Insomnia in Postmenopausal Women: How to Approach and Treat It?
Abstract
:1. Introduction
2. Insomnia and Sleep Disorders: Definition
3. Sleep Disturbance across Menopause: Epidemiology
4. Insomnia and Menopause: Pathogenesis and Etiology
4.1. Reproductive Hormonal Changes
4.2. Vasomotor Symptoms
4.3. Mood Disorders
4.4. Circadian Rhythm Modifications/Decreased Melatonin Secretion
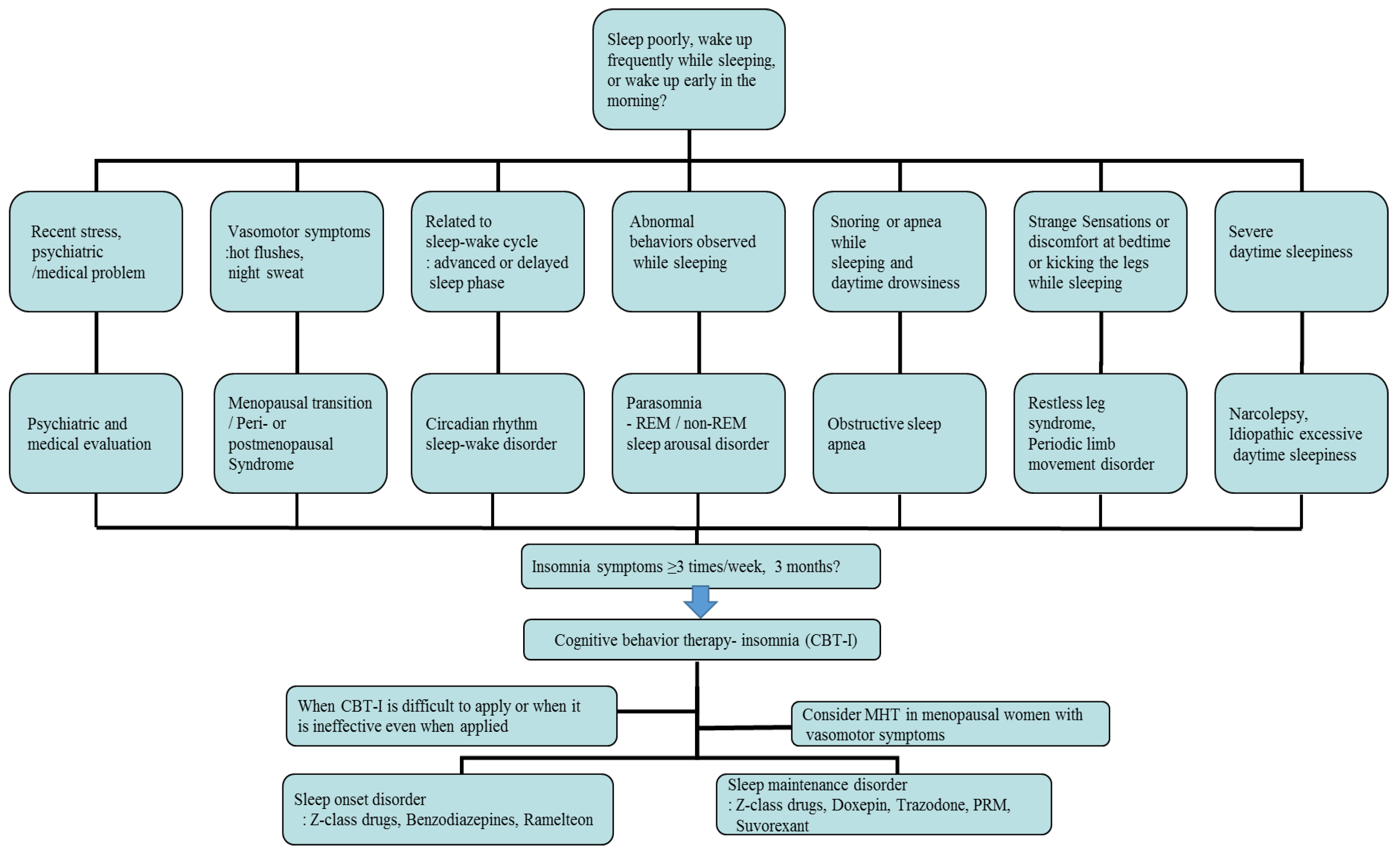
5. Assessment of Insomnia in Menopausal Women
6. Management of Insomnia in Menopausal Women
6.1. Non-Pharmacological Treatment
6.1.1. Sleep Hygiene Education
6.1.2. Sleep Restriction Therapy and Stimulus Control Therapy
6.1.3. Relaxation Training
6.1.4. Cognitive Therapy
6.2. Menopausal Hormone Therapy
6.3. Non-Hormonal Pharmacological Treatment
6.3.1. Benzodiazepines and Z-Class Drugs
6.3.2. Antidepressants
6.3.3. Melatonin
6.3.4. Orexin Antagonist and Gabapentin
7. Conclusions
Funding
Conflicts of Interest
References
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Masters-Isarilov, A.; Salifu, I.; Zizi, F.; Jean-Louis, G.; Pandi-Perumal, S.R.; Gupta, R.; Brzezinski, A.; McFarlane, S.I. Sleep disorders in postmenopausal women. J. Sleep Disord. Ther. 2015, 4, 212. [Google Scholar] [PubMed]
- Kravitz, H.M.; Zhao, X.; Bromberger, J.T.; Gold, E.B.; Hall, M.H.; Matthews, K.A.; Sowers, M.R. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 2008, 31, 979–990. [Google Scholar] [PubMed]
- Edinger, J.D.; Bonnet, M.H.; Bootzin, R.R.; Doghramji, K.; Dorsey, C.M.; Espie, C.A.; Jamieson, A.O.; McCall, W.V.; Morin, C.M.; Stepanski, E.J. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep 2004, 27, 1567–1596. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Aouad, R. The Effects of Insomnia and Sleep Loss on Cardiovascular Disease. Sleep Med. Clin. 2017, 12, 167–177. [Google Scholar] [CrossRef]
- Lee, K.A.; Baker, F.C. Sleep and Women’s Health Across the Lifespan. Sleep Med. Clin. 2018, 13, xv–xvi. [Google Scholar] [CrossRef]
- Zhang, B.; Wing, Y.K. Sex differences in insomnia: A meta-analysis. Sleep 2006, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, P.; Marra, S.; Campana, C.; Agostoni, E.C.; Palagini, L.; Nobili, L.; Nappi, R.E. Insomnia and menopause: A narrative review on mechanisms and treatments. Climacteric 2020, 23, 539–549. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Bruyneel, M. Sleep disturbances in menopausal women: Aetiology and practical aspects. Maturitas 2015, 81, 406–409. [Google Scholar] [CrossRef]
- Tandon, V.R.; Sharma, S.; Mahajan, A.; Mahajan, A.; Tandon, A. Menopause and Sleep Disorders. J. Midlife Health 2022, 13, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.B.; Sternfeld, B.; Kelsey, J.L.; Brown, C.; Mouton, C.; Reame, N.; Salamone, L.; Stellato, R. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am. J. Epidemiol. 2000, 152, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lang, C.P. Examining the relationship between subjective sleep disturbance and menopause: A systematic review and meta-analysis. Menopause 2014, 21, 1301–1318. [Google Scholar] [CrossRef]
- Lampio, L.; Saaresranta, T.; Engblom, J.; Polo, O.; Polo-Kantola, P. Predictors of sleep disturbance in menopausal transition. Maturitas 2016, 94, 137–142. [Google Scholar] [CrossRef]
- LeBlanc, M.; Mérette, C.; Savard, J.; Ivers, H.; Baillargeon, L.; Morin, C.M. Incidence and risk factors of insomnia in a population-based sample. Sleep 2009, 32, 1027–1037. [Google Scholar] [CrossRef]
- Ohayon, M.M. Severe hot flashes are associated with chronic insomnia. Arch. Intern. Med. 2006, 166, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.F.; Mitchell, E.S. Sleep symptoms during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Sleep 2010, 33, 539–549. [Google Scholar] [CrossRef]
- Walters, J.F.; Hampton, S.M.; Ferns, G.A.; Skene, D.J. Effect of menopause on melatonin and alertness rhythms investigated in constant routine conditions. Chronobiol. Int. 2005, 22, 859–872. [Google Scholar] [CrossRef]
- Eichling, P.S.; Sahni, J. Menopause related sleep disorders. J. Clin. Sleep Med. 2005, 1, 291–300. [Google Scholar] [CrossRef]
- Freedman, R.R.; Woodward, S. Core body temperature during menopausal hot flushes. Fertil. Steril. 1996, 65, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Gervais, N.J.; Mong, J.A.; Lacreuse, A. Ovarian hormones, sleep and cognition across the adult female lifespan: An integrated perspective. Front. Neuroendocr. 2017, 47, 134–153. [Google Scholar] [CrossRef]
- Herson, M.; Kulkarni, J. Hormonal Agents for the Treatment of Depression Associated with the Menopause. Drugs Aging 2022, 39, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Lancel, M.; Faulhaber, J.; Schiffelholz, T.; Romeo, E.; Di Michele, F.; Holsboer, F.; Rupprecht, R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J. Pharmacol. Exp. Ther. 1997, 282, 1213–1218. [Google Scholar] [PubMed]
- Kapur, J.; Joshi, S. Progesterone modulates neuronal excitability bidirectionally. Neurosci. Lett. 2021, 744, 135619. [Google Scholar] [CrossRef] [PubMed]
- Bairam, A.; Lumbroso, D.; Joseph, V. Effect of progesterone on respiratory response to moderate hypoxia and apnea frequency in developing rats. Respir. Physiol. Neurobiol. 2013, 185, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Pien, G.W.; Sammel, M.D.; Freeman, E.W.; Lin, H.; DeBlasis, T.L. Predictors of sleep quality in women in the menopausal transition. Sleep 2008, 31, 991–999. [Google Scholar]
- Polo-Kantola, P.; Erkkola, R.; Irjala, K.; Helenius, H.; Pullinen, S.; Polo, O. Climacteric symptoms and sleep quality. Obs. Gynecol. 1999, 94, 219–224. [Google Scholar] [CrossRef]
- Woods, N.F.; Smith-Dijulio, K.; Percival, D.B.; Tao, E.Y.; Taylor, H.J.; Mitchell, E.S. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: Observations from the Seattle Midlife Women’s Health Study. J. Womens Health 2007, 16, 667–677. [Google Scholar] [CrossRef]
- Kravitz, H.M.; Janssen, I.; Santoro, N.; Bromberger, J.T.; Schocken, M.; Everson-Rose, S.A.; Karavolos, K.; Powell, L.H. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch. Intern. Med. 2005, 165, 2370–2376. [Google Scholar] [CrossRef]
- Avis, N.E.; Crawford, S.L.; Greendale, G.; Bromberger, J.T.; Everson-Rose, S.A.; Gold, E.B.; Hess, R.; Joffe, H.; Kravitz, H.M.; Tepper, P.G.; et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern. Med. 2015, 175, 531–539. [Google Scholar] [CrossRef]
- de Zambotti, M.; Colrain, I.M.; Javitz, H.S.; Baker, F.C. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil. Steril. 2014, 102, 1708–1715.e1701. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.T.; Kim, S.; Galvan, T.; White, D.P.; Joffe, H. Nocturnal Hot Flashes: Relationship to Objective Awakenings and Sleep Stage Transitions. J. Clin. Sleep Med. 2016, 12, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, H.M.; Joffe, H. Sleep during the perimenopause: A SWAN story. Obs. Gynecol. Clin. N. Am. 2011, 38, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J.; Campbell, S.S. Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep 2007, 30, 1788–1794. [Google Scholar] [CrossRef]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Coo Calcagni, S.; Bei, B.; Milgrom, J.; Trinder, J. The relationship between sleep and mood in first-time and experienced mothers. Behav. Sleep Med. 2012, 10, 167–179. [Google Scholar] [CrossRef]
- Thurston, R.C.; Bromberger, J.T.; Joffe, H.; Avis, N.E.; Hess, R.; Crandall, C.J.; Chang, Y.; Green, R.; Matthews, K.A. Beyond frequency: Who is most bothered by vasomotor symptoms? Menopause 2008, 15, 841–847. [Google Scholar] [CrossRef]
- Vousoura, E.; Spyropoulou, A.C.; Koundi, K.L.; Tzavara, C.; Verdeli, H.; Paparrigopoulos, T.; Augoulea, A.; Lambrinoudaki, I.; Zervas, I.M. Vasomotor and depression symptoms may be associated with different sleep disturbance patterns in postmenopausal women. Menopause 2015, 22, 1053–1057. [Google Scholar] [CrossRef]
- Pines, A. Circadian rhythm and menopause. Climacteric 2016, 19, 551–552. [Google Scholar] [CrossRef]
- Cagnacci, A. Role of melatonin in circadian rhythm at menopause. Climacteric 2017, 20, 183. [Google Scholar] [CrossRef]
- Carranza-Lira, S.; García López, F. Melatonin and climactery. Med. Sci. Monit. 2000, 6, 1209–1212. [Google Scholar] [PubMed]
- Baker, F.C.; de Zambotti, M.; Colrain, I.M.; Bei, B. Sleep problems during the menopausal transition: Prevalence, impact, and management challenges. Nat. Sci. Sleep 2018, 10, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Caretto, M.; Giannini, A.; Simoncini, T. An integrated approach to diagnosing and managing sleep disorders in menopausal women. Maturitas 2019, 128, 1–3. [Google Scholar] [CrossRef]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Haynes, J.; Talbert, M.; Fox, S.; Close, E. Cognitive Behavioral Therapy in the Treatment of Insomnia. South. Med. J. 2018, 111, 75–80. [Google Scholar] [CrossRef]
- Dopheide, J.A. Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am. J. Manag. Care 2020, 26, S76–S84. [Google Scholar] [CrossRef]
- Trauer, J.M.; Qian, M.Y.; Doyle, J.S.; Rajaratnam, S.M.; Cunnington, D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 191–204. [Google Scholar] [CrossRef]
- McCurry, S.M.; Guthrie, K.A.; Morin, C.M.; Woods, N.F.; Landis, C.A.; Ensrud, K.E.; Larson, J.C.; Joffe, H.; Cohen, L.S.; Hunt, J.R.; et al. Telephone-Based Cognitive Behavioral Therapy for Insomnia in Perimenopausal and Postmenopausal Women with Vasomotor Symptoms: A MsFLASH Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 913–920. [Google Scholar] [CrossRef]
- Guthrie, K.A.; Larson, J.C.; Ensrud, K.E.; Anderson, G.L.; Carpenter, J.S.; Freeman, E.W.; Joffe, H.; LaCroix, A.Z.; Manson, J.E.; Morin, C.M.; et al. Effects of Pharmacologic and Nonpharmacologic Interventions on Insomnia Symptoms and Self-reported Sleep Quality in Women with Hot Flashes: A Pooled Analysis of Individual Participant Data from Four MsFLASH Trials. Sleep 2018, 41, zsx190. [Google Scholar] [CrossRef]
- Kim, S.J.; Benloucif, S.; Reid, K.J.; Weintraub, S.; Kennedy, N.; Wolfe, L.F.; Zee, P.C. Phase-shifting response to light in older adults. J. Physiol. 2014, 592, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Cintron, D.; Lipford, M.; Larrea-Mantilla, L.; Spencer-Bonilla, G.; Lloyd, R.; Gionfriddo, M.R.; Gunjal, S.; Farrell, A.M.; Miller, V.M.; Murad, M.H. Efficacy of menopausal hormone therapy on sleep quality: Systematic review and meta-analysis. Endocrine 2017, 55, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Welton, A.J.; Vickers, M.R.; Kim, J.; Ford, D.; Lawton, B.A.; MacLennan, A.H.; Meredith, S.K.; Martin, J.; Meade, T.W. Health related quality of life after combined hormone replacement therapy: Randomised controlled trial. BMJ 2008, 337, a1190. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Monteleone, P.; Benussi, C.; Bevilacqua, G.; Vacca, F.; Genazzani, A.R. Effects of low-dose, continuous combined hormone replacement therapy on sleep in symptomatic postmenopausal women. Maturitas 2005, 50, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Rosano, G.; Cappagli, B.; Pepe, A.; Vitale, C.; Genazzani, A.R. Clinical and metabolic effects of drospirenone-estradiol in menopausal women: A prospective study. Climacteric 2011, 14, 18–24. [Google Scholar] [CrossRef]
- Kagan, R.; Constantine, G.; Kaunitz, A.M.; Bernick, B.; Mirkin, S. Improvement in sleep outcomes with a 17β-estradiol-progesterone oral capsule (TX-001HR) for postmenopausal women. Menopause 2018, 26, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Guthrie, K.A.; Hohensee, C.; Caan, B.; Carpenter, J.S.; Freeman, E.W.; LaCroix, A.Z.; Landis, C.A.; Manson, J.; Newton, K.M.; et al. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep 2015, 38, 97–108. [Google Scholar] [CrossRef]
- Lindberg, E.; Bonsignore, M.R.; Polo-Kantola, P. Role of menopause and hormone replacement therapy in sleep-disordered breathing. Sleep Med. Rev. 2020, 49, 101225. [Google Scholar] [CrossRef]
- Mirer, A.G.; Peppard, P.E.; Palta, M.; Benca, R.M.; Rasmuson, A.; Young, T. Menopausal hormone therapy and sleep-disordered breathing: Evidence for a healthy user bias. Ann. Epidemiol. 2015, 25, 779–784.e771. [Google Scholar] [CrossRef]
- Scharf, M.B.; McDannold, M.D.; Stover, R.; Zaretsky, N.; Berkowitz, D.V. Effects of estrogen replacement therapy on rates of cyclic alternating patterns and hot-flush events during sleep in postmenopausal women: A pilot study. Clin. Ther. 1997, 19, 304–311. [Google Scholar] [CrossRef]
- Bliwise, N.G. Factors related to sleep quality in healthy elderly women. Psychol. Aging 1992, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Saletu, B.; Anderer, P.; Gruber, G.; Mandl, M.; Gruber, D.; Metka, M.; Johannes, H.; Loeffler-Stastka, H.; Hassan, B.; Saletu-Zyhlarz, G. Insomnia related to postmenopausal syndrome: Sleep laboratory studies on differences between patients and normal controls, and influence of an estrogen-progestogen combination with dienogest Versus estrogen alone and placebo. Drugs Today 2001, 37, 39–62. [Google Scholar]
- Tal, J.Z.; Suh, S.A.; Dowdle, C.L.; Nowakowski, S. Treatment of Insomnia, Insomnia Symptoms, and Obstructive Sleep Apnea During and After Menopause: Therapeutic Approaches. Curr. Psychiatry Rev. 2015, 11, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Polo-Kantola, P.; Erkkola, R.; Helenius, H.; Irjala, K.; Polo, O. When does estrogen replacement therapy improve sleep quality? Am. J. Obs. Gynecol. 1998, 178, 1002–1009. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Use of hormone replacement therapy and risk of venous thromboembolism: Nested case-control studies using the QResearch and CPRD databases. BMJ 2019, 364, k4810. [Google Scholar] [CrossRef]
- Prior, J.C. Progesterone for treatment of symptomatic menopausal women. Climacteric 2018, 21, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.E., 3rd; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- MacFarlane, J.; Morin, C.M.; Montplaisir, J. Hypnotics in insomnia: The experience of zolpidem. Clin. Ther. 2014, 36, 1676–1701. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef]
- Dorsey, C.M.; Lee, K.A.; Scharf, M.B. Effect of zolpidem on sleep in women with perimenopausal and postmenopausal insomnia: A 4-week, randomized, multicenter, double-blind, placebo-controlled study. Clin. Ther. 2004, 26, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; Petrillo, L.; Viguera, A.; Koukopoulos, A.; Silver-Heilman, K.; Farrell, A.; Yu, G.; Silver, M.; Cohen, L.S. Eszopiclone improves insomnia and depressive and anxious symptoms in perimenopausal and postmenopausal women with hot flashes: A randomized, double-blinded, placebo-controlled crossover trial. Am. J. Obs. Gynecol. 2010, 202, 171.e1–171.e11. [Google Scholar] [CrossRef]
- French, D.D.; Spehar, A.M.; Campbell, R.R.; Palacios, P.; Coakley, R.W.; Coblio, N.; Means, H.; Werner, D.C.; Angaran, D.M. Advances in Patient Safety Outpatient Benzodiazepine Prescribing, Adverse Events, and Costs. In Advances in Patient Safety: From Research to Implementation (Volume 1: Research Findings); Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2005. [Google Scholar]
- Capiau, A.; Huys, L.; van Poelgeest, E.; van der Velde, N.; Petrovic, M.; Somers, A. Therapeutic dilemmas with benzodiazepines and Z-drugs: Insomnia and anxiety disorders versus increased fall risk: A clinical review. Eur. Geriatr. Med. 2023, 14, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Ma, X.L.; Ma, J.X.; Wang, J.; Yang, Y.; Chen, Y. Association between use of benzodiazepines and risk of fractures: A meta-analysis. Osteoporos. Int. 2014, 25, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Treves, N.; Perlman, A.; Kolenberg Geron, L.; Asaly, A.; Matok, I. Z-drugs and risk for falls and fractures in older adults-a systematic review and meta-analysis. Age Ageing 2018, 47, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Devnani, P.; Fernandes, R. Management of REM sleep behavior disorder: An evidence based review. Ann. Indian Acad. Neurol. 2015, 18, 1–5. [Google Scholar] [CrossRef]
- Garcia-Borreguero, D.; Stillman, P.; Benes, H.; Buschmann, H.; Chaudhuri, K.R.; Gonzalez Rodríguez, V.M.; Högl, B.; Kohnen, R.; Monti, G.C.; Stiasny-Kolster, K.; et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011, 11, 28. [Google Scholar] [CrossRef]
- Mason, M.; Cates, C.J.; Smith, I. Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea. Cochrane Database Syst. Rev. 2015, 14, CD011090. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Sloan, J.; Stearns, V.; Slack, R.; Iyengar, M.; Diekmann, B.; Kimmick, G.; Lovato, J.; Gordon, P.; Pandya, K.; et al. Newer antidepressants and gabapentin for hot flashes: An individual patient pooled analysis. J. Clin. Oncol. 2009, 27, 2831–2837. [Google Scholar] [CrossRef]
- Biglia, N.; Bounous, V.E.; De Seta, F.; Lello, S.; Nappi, R.E.; Paoletti, A.M. Non-hormonal strategies for managing menopausal symptoms in cancer survivors: An update. Ecancermedicalscience 2019, 13, 909. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Joffe, H.; Guthrie, K.A.; Larson, J.C.; Reed, S.D.; Newton, K.M.; Sternfeld, B.; Lacroix, A.Z.; Landis, C.A.; Woods, N.F.; et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: A randomized controlled trial. Menopause 2012, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, F.; Chen, Y.; Kong, S.; Huang, Q.; Lyu, D.; Yang, W.; Wei, Z.; Qian, N.; Zhang, M.; et al. Difference in the regulation of biological rhythm symptoms of Major depressive disorder between escitalopram and mirtazapine. J. Affect. Disord. 2022, 296, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.F.; Chung, K.F.; Yung, K.P.; Ng, T.H. Doxepin for insomnia: A systematic review of randomized placebo-controlled trials. Sleep Med. Rev. 2015, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci. 2017, 14, 24–34. [Google Scholar] [PubMed]
- Generali, J.A.; Cada, D.J. Trazodone: Insomnia (Adults). Hosp. Pharm. 2015, 50, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Bellipanni, G.; Di Marzo, F.; Blasi, F.; Di Marzo, A. Effects of melatonin in perimenopausal and menopausal women: Our personal experience. Ann. N. Y. Acad. Sci. 2005, 1057, 393–402. [Google Scholar] [CrossRef]
- Wilson, S.J.; Nutt, D.J.; Alford, C.; Argyropoulos, S.V.; Baldwin, D.S.; Bateson, A.N.; Britton, T.C.; Crowe, C.; Dijk, D.J.; Espie, C.A.; et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J. Psychopharmacol. 2010, 24, 1577–1601. [Google Scholar] [CrossRef]
- Wade, A.G.; Ford, I.; Crawford, G.; McMahon, A.D.; Nir, T.; Laudon, M.; Zisapel, N. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: Quality of sleep and next-day alertness outcomes. Curr. Med. Res. Opin. 2007, 23, 2597–2605. [Google Scholar] [CrossRef]
- Yi, M.; Wang, S.; Wu, T.; Zhang, X.; Jiang, L.; Fang, X. Effects of exogenous melatonin on sleep quality and menopausal symptoms in menopausal women: A systematic review and meta-analysis of randomized controlled trials. Menopause 2021, 28, 717–725. [Google Scholar] [CrossRef]
- Chung, S.; Youn, S.; Park, B.; Lee, S.; Kim, C. The Effectiveness of Prolonged-Release Melatonin in Primary Insomnia Patients with a Regular Sleep-Wake Cycle. Sleep Med. Res. 2016, 7, 16–20. [Google Scholar] [CrossRef]
- Lemoine, P.; Zisapel, N. Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia. Expert. Opin. Pharmacother. 2012, 13, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Nathan, M.D.; Wiley, A.; Crawford, S.; Cohn, A.Y.; Harder, J.A.; Grant, L.K.; Erickson, A.; Srivastava, A.; McCormick, K.; et al. A double-blind, randomized, placebo-controlled trial of suvorexant for the treatment of vasomotor symptom-associated insomnia disorder in midlife women. Sleep 2022, 45, zsac007. [Google Scholar] [CrossRef] [PubMed]
- Yurcheshen, M.E.; Guttuso, T., Jr.; McDermott, M.; Holloway, R.G.; Perlis, M. Effects of gabapentin on sleep in menopausal women with hot flashes as measured by a Pittsburgh Sleep Quality Index factor scoring model. J. Womens Health 2009, 18, 1355–1360. [Google Scholar] [CrossRef]
- Guttuso, T., Jr. Nighttime awakenings responding to gabapentin therapy in late premenopausal women: A case series. J. Clin. Sleep Med. 2012, 8, 187–189. [Google Scholar] [CrossRef]
| Physiologic/Physical |
|---|
| Age Circadian rhythm modifications Decreased melatonin secretion Female sexual hormone changes Decreased estrogen and progesterone, increased FSH Menopausal symptoms Hot flushes, night sweats Others Bladder problems, Ill health, chronic pain—musculoskeletal disorders, osteoarthritis, fibromyalgia, cancer, etc. Poor sleep hygiene/circumstances Medication, coffee, smoking |
| Psychiatric/Psycho-social |
| Mood disorder—depression Anxiety Illegal drugs, alcohol intake Others—familial/economic/social problem: stress, bereavement, divorce, unemployment, finances, etc. |
| Comorbid diseases with sleep disorders |
| Obstructive sleep apnea Restless legs syndrome Periodic limb movement syndrome |
| Others |
| Circadian rhythm sleep–wake disorder Narcolepsy, idiopathic hypersomnia Parasomnias |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, G.-H. Insomnia in Postmenopausal Women: How to Approach and Treat It? J. Clin. Med. 2024, 13, 428. https://doi.org/10.3390/jcm13020428
Jeon G-H. Insomnia in Postmenopausal Women: How to Approach and Treat It? Journal of Clinical Medicine. 2024; 13(2):428. https://doi.org/10.3390/jcm13020428
Chicago/Turabian StyleJeon, Gyun-Ho. 2024. "Insomnia in Postmenopausal Women: How to Approach and Treat It?" Journal of Clinical Medicine 13, no. 2: 428. https://doi.org/10.3390/jcm13020428





