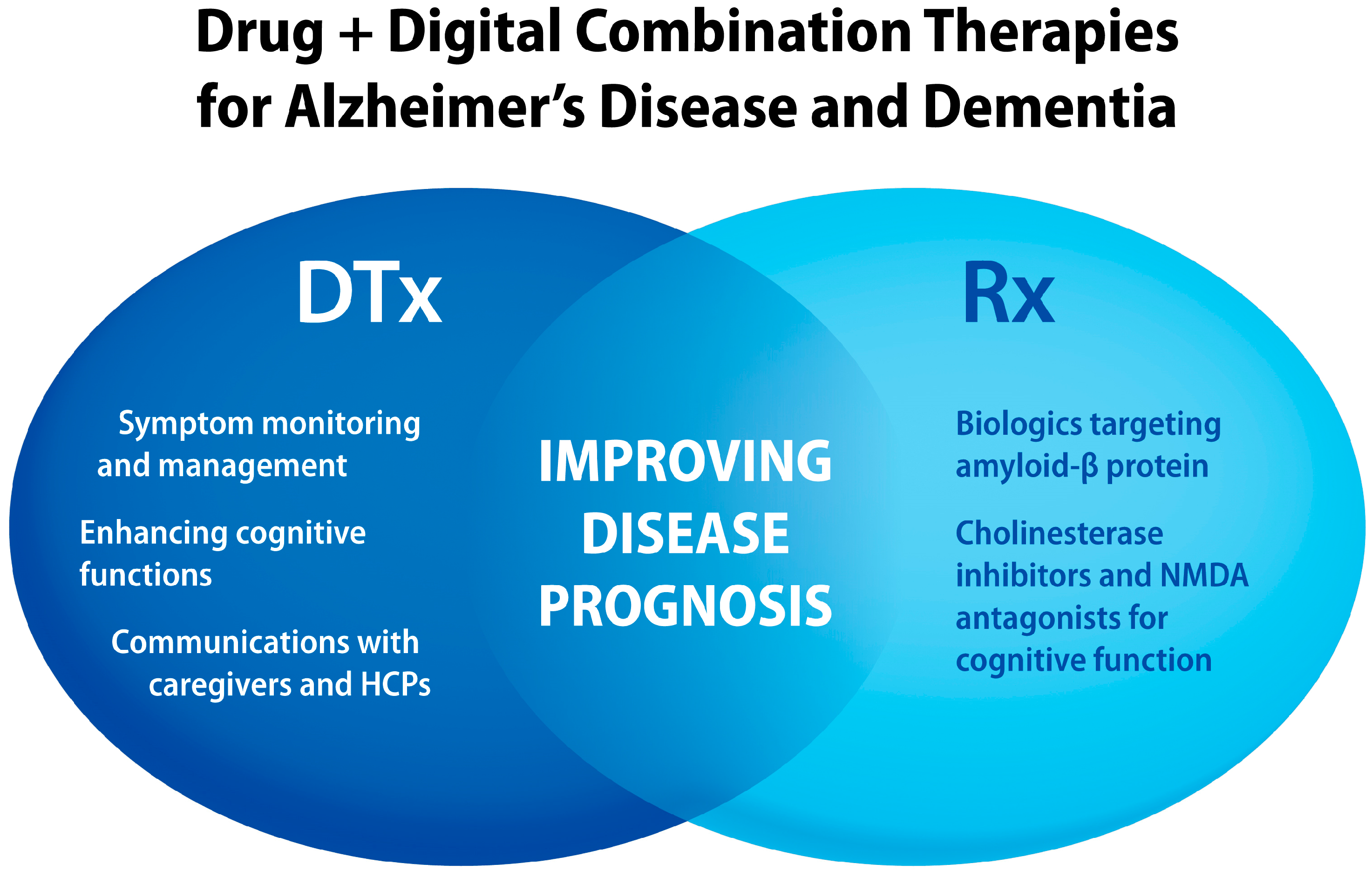
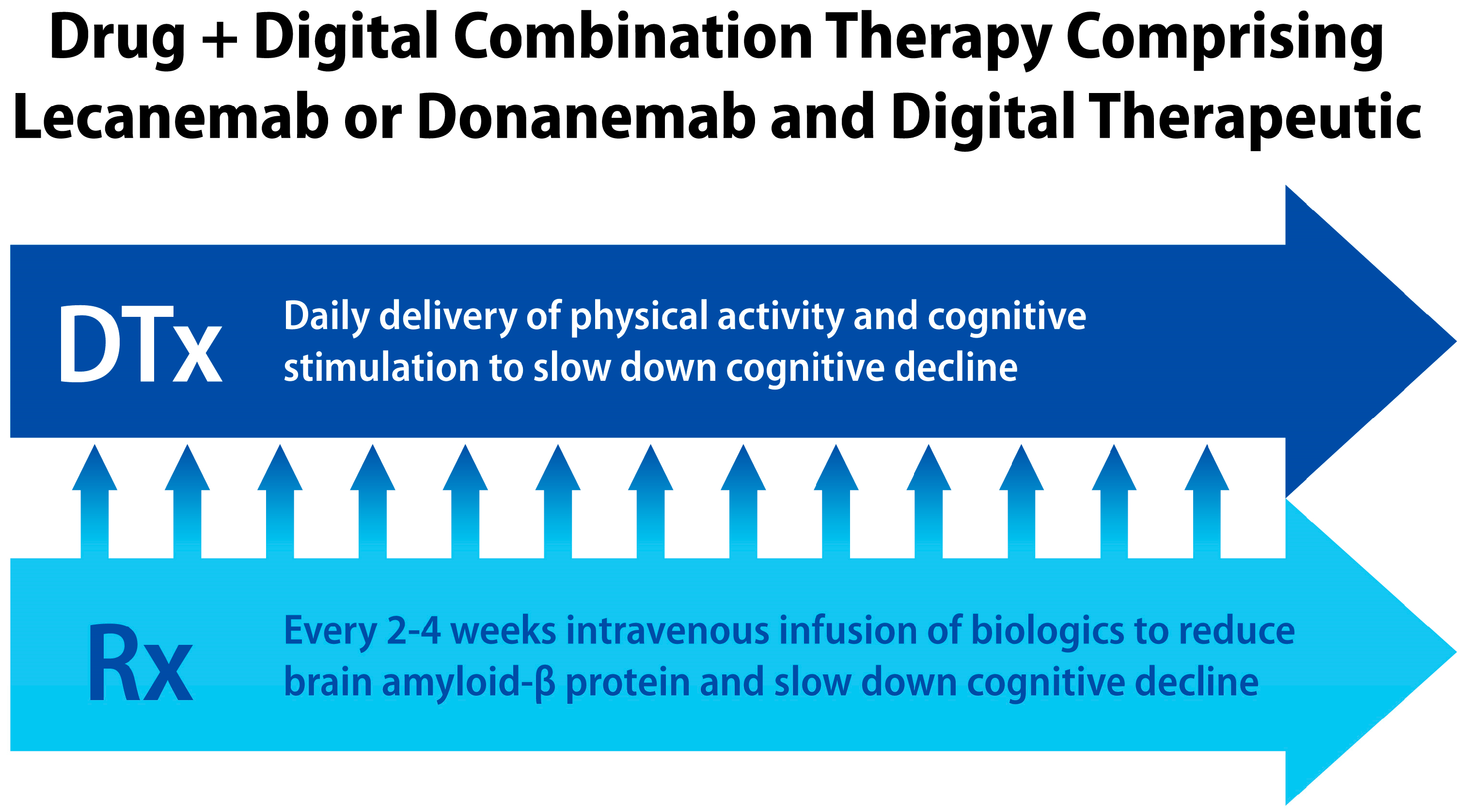

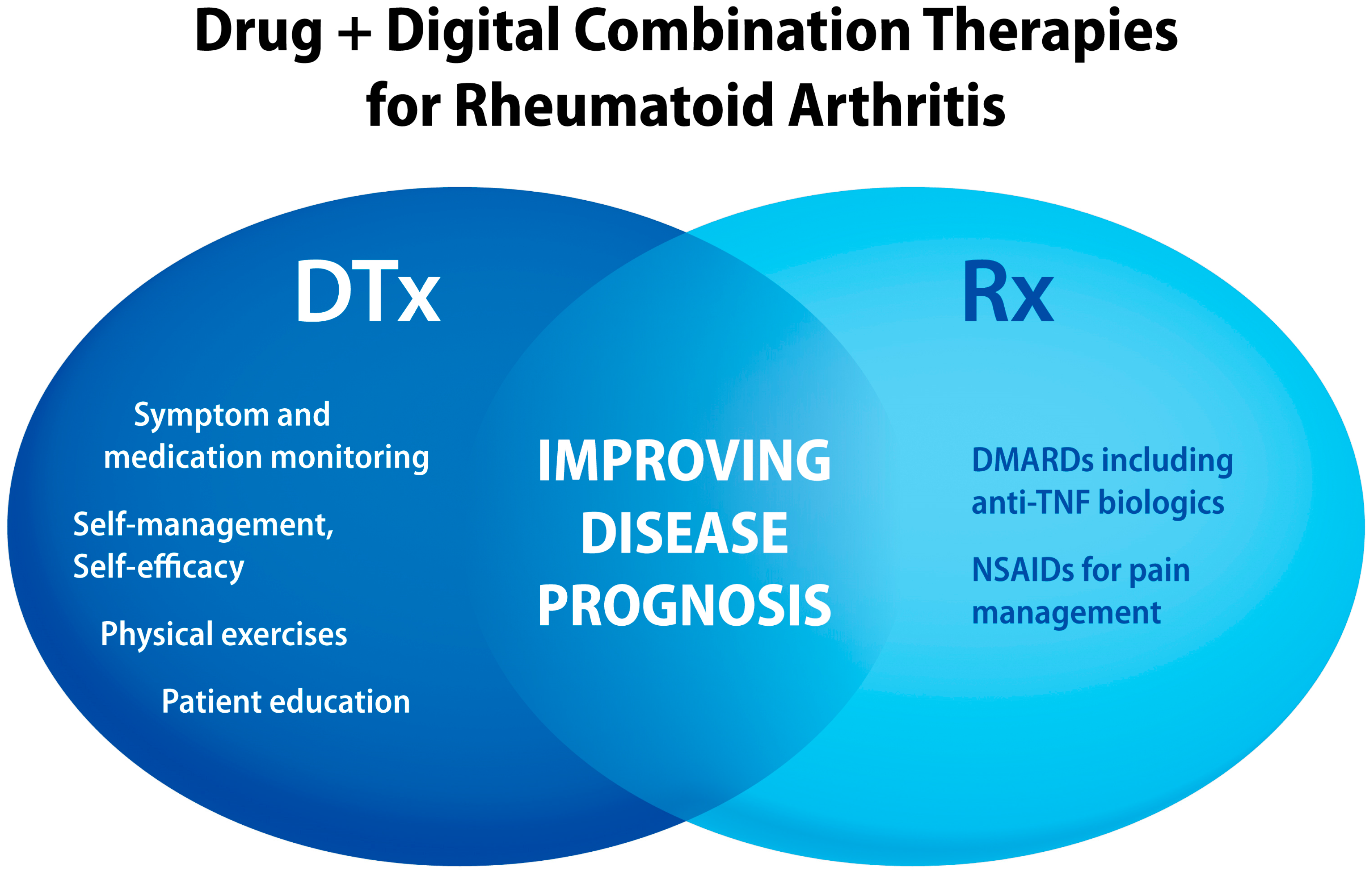
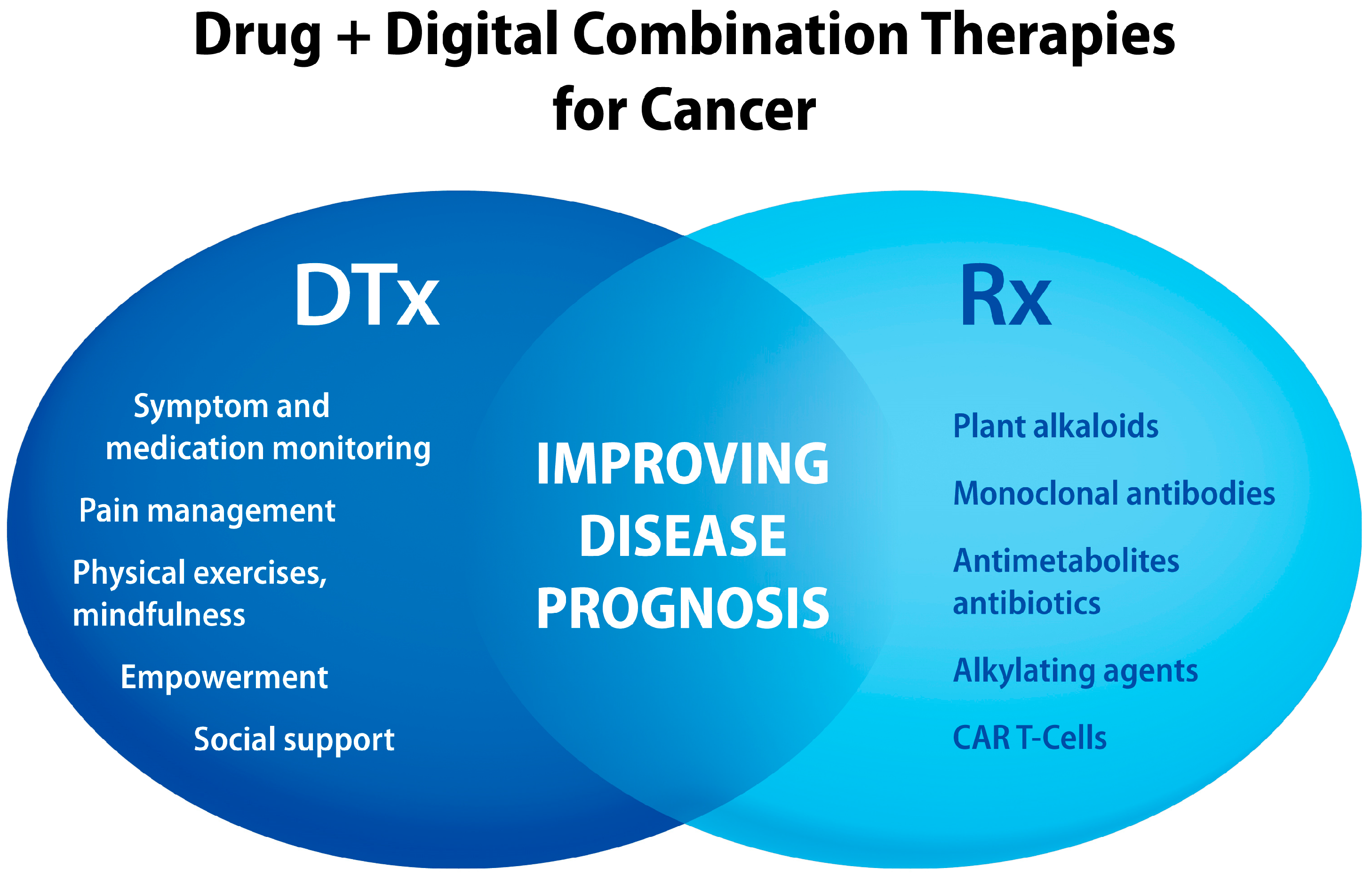
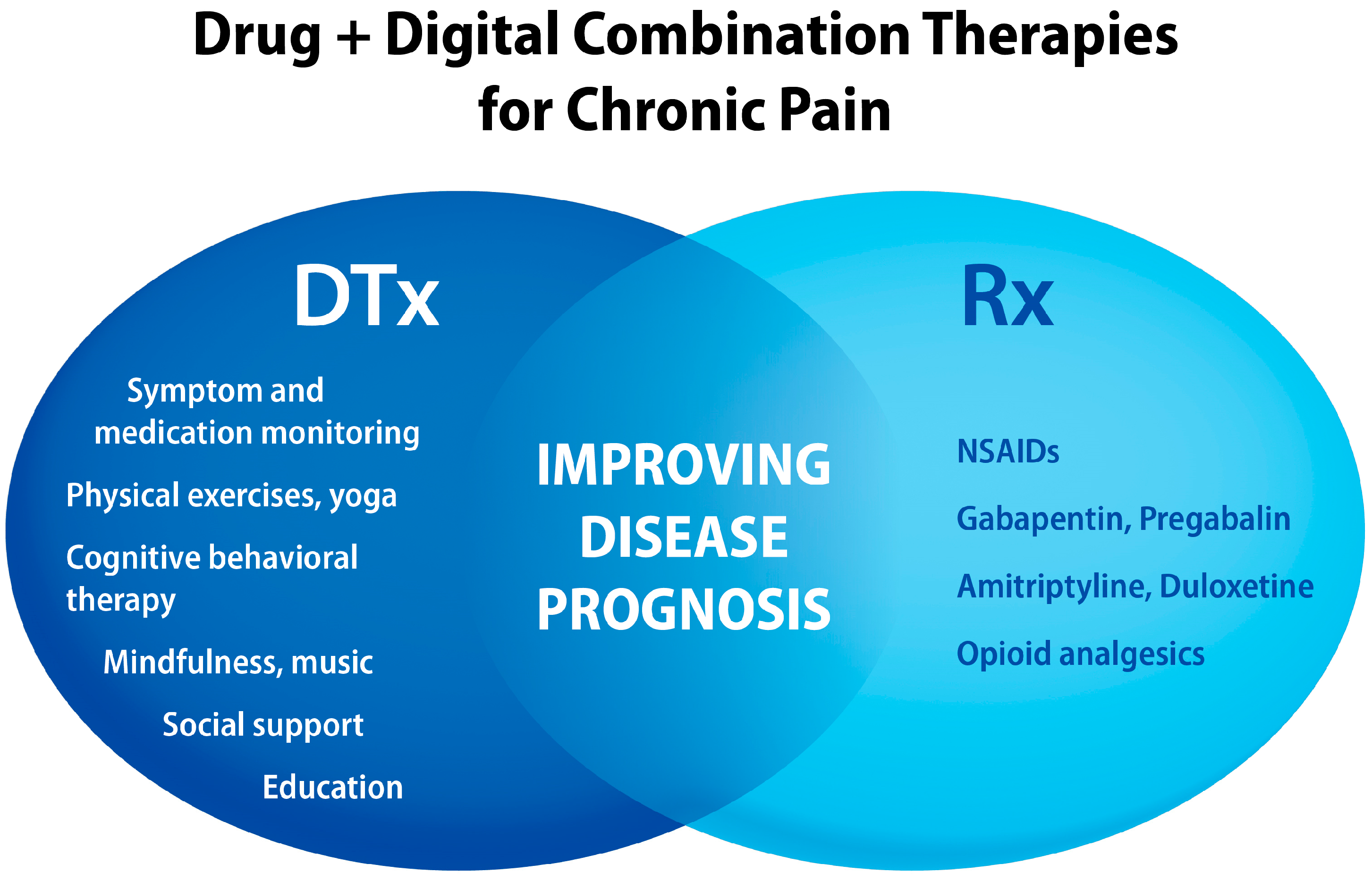
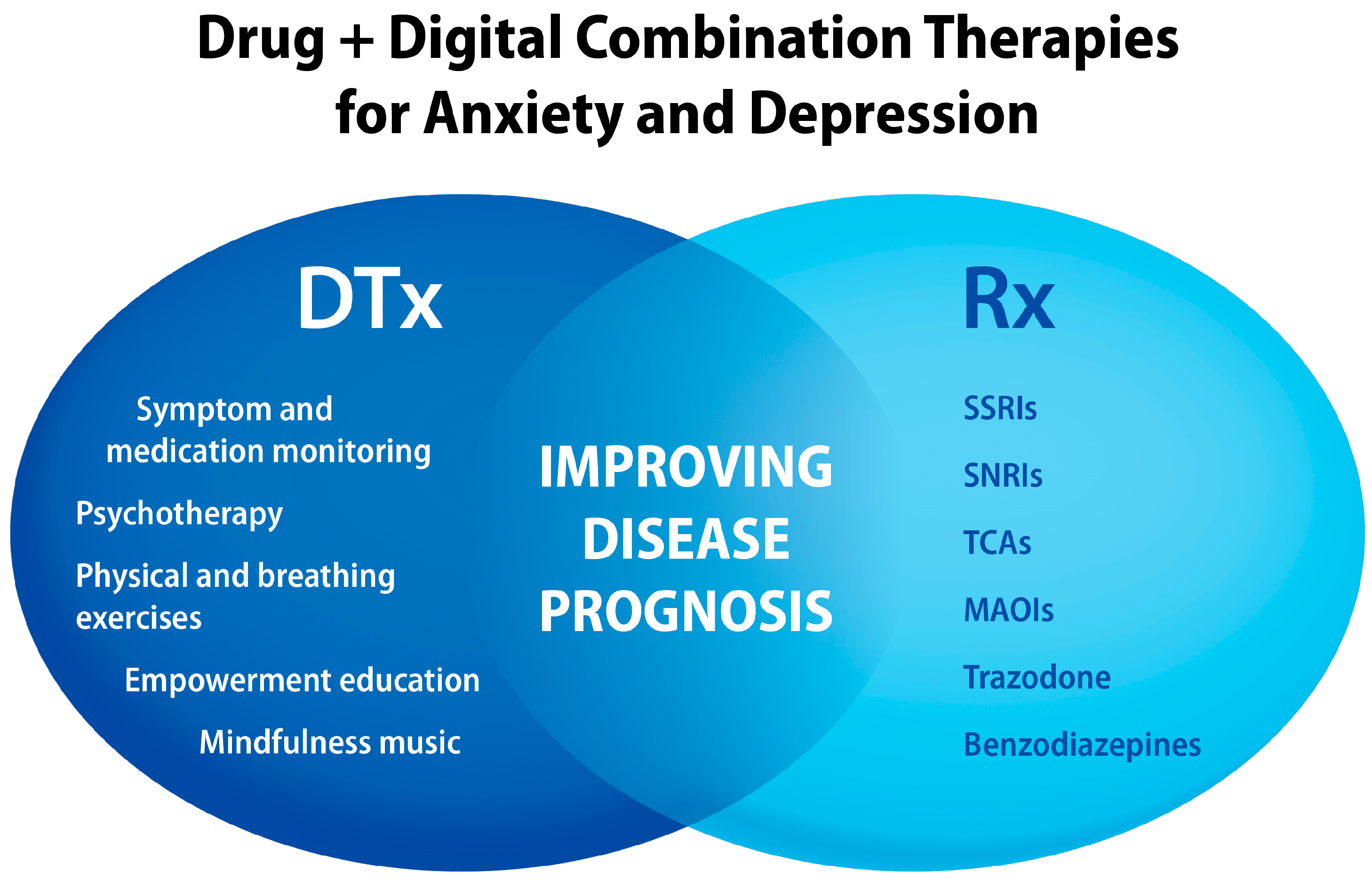
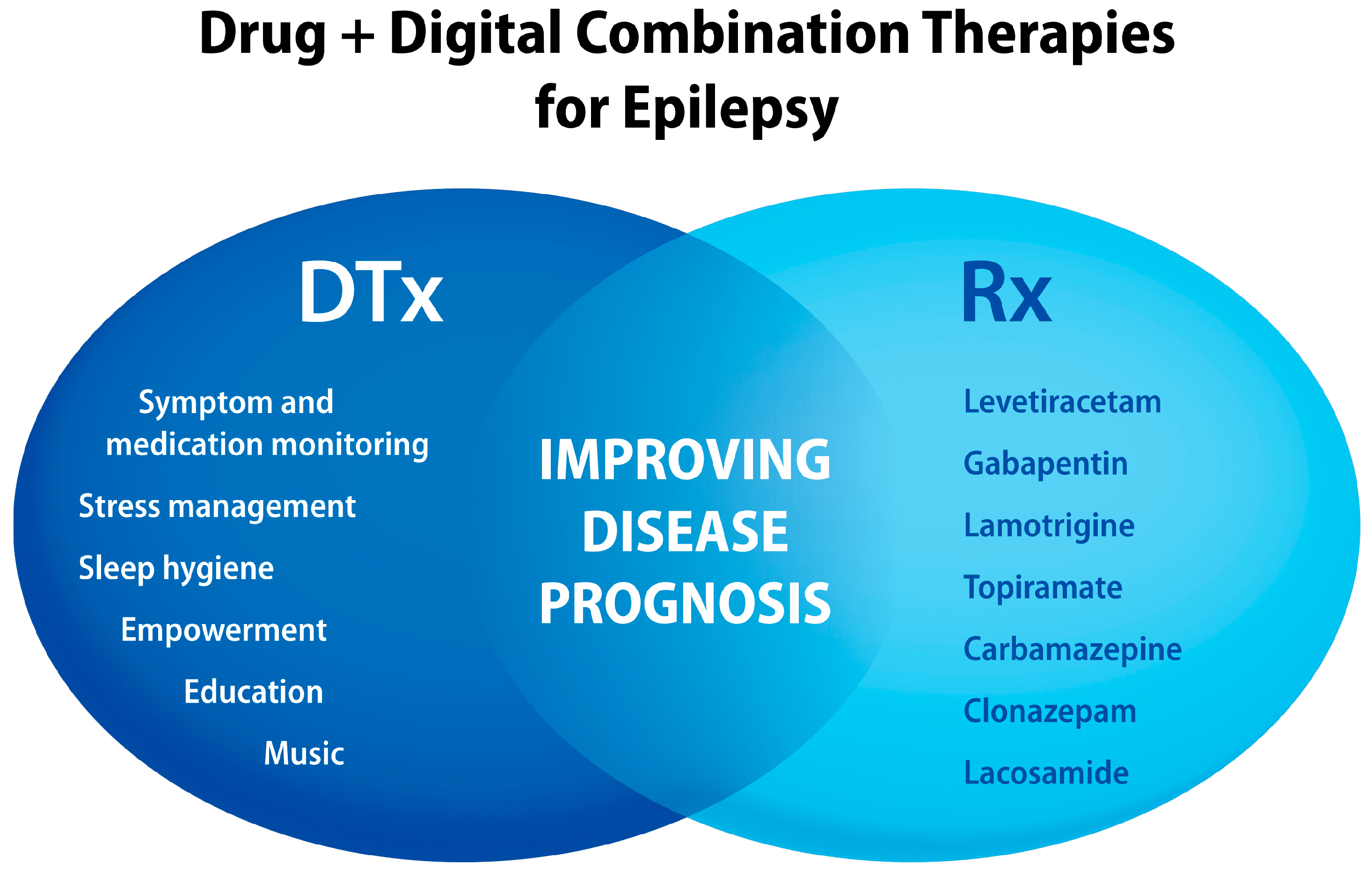

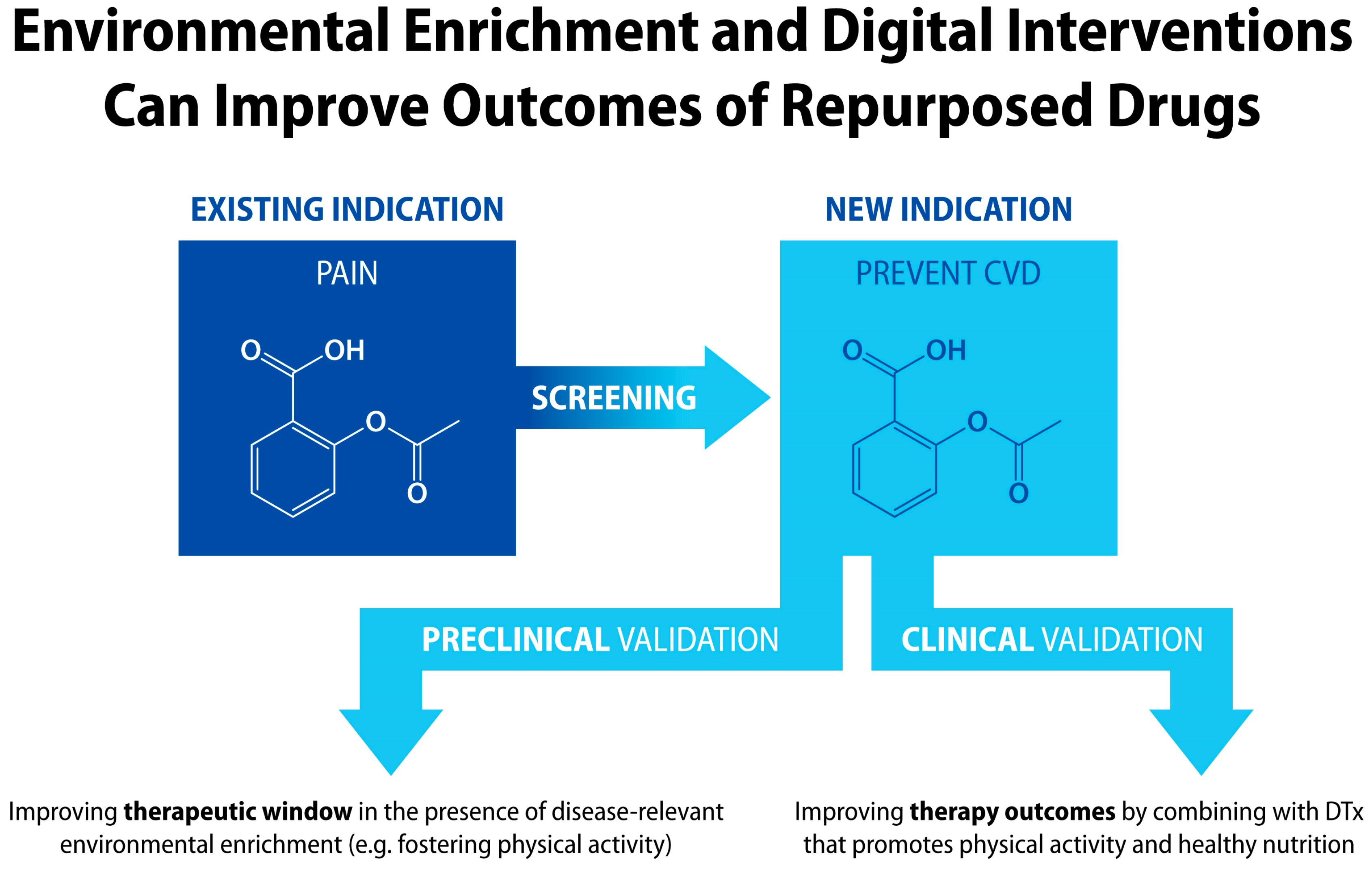
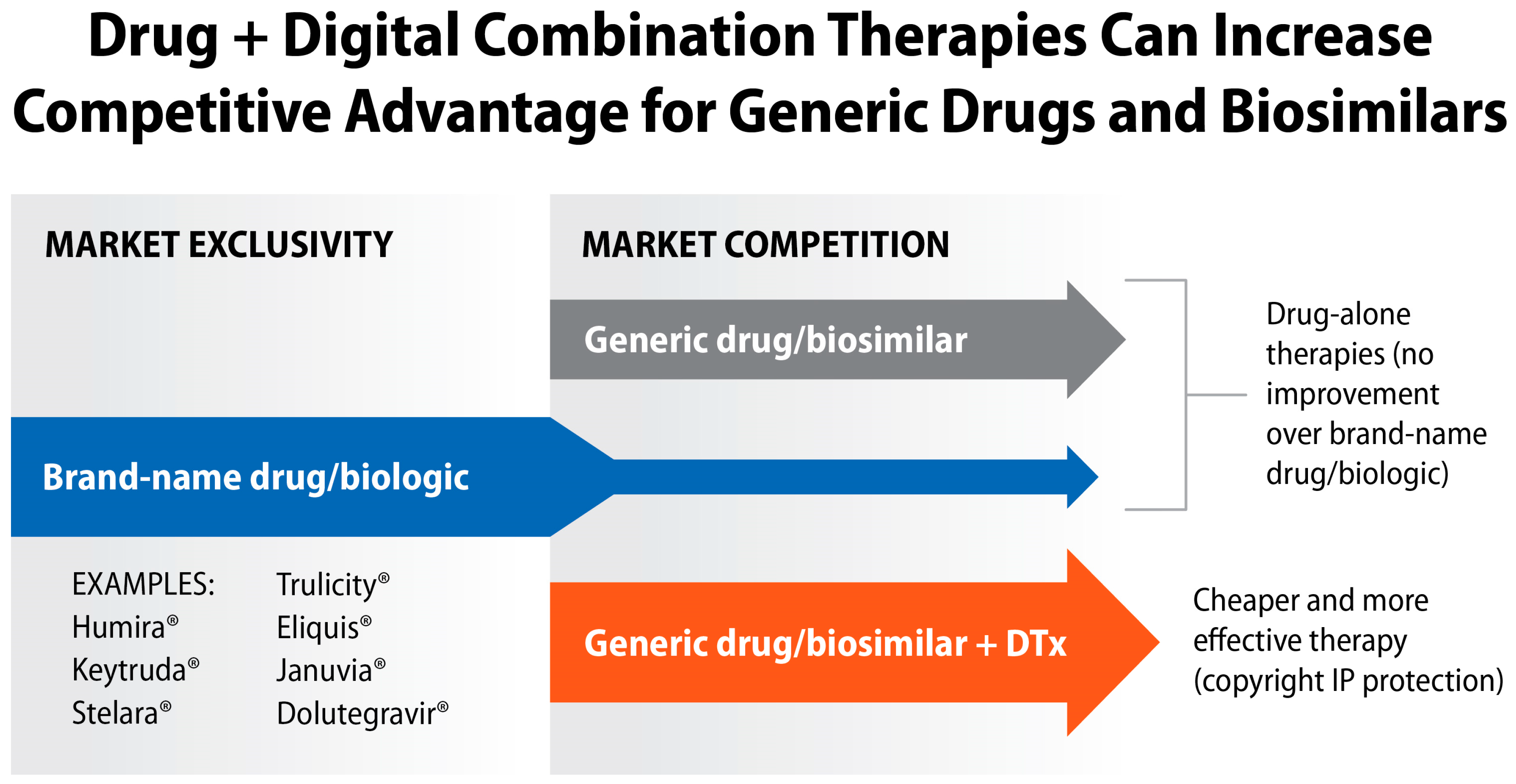
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biskupiak, Z.; Ha, V.V.; Rohaj, A.; Bulaj, G. Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases. J. Clin. Med. 2024, 13, 403. https://doi.org/10.3390/jcm13020403
Biskupiak Z, Ha VV, Rohaj A, Bulaj G. Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases. Journal of Clinical Medicine. 2024; 13(2):403. https://doi.org/10.3390/jcm13020403
Chicago/Turabian StyleBiskupiak, Zack, Victor Vinh Ha, Aarushi Rohaj, and Grzegorz Bulaj. 2024. "Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases" Journal of Clinical Medicine 13, no. 2: 403. https://doi.org/10.3390/jcm13020403
APA StyleBiskupiak, Z., Ha, V. V., Rohaj, A., & Bulaj, G. (2024). Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases. Journal of Clinical Medicine, 13(2), 403. https://doi.org/10.3390/jcm13020403




