The Prevalence of Endometriosis in Patients with Unexplained Infertility
Abstract
1. Introduction
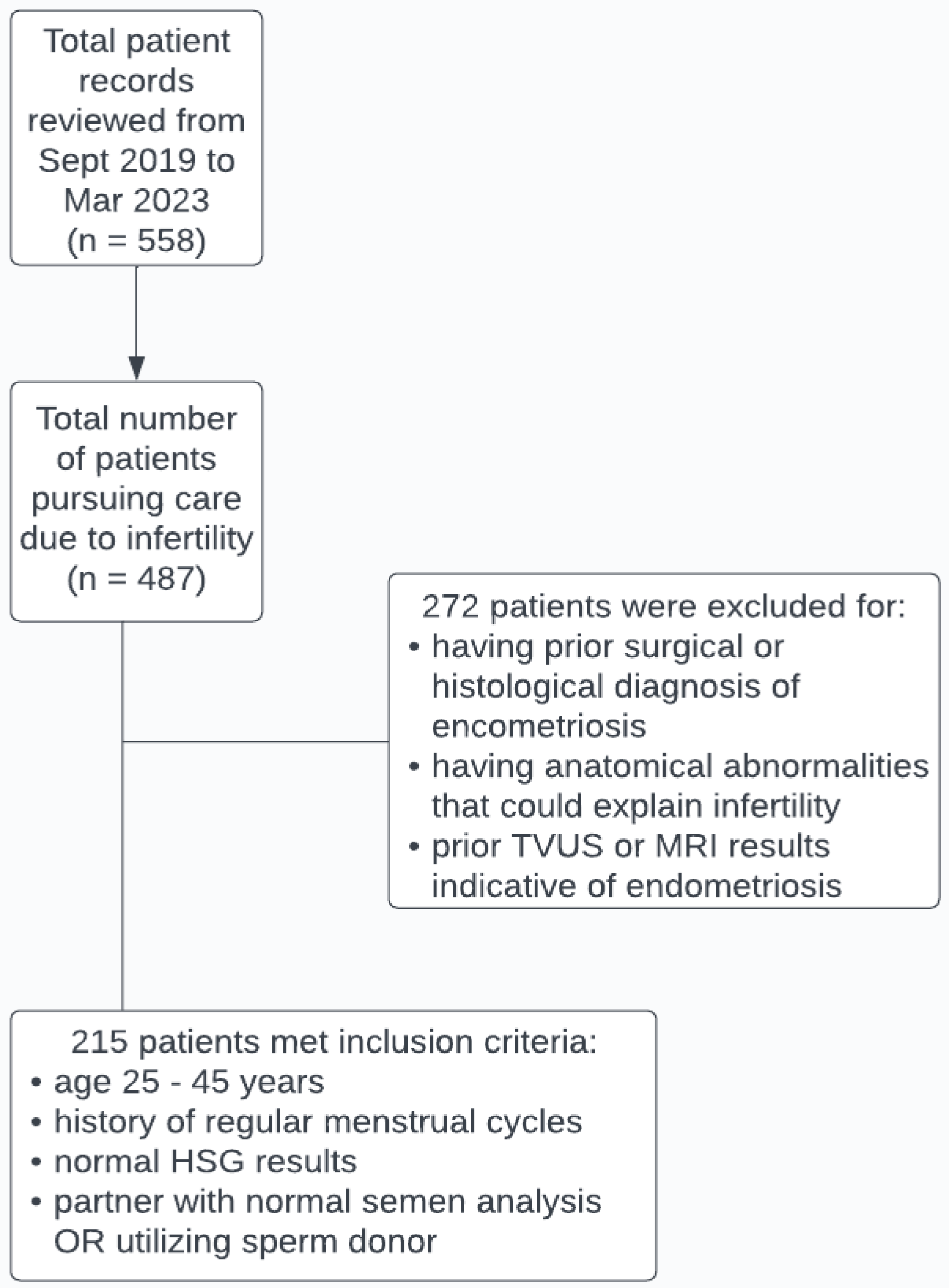
2. Materials and Methods
2.1. Subjects and Data Collection
2.2. Surgical and Histological Evaluation
2.3. Statistical Analysis
3. Results
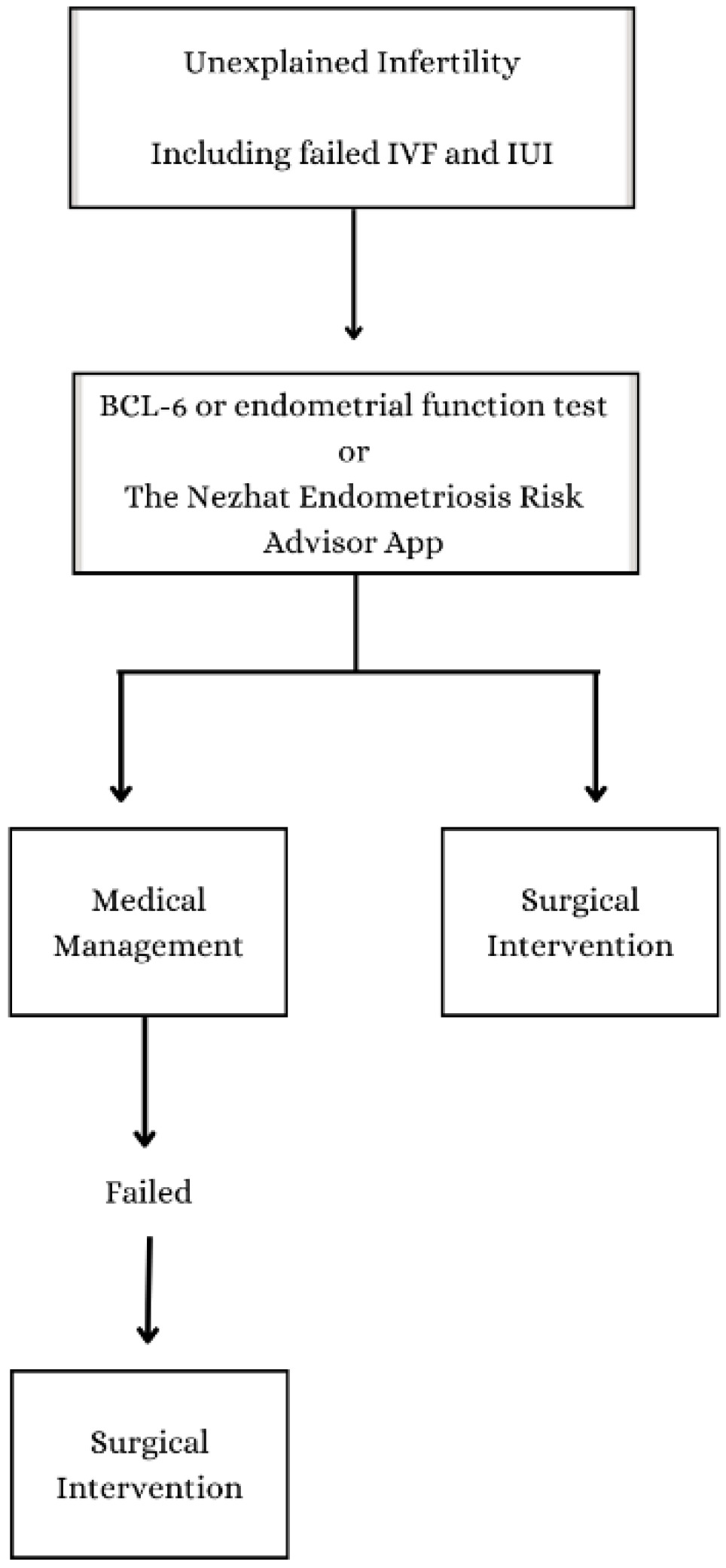
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Noël, J.-C.; Chapron, C.; Bucella, D.; Buxant, F.; Peny, M.-O.; Fayt, I.; Borghese, B.; Anaf, V. Estrogen and progesterone receptors in smooth muscle component of deep infiltrating endometriosis. Fertil. Steril. 2010, 93, 1774–1777. [Google Scholar] [CrossRef]
- Donnez, J. Introduction: From pathogenesis to therapy, deep endometriosis remains a source of controversy. Fertil. Steril. 2017, 108, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.E. Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J. Am. Assoc. Gynecol. Laparoscopists 1994, 2, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.; Munro, D.; Clarke, J. Endometriosis Is Undervalued: A Call to Action. Front. Glob. Women’s Health 2022, 3, 902371. [Google Scholar] [CrossRef]
- Huang, J.Q.; Lathi, R.B.; Lemyre, M.; Rodriguez, H.E.; Nezhat, C.H.; Nezhat, C. Coexistence of endometriosis in women with symptomatic leiomyomas. Fertil. Steril. 2010, 94, 720–723. [Google Scholar] [CrossRef]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef]
- Nezhat, F.R.; Pejovic, T.; Reis, F.M.; Guo, S.-W. The Link between Endometriosis and Ovarian Cancer: Clinical Implications. Int. J. Gynecol. Cancer 2014, 24, 623–628. [Google Scholar] [CrossRef]
- Nezhat, C.; Li, A.; Abed, S.; Balassiano, E.; Soliemannjad, R.; Nezhat, A.; Nezhat, C.H.; Nezhat, F. Strong Association between Endometriosis and Symptomatic Leiomyomas. JSLS 2016, 20, e2016.00053. [Google Scholar] [CrossRef]
- Nezhat, C.; Nezhat, F.; Nezhat, C. Endometriosis: Ancient Disease, Ancient Treatments. Fertil. Steril. 2012, 98, S1–S62. [Google Scholar] [CrossRef]
- Nezhat, F.; Nezhat, C.; Nezhat, C.H.; Levy, J.S.; Smith, E.; Katz, L. Use of Hysteroscopy in Addition to Laparoscopy for Evaluating Chronic Pelvic Pain. Obstet. Gynecol. Surv. 1995, 50, 721–722. [Google Scholar] [CrossRef]
- Zullo, F.; Spagnolo, E.; Saccone, G.; Acunzo, M.; Xodo, S.; Ceccaroni, M.; Berghella, V. Endometriosis and obstetrics complications: A systematic review and meta-analysis. Fertil. Steril. 2017, 108, 667–672.e5. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, F.; Datta, M.S.; Hanson, V.; Pejovic, T.; Nezhat, C.; Nezhat, C. The relationship of endometriosis and ovarian malignancy: A review. Fertil. Steril. 2008, 90, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, F.; Apostol, R.; Mahmoud, M.; el Daouk, M. Malignant transformation of endometriosis and its clinical significance. Fertil. Steril. 2014, 102, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-C.; Yang, Y.-C.; Wang, J.-H.; Lin, S.-Z.; Ding, D.-C. Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women. J. Clin. Med. 2021, 10, 4173. [Google Scholar] [CrossRef]
- Melo, A.S.; Rosa-E-Silva, J.C.; Rosa-E-Silva, A.C.J.D.S.; Poli-Neto, O.B.; Ferriani, R.A.; Vieira, C.S. Unfavorable lipid profile in women with endometriosis. Fertil. Steril. 2010, 93, 2433–2436. [Google Scholar] [CrossRef]
- Okoth, K.; Wang, J.; Zemedikun, D.; Thomas, G.N.; Nirantharakumar, K.; Adderley, N.J. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: A retrospective matched cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1598–1609. [Google Scholar] [CrossRef]
- Sinaii, N.; Cleary, S.; Ballweg, M.; Nieman, L.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; A Missmer, S.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [PubMed]
- Sims, O.T.; Gupta, J.; Missmer, S.A.; Aninye, I.O. Stigma and Endometriosis: A Brief Overview and Recommendations to Improve Psychosocial Well-Being and Diagnostic Delay. Int. J. Environ. Res. Public Health 2021, 18, 8210. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, V.H.; Decter, D.H.; Chodick, G.; Shalev, V.; Weil, C. Burden of Endometriosis: Infertility, Comorbidities, and Healthcare Resource Utilization. J. Clin. Med. 2022, 11, 1133. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef]
- Moradi, M.; Parker, M.; Sneddon, A.; Lopez, V.; Ellwood, D. Impact of endometriosis on women’s lives: A qualitative study. BMC Women’s Health 2014, 14, 123. [Google Scholar] [CrossRef]
- Soliman, A.M.; Coyne, K.S.; Gries, K.S.; Castelli-Haley, J.; Snabes, M.C.; Surrey, E.S. The Effect of Endometriosis Symptoms on Absenteeism and Presenteeism in the Workplace and at Home. J. Manag. Care Spéc. Pharm. 2017, 23, 745–754. [Google Scholar] [CrossRef]
- Soliman, A.M.; Surrey, E.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Real-World Evaluation of Direct and Indirect Economic Burden Among Endometriosis Patients in the United States. Adv. Ther. 2018, 35, 408–423. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.R. Unexplained Infertility, the Controversial Matter in Management of Infertile Couples. J. Reprod. Infertil. 2015, 16, 1–2. [Google Scholar] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Ray, A.; Shah, A.; Gudi, A.; Homburg, R. Unexplained infertility: An update and review of practice. Reprod. Biomed. Online 2012, 24, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.; Showstack, J.; Smith, J.F.; Nachtigall, R.D.; Millstein, S.G.; Wing, H.; Eisenberg, M.L.; Pasch, L.A.; Croughan, M.S.; Adler, N. Costs of infertility treatment: Results from an 18-month prospective cohort study. Fertil. Steril. 2011, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- The Guideline Group on Unexplained Infertility; Romualdi, D.; Ata, B.; Bhattacharya, S.; Bosch, E.; Costello, M.; Gersak, K.; Homburg, R.; Mincheva, M.; Norman, R.J.; et al. Evidence-based guideline: Unexplained infertility. Hum. Reprod. 2023, 38, 1881–1890. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef]
- Nisenblat, V.; Prentice, L.; Bossuyt, P.M.; Farquhar, C.; Hull, M.L.; Johnson, N. Combination of the non-invasive tests for the diagnosis of endometriosis. Emergencias 2016, 2016, CD012281. [Google Scholar] [CrossRef]
- Stratton, P.; A Winkel, C.; Sinaii, N.; Merino, M.J.; Zimmer, C.; Nieman, L.K. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertil. Steril. 2002, 78, 743–749. [Google Scholar] [CrossRef]
- Nezhat, C.; Armani, E.; Chen, H.-C.C.; Najmi, Z.; Lindheim, S.R.; Nezhat, C. Use of the Free Endometriosis Risk Advisor App as a Non-Invasive Screening Test for Endometriosis in Patients with Chronic Pelvic Pain and/or Unexplained Infertility. J. Clin. Med. 2023, 12, 5234. [Google Scholar] [CrossRef]
- Nezhat, C.; Rambhatla, A.; Miranda-Silva, C.; Asiaii, A.; Nguyen, K.; Eyvazzadeh, A.; Tazuke, S.; Agarwal, S.; Jun, S.; Nezhat, A.; et al. BCL-6 Overexpression as a Predictor for Endometriosis in Patients Undergoing In Vitro Fertilization. JSLS J. Soc. Laparosc. Robot. Surg. 2020, 24, e2020.00064. [Google Scholar] [CrossRef] [PubMed]
- Kliman, H.J.; Frankfurter, D. Clinical approach to recurrent implantation failure: Evidence-based evaluation of the en-dometrium. Fertil. Steril. 2019, 111, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Guleken, Z.; Bulut, H.; Depciuch, J.; Tarhan, N. Diagnosis of endometriosis using endometrioma volume and vibrational spectroscopy with multivariate methods as a noninvasive method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120246. [Google Scholar] [CrossRef]
- Janša, V.; Novak, M.P.; Frangež, H.B.; Rižner, T.L. TGFBI as a candidate biomarker for non-invasive diagnosis of early-stage endometriosis. Hum. Reprod. 2023, 38, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.; Kulkarni, M.T.; Missmer, S.A. Is Endometriosis More Common and More Severe than It Was 30 Years Ago? J. Minim. Invasive Gynecol. 2020, 27, 452–461. [Google Scholar] [CrossRef]
- Göçmen, A.; Atak, T. Diagnostic laparoscopy findings in unexplained infertility cases. Clin. Exp. Obstet. Gynecol. 2012, 39, 452–453. [Google Scholar] [PubMed]
- Littman, E.; Giudice, L.; Lathi, R.; Berker, B.; Milki, A.; Nezhat, C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil. Steril. 2005, 84, 1574–1578. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ohgi, S.; Horikawa, T.; Kojima, R.; Ito, M.; Saito, H. Laparoscopy should be strongly considered for women with unexplained infertility. J. Obstet. Gynaecol. Res. 2007, 33, 665–670. [Google Scholar] [CrossRef]
- Tsuji, I.; Ami, K.; Miyazaki, A.; Hujinami, N.; Hoshiai, H. Benefit of Diagnostic Laparoscopy for Patients with Unexplained Infertility and Normal Hysterosalpingography Findings. Tohoku J. Exp. Med. 2009, 219, 39–42. [Google Scholar] [CrossRef][Green Version]
- Naess, K. [Pharmacology (2). Chemotherapy]. Fag Tidsskr Sykepl. 1990, 78, 21–23. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.J.; Hentz, J.G.; Magtibay, P.M.; Cornella, J.L.; Magrina, J.F. Endometriosis: Correlation between histologic and visual findings at laparoscopy. Am. J. Obstet. Gynecol. 2001, 184, 1407–1413. [Google Scholar] [CrossRef]
- Gichuhi, J.; Ogengo, J.; Gichangi, P. Endometriosis Diagnosis Correlation of Laparoscopic Visualization and Histopathology Confirmation in Low Resource Setting. Open J. Obstet. Gynecol. 2021, 11, 845–852. [Google Scholar] [CrossRef]
- Clement, P.B. The pathology of endometriosis: A survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef]
- Nezhat, C.H.; Hincapie, M.; Hauser, K.; Nezhat, F.R. Cancer Arising from Endometriosis. 2018. Available online: Ciné-Medcine-med.com/shoppingcart/index.php?nav=nursing&id=ACS-5616 (accessed on 31 December 2023).
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Gerbie, A.B.; Merrill, J.A. Pathology of Endometriosis. Clin. Obstet. Gynecol. 1988, 31, 779. [Google Scholar] [CrossRef]
- Portuondo, J.A.; Herrán, C.; Echanojauregui, A.D.; Riego, A.G. Peritoneal flushing and biopsy in laparoscopically diagnosed endometriosis. Fertil. Steril. 1982, 38, 538–541. [Google Scholar] [CrossRef]
- Stephen, N.; Gochhait, D.; Anbalagan, A.; Siddaraju, N. Haemosiderin-laden mesothelial cells in ascitic fluid: A soft point-er for endometriosis in a female of reproductive age. Cytopathology 2021, 32, 553–554. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.; Xu, H.; Guo, S.-W. Mesothelial Cells Participate in Endometriosis Fibrogenesis Through Platelet-Induced Mesothelial-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2020, 105, e4124–e4147. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar]
- Hudelist, G.; Valentin, L.; Saridogan, E.; Condous, G.; Malzoni, M.; Roman, H.; Jurkovic, D.; Keckstein, J. What to choose and why to use—A critical review on the clinical relevance of rASRM, EFI and Enzian classifications of endometriosis. Facts Views Vis. Obgyn 2023, 13, 331–338. [Google Scholar] [CrossRef]
- Holoch, K.J.; Lessey, B.A. Endometriosis and Infertility. Clin. Obstet. Gynecol. 2010, 53, 429. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Falcone, T.; Flyckt, R. Surgical Management of Endometriosis in Patients with Chronic Pelvic Pain. Semin. Reprod. Med. 2017, 35, 054–064. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Gallos, I.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Králíčková, M.; Laganà, A.S.; Ghezzi, F.; Vetvicka, V. Endometriosis and risk of ovarian cancer: What do we know? Arch. Gynecol. Obstet. 2020, 301, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hermens, M.; van Altena, A.M.; Bulten, J.; Siebers, A.G.; Bekkers, R.L. Increased association of ovarian cancer in women with histological proven endosalpingiosis. Cancer Epidemiol. 2020, 65, 101700. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Pejovic, T.; Nezhat, F. Induction of ovulation and ovarian cancer: A critical review of the literature. Fertil. Steril. 2006, 85, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.; Yamada, R.; Lee, R.; Sauer, M.V.; Ananth, C.V. Risk of Stroke Hospitalization After Infertility Treatment. JAMA Netw. Open 2023, 6, e2331470. [Google Scholar] [CrossRef]
- Schwartz, A.S.K.; Wölfler, M.M.; Mitter, V.; Rauchfuss, M.; Haeberlin, F.; Eberhard, M.; von Orelli, S.; Imthurn, B.; Imesch, P.; Fink, D.; et al. Endometriosis, especially mild disease: A risk factor for miscarriages. Fertil. Steril. 2017, 108, 806–814.e2. [Google Scholar] [CrossRef]
- Dun, E.C. Endometriosis in Adolescents. JSLS 2015, 19, e2015.00019. [Google Scholar] [CrossRef] [PubMed]

| Variable | Number of Patients (%) | Mean Age ± SD |
|---|---|---|
| Mean age of patient cohort | 215 | 36.2 ± 4.1 |
| Endometriosis stage (rASRM) I II III IV | 5 (2.4) 67 (31.6) 52 (24.5) 88 (41.5) | 39.2 ± 2.8 36.1 ± 4.2 36.5 ± 4.3 36.0 ± 3.9 |
| Had a history of taking hormonal treatments | 133 (61.9) | 36.2 ± 4.1 |
| Had a history of one or more failed IUI and/or IVF cycles | 180 (83.7) | 35.8 ± 3.7 |
| Histological Findings | Number of Patients (%) | Mean Age ± SD |
|---|---|---|
| Prevalence of histologically-proven tissue abnormalities | 212 (98.6) | 36.2 ± 4.1 |
| Prevalence of histologically-proven endometriosis | 195 (90.7) | 36.3 ± 4.0 |
| Presence of endometrial-like glands and stroma | 195 | 36.3 ± 4.0 |
| No presence of endometrial-like glands and stroma, while: | ||
| 4 | 32.8 ± 5.4 |
| 13 | 35.8 ± 4.5 |
| 3 | 37.3 ± 6.7 |
| Presence of plasma cells in the endometrium as a possible sign of endometritis | 30 (14.0) | 34.7 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nezhat, C.; Khoyloo, F.; Tsuei, A.; Armani, E.; Page, B.; Rduch, T.; Nezhat, C. The Prevalence of Endometriosis in Patients with Unexplained Infertility. J. Clin. Med. 2024, 13, 444. https://doi.org/10.3390/jcm13020444
Nezhat C, Khoyloo F, Tsuei A, Armani E, Page B, Rduch T, Nezhat C. The Prevalence of Endometriosis in Patients with Unexplained Infertility. Journal of Clinical Medicine. 2024; 13(2):444. https://doi.org/10.3390/jcm13020444
Chicago/Turabian StyleNezhat, Camran, Farrah Khoyloo, Angie Tsuei, Ellie Armani, Barbara Page, Thomas Rduch, and Ceana Nezhat. 2024. "The Prevalence of Endometriosis in Patients with Unexplained Infertility" Journal of Clinical Medicine 13, no. 2: 444. https://doi.org/10.3390/jcm13020444
APA StyleNezhat, C., Khoyloo, F., Tsuei, A., Armani, E., Page, B., Rduch, T., & Nezhat, C. (2024). The Prevalence of Endometriosis in Patients with Unexplained Infertility. Journal of Clinical Medicine, 13(2), 444. https://doi.org/10.3390/jcm13020444






