Unveiling the Web: Exploring the Multifaceted Role of Neutrophil Extracellular Traps in Ocular Health and Disease
Abstract
:1. Introduction
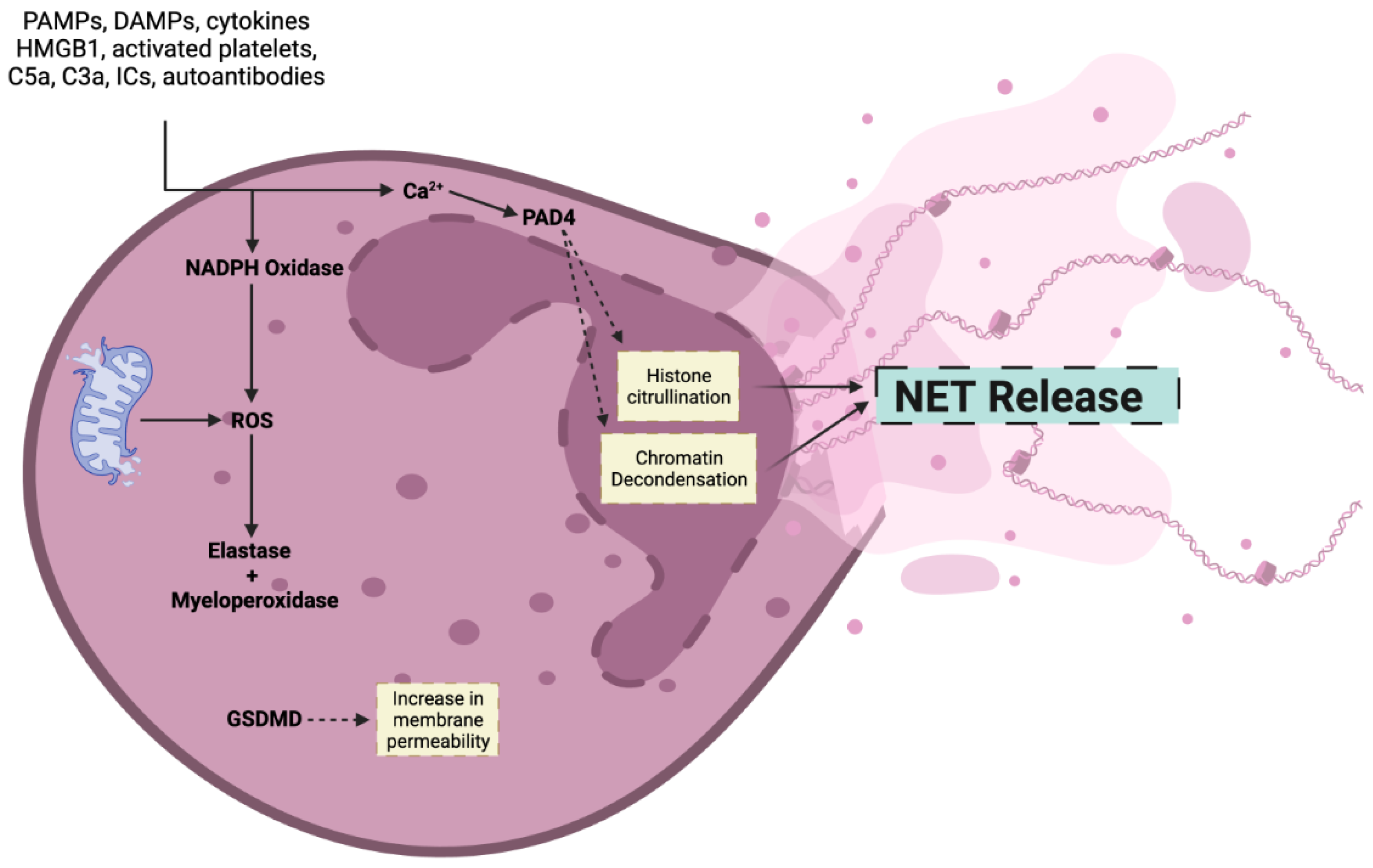
2. Mechanism of NET Formation
3. NETs in Ocular Homeostasis
4. NETs in Bacterial Infections of the Eye
5. NETs in Fungal Infections of the Eye
6. NETs in Protozoal Infections of the Eye
7. NETs in Non-Infectious Corneal Diseases
8. NETs in Dacryolithiasis
9. NETs in Non-Infectious Uveitis
9.1. Acute Anterior Uveitis
9.2. Behcet’s Disease
9.3. Juvenile Idiopathic Arthritis
10. NETs in Diabetic Retinopathy
11. Age-Related Macular Degeneration
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J.; White, P.C.; Milward, M.R.; Cooper, P.R.; Chapple, I.L.C. Modulation of Neutrophil Extracellular Trap and Reactive Oxygen Species Release by Periodontal Bacteria. Infect. Immun. 2017, 85, 10–128. [Google Scholar] [CrossRef]
- Meyers, S.; Lox, M.; Kraisin, S.; Liesenborghs, L.; Martens, C.P.; Frederix, L.; Bruggen, S.V.; Crescente, M.; Missiakas, D.; Baatsen, P.; et al. Neutrophils Protect Against Staphylococcus aureus Endocarditis Progression Independent of Extracellular Trap Release. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.P.C.; Celestrino, G.A.; Igoa, M.V.B.; Jesus, T.M.; França, T.T.; Moreira, D.V.S.; Rigato, P.O.; Sato, P.K.; Condino-Neto, A.; Noronha, I.L.; et al. The Dermatophyte Trichophyton rubrum Induces Neutrophil Extracellular Traps Release by Human Neutrophils. J. Fungi. 2022, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Nett, J.E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 2019, 89, 47–57. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Abbate, A.; Montecucco, F. Neutrophil Extracellular Traps and Cardiovascular Diseases: An Update. Cells 2020, 9, 231. [Google Scholar] [CrossRef]
- Berezin, A. Neutrophil extracellular traps: The core player in vascular complications of diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Ma, L.; Li, X.; Wang, Z.; Gao, R.; Peng, C.; Kang, B.; Wang, Y.; Luo, T.; Wu, J.; et al. Neutrophil Extracellular Traps Induce Glomerular Endothelial Cell Dysfunction and Pyroptosis in Diabetic Kidney Disease. Diabetes 2022, 71, 2739–2750. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Yang, X.; Yan, C.; Feng, Y. Recent Insights into Neutrophil Extracellular Traps in Cardiovascular Diseases. J. Clin. Med. 2022, 11, 6662. [Google Scholar] [CrossRef] [PubMed]
- Parackova, Z.; Zentsova, I.; Malcova, H.; Cebecauerova, D.; Sediva, A.; Horvath, R. Increased histone citrullination in juvenile idiopathic arthritis. Front. Med. 2022, 9, 971121. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, L.; Yücel, L.; Strutzberg-Minder, K.; von Köckritz-Blickwede, M.; Ohnesorge, B.; de Buhr, N. Ex Vivo and In Vitro Analysis Identify a Detrimental Impact of Neutrophil Extracellular Traps on Eye Structures in Equine Recurrent Uveitis. Front. Immunol. 2022, 13, 830871. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, M.; Zhou, Y.; Zhu, M.; Liu, X. Neutrophil Extracellular Traps (NETs) in Ocular Diseases: An Update. Biomolecules 2022, 12, 1440. [Google Scholar] [CrossRef]
- Shafqat, A.; Omer, M.H.; Albalkhi, I.; Alabdul Razzak, G.; Abdulkader, H.; Abdul Rab, S.; Sabbah, B.N.; Alkattan, K.; Yaqinuddin, A. Neutrophil extracellular traps and long COVID. Front. Immunol. 2023, 14, 1254310. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, A.; Abdul Rab, S.; Ammar, O.; Al Salameh, S.; Alkhudairi, A.; Kashir, J.; Alkattan, K.; Yaqinuddin, A. Emerging role of neutrophil extracellular traps in the complications of diabetes mellitus. Front. Med. 2022, 9, 995993. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front. Immunol. 2018, 9, 3076. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Douda, D.N.; Yip, L.; Khan, M.A.; Grasemann, H.; Palaniyar, N. Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 2014, 123, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Salma, U.; Irahara, T.; Watanabe, E.; Takeyama, N. Quantifying Myeloperoxidase-DNA and Neutrophil Elastase-DNA Complexes from Neutrophil Extracellular Traps by Using a Modified Sandwich ELISA. J. Vis. Exp. 2023, 195, e64644. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, X.; Wang, X.; Wang, Y.; Zhang, X.; Zhang, N.; Wang, J.; Yang, J.; Zhang, X.; Gong, P.; et al. Trypanosoma evansi triggered neutrophil extracellular traps formation dependent on myeloperoxidase, neutrophil elastase, and extracellular signal-regulated kinase 1/2 signaling pathways. Vet. Parasitol. 2021, 296, 109502. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Guiducci, E.; Lemberg, C.; Küng, N.; Schraner, E.; Theocharides, A.P.A.; LeibundGut-Landmann, S. Candida albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4. Front. Immunol. 2018, 9, 1573. [Google Scholar] [CrossRef]
- Shen, Y.; You, Q.; Wu, Y.; Wu, J. Inhibition of PAD4-mediated NET formation by cl-amidine prevents diabetes development in nonobese diabetic mice. Eur. J. Pharmacol. 2022, 916, 174623. [Google Scholar] [CrossRef]
- Gajendran, C.; Fukui, S.; Sadhu, N.M.; Zainuddin, M.; Rajagopal, S.; Gosu, R.; Gutch, S.; Fukui, S.; Sheehy, C.E.; Chu, L.; et al. Alleviation of arthritis through prevention of neutrophil extracellular traps by an orally available inhibitor of protein arginine deiminase 4. Sci. Rep. 2023, 13, 3189. [Google Scholar] [CrossRef]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar] [CrossRef]
- Bruschi, M.; Petretto, A.; Santucci, L.; Vaglio, A.; Pratesi, F.; Migliorini, P.; Bertelli, R.; Lavarello, C.; Bartolucci, M.; Candiano, G.; et al. Neutrophil Extracellular Traps protein composition is specific for patients with Lupus nephritis and includes methyl-oxidized αenolase (methionine sulfoxide 93). Sci. Rep. 2019, 9, 7934. [Google Scholar] [CrossRef]
- Sosa-Luis, S.A.; Ríos-Ríos, W.J.; Gómez-Bustamante, Á.E.; Romero-Tlalolini, M.; Aguilar-Ruiz, S.R.; Baltierez-Hoyos, R.; Torres-Aguilar, H. Structural differences of neutrophil extracellular traps induced by biochemical and microbiologic stimuli under healthy and autoimmune milieus. Immunol. Res. 2021, 69, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Grüneboom, A.; Petru, L.; Podolska, M.J.; Kling, L.; Maueröder, C.; Dahms, F.; Christiansen, S.; Günter, L.; Krenn, V.; et al. Frontline Science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J. Leukoc. Biol. 2019, 105, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Postnikoff, C.K.; Nichols, K.K. Neutrophil and T-Cell Homeostasis in the Closed Eye. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6212–6220. [Google Scholar] [CrossRef]
- Postnikoff, C.K.; Held, K.; Viswanath, V.; Nichols, K.K. Enhanced closed eye neutrophil degranulation in dry eye disease. Ocul. Surf. 2020, 18, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Lacy, H.M.; Bowlin, A.K.; Hennings, L.; Scurlock, A.M.; Nagarajan, U.M.; Rank, R.G. Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections. Infect. Immun. 2011, 79, 1889–1897. [Google Scholar] [CrossRef]
- Thanabalasuriar, A.; Scott, B.N.V.; Peiseler, M.; Willson, M.E.; Zeng, Z.; Warrener, P.; Keller, A.E.; Surewaard, B.G.J.; Dozier, E.A.; Korhonen, J.T.; et al. Neutrophil Extracellular Traps Confine Pseudomonas aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 2019, 25, 526–536.e524. [Google Scholar] [CrossRef]
- Sousa, A.; Pereira, M. Pseudomonas aeruginosa Diversification during Infection Development in Cystic Fibrosis Lungs—A Review. Pathogens 2014, 3, 680–703. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.; Yuan, K.; Huang, X.; Hu, R.; Jin, X. Neutrophil extracellular traps may have a dual role in Pseudomonas aeruginosa keratitis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 169–180. [Google Scholar] [CrossRef]
- Sharma, N.; Bagga, B.; Singhal, D.; Nagpal, R.; Kate, A.; Saluja, G.; Maharana, P.K. Fungal keratitis: A review of clinical presentations, treatment strategies and outcomes. Ocul. Surf. 2022, 24, 22–30. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Liu, S.; Chen, G.; He, S.; Li, Z.; Wang, L. Mast Cell Activation Protects Cornea by Promoting Neutrophil Infiltration via Stimulating ICAM-1 and Vascular Dilation in Fungal Keratitis. Sci. Rep. 2018, 8, 8365. [Google Scholar] [CrossRef]
- Pearlman, E.; Sun, Y.; Roy, S.; Karmakar, M.; Hise, A.G.; Szczotka-Flynn, L.; Ghannoum, M.; Chinnery, H.R.; McMenamin, P.G.; Rietsch, A. Host Defense at the Ocular Surface. Int. Rev. Immunol. 2013, 32, 4–18. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, Y.; Zhang, F.; Wan, T.; Fan, F.; Xie, X.; Lin, Z. Neutrophil extracellular traps involvement in corneal fungal infection. Mol. Vis. 2016, 22, 944–952. [Google Scholar] [PubMed]
- Fan, F.; Huang, X.; Yuan, K.; Zhu, B.; Zhao, Y.; Hu, R.; Wan, T.; Zhu, L.; Jin, X. Glucocorticoids May Exacerbate Fungal Keratitis by Increasing Fungal Aggressivity and Inhibiting the Formation of Neutrophil Extracellular Traps. Curr. Eye Res. 2020, 45, 124–133. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The biology of Acanthamoeba keratitis. Exp. Eye Res. 2021, 202, 108365. [Google Scholar] [CrossRef]
- Carvalho-Kelly, L.F.; Freitas-Mesquita, A.L.; Nascimento, M.T.C.; Dick, C.F.; de Souza-Maciel, E.; Rochael, N.C.; Saraiva, E.M.; Meyer-Fernandes, J.R. Acanthamoeba castellanii trophozoites escape killing by neutrophil extracellular traps using their 3′-nucleotidase/nuclease activity. Eur. J. Protistol. 2023, 91, 126032. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Sonawane, S.; Khanolkar, V.; Namavari, A.; Chaudhary, S.; Gandhi, S.; Tibrewal, S.; Jassim, S.H.; Shaheen, B.; Hallak, J.; Horner, J.H.; et al. Ocular surface extracellular DNA and nuclease activity imbalance: A new paradigm for inflammation in dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8253–8263. [Google Scholar] [CrossRef] [PubMed]
- Tibrewal, S.; Ivanir, Y.; Sarkar, J.; Nayeb-Hashemi, N.; Bouchard, C.S.; Kim, E.; Jain, S. Hyperosmolar Stress Induces Neutrophil Extracellular Trap Formation: Implications for Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7961–7969. [Google Scholar] [CrossRef]
- Goto, E.; Monden, Y.; Takano, Y.; Mori, A.; Shimmura, S.; Shimazaki, J.; Tsubota, K. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br. J. Ophthalmol. 2002, 86, 1403–1407. [Google Scholar] [CrossRef]
- An, S.; Raju, I.; Surenkhuu, B.; Kwon, J.-E.; Gulati, S.; Karaman, M.; Pradeep, A.; Sinha, S.; Mun, C.; Jain, S. Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: Implications for novel biomarkers and therapeutic strategies. Ocul. Surf. 2019, 17, 589–614. [Google Scholar] [CrossRef]
- Chi, H.; Hao, W.; Qi, X.; Zhang, T.; Dong, Y.; Gao, H.; Wei, C.; Shi, W. A proteomic approach towards understanding the pathogenesis of Mooren’s ulcer. Exp. Eye Res. 2021, 205, 108509. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, H.; Wang, Z.; Yang, B.; Liu, Z.; Chen, L.; Gong, X.; Lin, Y. Mooren’s ulcer in China: A study of clinical characteristics and treatment. Br. J. Ophthalmol. 2000, 84, 1244–1249. [Google Scholar] [CrossRef]
- Murray, P.I.; Rahi, A.H. Pathogenesis of Mooren’s ulcer: Some new concepts. Br. J. Ophthalmol. 1984, 68, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Naffah de Souza, C.; Breda, L.C.D.; Khan, M.A.; de Almeida, S.R.; Câmara, N.O.S.; Sweezey, N.; Palaniyar, N. Alkaline pH Promotes NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation: A Matter of Mitochondrial Reactive Oxygen Species Generation and Citrullination and Cleavage of Histone. Front. Immunol. 2017, 8, 1849. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhang, Y.; Yuan, K.; Min, J.; Mou, Y.; Jin, X. Acetylsalicylic Acid Promotes Corneal Epithelium Migration by Regulating Neutrophil Extracellular Traps in Alkali Burn. Front. Immunol. 2020, 11, 551057. [Google Scholar] [CrossRef]
- Mun, C.; Gulati, S.; Tibrewal, S.; Chen, Y.F.; An, S.; Surenkhuu, B.; Raju, I.; Buwick, M.; Ahn, A.; Kwon, J.E.; et al. A Phase I/II Placebo-Controlled Randomized Pilot Clinical Trial of Recombinant Deoxyribonuclease (DNase) Eye Drops Use in Patients with Dry Eye Disease. Transl. Vis. Sci. Technol. 2019, 8, 10. [Google Scholar] [CrossRef]
- Napirei, M.; Ludwig, S.; Mezrhab, J.; Klöckl, T.; Mannherz, H.G. Murine serum nucleases--contrasting effects of plasmin and heparin on the activities of DNase1 and DNase1-like 3 (DNase1l3). FEBS J. 2009, 276, 1059–1073. [Google Scholar] [CrossRef]
- Nan, W.; He, Y.; Shen, S.; Wu, M.; Wang, S.; Zhang, Y. BMP4 inhibits corneal neovascularization by interfering with tip cells in angiogenesis. Exp. Eye Res. 2023, 237, 109680. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Oguido, A.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Crespigio, J.; Domiciano, T.P.; Verri, W.A., Jr.; Casella, A.M.B. Naringenin Eye Drops Inhibit Corneal Neovascularization by Anti-Inflammatory and Antioxidant Mechanisms. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5764–5776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, J.; Shi, Y.; Liu, M. Rapamycin inhibits corneal inflammatory response and neovascularization in a mouse model of corneal alkali burn. Exp. Eye Res. 2023, 233, 109539. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Hu, K.Y.; Kamal, S.; Andron, A.; Della Rocca, R.C.; Ali, M.J.; Nair, A.G. Dacryolithiasis: A Review. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 83–89. [Google Scholar] [CrossRef]
- Zlatar, L.; Timm, T.; Lochnit, G.; Bilyy, R.; Bäuerle, T.; Munoz-Becerra, M.; Schett, G.; Knopf, J.; Heichel, J.; Ali, M.J.; et al. Neutrophil Extracellular Traps Drive Dacryolithiasis. Cells 2023, 12, 1857. [Google Scholar] [CrossRef]
- Krishna, U.; Ajanaku, D.; Denniston, A.K.; Gkika, T. Uveitis: A sight-threatening disease which can impact all systems. Postgrad. Med. J. 2017, 93, 766–773. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Bodaghi, B.; Couto, C.; Zierhut, M.; Acharya, N.; Pavesio, C.; Tay-Kearney, M.L.; Neri, P.; Douglas, K.; Pathai, S.; et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review. Semin. Arthritis Rheum. 2019, 49, 438–445. [Google Scholar] [CrossRef]
- Takeuchi, M.; Mizuki, N.; Ohno, S. Pathogenesis of Non-Infectious Uveitis Elucidated by Recent Genetic Findings. Front. Immunol. 2021, 12, 640473. [Google Scholar] [CrossRef]
- Tsirouki, T.; Dastiridou, A.; Symeonidis, C.; Tounakaki, O.; Brazitikou, I.; Kalogeropoulos, C.; Androudi, S. A Focus on the Epidemiology of Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 2–16. [Google Scholar] [CrossRef]
- Khan, M.A.; Haroon, M.; Rosenbaum, J.T. Acute Anterior Uveitis and Spondyloarthritis: More Than Meets the Eye. Curr. Rheumatol. Rep. 2015, 17, 59. [Google Scholar] [CrossRef]
- Wakefield, D.; Yates, W.; Amjadi, S.; McCluskey, P. HLA-B27 Anterior Uveitis: Immunology and Immunopathology. Ocul. Immunol. Inflamm. 2016, 24, 450–459. [Google Scholar] [CrossRef]
- Smith, J.R.; Hart, P.H.; Williams, K.A. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol. Cell Biol. 1998, 76, 497–512. [Google Scholar] [CrossRef]
- Tong, B.; Liu, X.; Xiao, J.; Su, G. Immunopathogenesis of Behcet’s Disease. Front. Immunol. 2019, 10, 665. [Google Scholar] [CrossRef]
- Bettiol, A.; Becatti, M.; Silvestri, E.; Argento, F.R.; Fini, E.; Mannucci, A.; Galora, S.; Mattioli, I.; Urban, M.L.; Malandrino, D.; et al. Neutrophil-mediated mechanisms of damage and in-vitro protective effect of colchicine in non-vascular Behçet’s syndrome. Clin. Exp. Immunol. 2021, 206, 410–421. [Google Scholar] [CrossRef]
- Ksiaa, I.; Abroug, N.; Kechida, M.; Zina, S.; Jelliti, B.; Khochtali, S.; Attia, S.; Khairallah, M. Eye and Behçet’s disease. J. Fr. Ophtalmol. 2019, 42, e133–e146. [Google Scholar] [CrossRef] [PubMed]
- Le Joncour, A.; Martos, R.; Loyau, S.; Lelay, N.; Dossier, A.; Cazes, A.; Fouret, P.; Domont, F.; Papo, T.; Jandrot-Perrus, M.; et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann. Rheum. Dis. 2019, 78, 1274–1282. [Google Scholar] [CrossRef]
- Li, L.; Yu, X.; Liu, J.; Wang, Z.; Li, C.; Shi, J.; Sun, L.; Liu, Y.; Zhang, F.; Chen, H.; et al. Neutrophil Extracellular Traps Promote Aberrant Macrophages Activation in Behçet’s Disease. Front. Immunol. 2020, 11, 590622. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bello, I.; López-Longo, F.J.; Arias-Salgado, E.G.; Jiménez-Yuste, V.; Butta, N.V. Behçet’s disease: New insight into the relationship between procoagulant state, endothelial activation/damage and disease activity. Orphanet J. Rare Dis. 2013, 8, 81. [Google Scholar] [CrossRef]
- Shu, Q.; Zhang, N.; Liu, Y.; Wang, X.; Chen, J.; Xie, H.; Pan, F.; Zhao, L.; Ding, X.; Wen, Y.; et al. IL-8 Triggers Neutrophil Extracellular Trap Formation Through an Nicotinamide Adenine Dinucleotide Phosphate Oxidase- and Mitogen-Activated Protein Kinase Pathway-Dependent Mechanism in Uveitis. Investig. Opthalmol. Vis. Sci. 2023, 64, 19. [Google Scholar] [CrossRef]
- Katsantonis, J.; Adler, Y.; Orfanos, C.E.; Zouboulis, C.C. Adamantiades-Behçet’s disease: Serum IL-8 is a more reliable marker for disease activity than C-reactive protein and erythrocyte sedimentation rate. Dermatology 2000, 201, 37–39. [Google Scholar] [CrossRef]
- Gür-Toy, G.; Lenk, N.; Yalcin, B.; Aksaray, S.; Alli, N. Serum interleukin-8 as a serologic marker of activity in Behçet’s disease. Int. J. Dermatol. 2005, 44, 657–660. [Google Scholar] [CrossRef]
- Kaur, I.; Singal, A.; Rohatgi, J. Conjunctival Ulcers in Behcet’s Disease and Response to Colchicine. Indian Dermatol. Online J. 2020, 11, 1005–1006. [Google Scholar] [CrossRef]
- Barut, K.; Adrovic, A.; Şahin, S.; Kasapçopur, Ö. Juvenile Idiopathic Arthritis. Balk. Med. J. 2017, 34, 90–101. [Google Scholar] [CrossRef]
- Sen, E.S.; Ramanan, A.V. Juvenile idiopathic arthritis-associated uveitis. Clin. Immunol. 2020, 211, 108322. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ahn, M.-H.; Jung, J.-Y.; Suh, C.-H.; Kim, H.-A. An Update on the Pathogenic Role of Neutrophils in Systemic Juvenile Idiopathic Arthritis and Adult-Onset Still’s Disease. Int. J. Mol. Sci. 2021, 22, 13038. [Google Scholar] [CrossRef]
- Hu, X.; Xie, Q.; Mo, X.; Jin, Y. The role of extracellular histones in systemic-onset juvenile idiopathic arthritis. Ital. J. Pediatr. 2019, 45, 14. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Taylor, A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 2011, 30, 18–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.W.; Zhang, D.D.; Fan, Z.G. Blood-retinal barrier as a converging pivot in understanding the initiation and development of retinal diseases. Chin. Med. J. 2020, 133, 2586–2594. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.E.; Gu, J.Y.; Yoo, H.J.; Park, S.H.; Kim, Y.I.; Nam-Goong, I.S.; Kim, E.S.; Kim, H.K. Evaluation of Circulating Markers of Neutrophil Extracellular Trap (NET) Formation as Risk Factors for Diabetic Retinopathy in a Case-Control Association Study. Exp. Clin. Endocrinol. Diabetes 2016, 124, 557–561. [Google Scholar] [CrossRef]
- Song, D.Y.; Gu, J.Y.; Yoo, H.J.; Kim, Y.I.; Nam-Goong, I.S.; Kim, E.S.; Kim, H.K. Activation of Factor XII and Kallikrein-Kinin System Combined with Neutrophil Extracellular Trap Formation in Diabetic Retinopathy. Exp. Clin. Endocrinol. Diabetes 2021, 129, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Guerrero, F.S.; Aguayo-Flores, J.E.; Buentello-Volante, B.; Zarco-Ávila, K.; Sánchez-Cisneros, P.; Castro-Salas, I.; De La Torre-Galván, E.; Rodríguez-Loaiza, J.L.; Jiménez-Corona, A.; Garfias, Y. Spontaneous Neutrophil Extracellular Traps Release Are Inflammatory Markers Associated with Hyperglycemia and Renal Failure on Diabetic Retinopathy. Biomedicines 2023, 11, 1791. [Google Scholar] [CrossRef]
- Chung, J.O.; Park, S.Y.; Cho, D.H.; Chung, D.J.; Chung, M.Y. Plasma neutrophil gelatinase-associated lipocalin levels are positively associated with diabetic retinopathy in patients with Type 2 diabetes. Diabet. Med. 2016, 33, 1649–1654. [Google Scholar] [CrossRef]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.M.; Buscarlet, M.; Mawambo, G.T.; Howard, J.P.; et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020, 369, eaay5356. [Google Scholar] [CrossRef]
- Barliya, T.; Dardik, R.; Nisgav, Y.; Dachbash, M.; Gaton, D.; Kenet, G.; Ehrlich, R.; Weinberger, D.; Livnat, T. Possible involvement of NETosis in inflammatory processes in the eye: Evidence from a small cohort of patients. Mol. Vis. 2017, 23, 922–932. [Google Scholar] [PubMed]
- Kumar-Singh, R. The role of complement membrane attack complex in dry and wet AMD—From hypothesis to clinical trials. Exp. Eye Res. 2019, 184, 266–277. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Shadrach, K.G.; Rayborn, M.E.; Li, Y.; Pauer, G.J.; Hagstrom, S.A.; Bhattacharya, S.K.; Hollyfield, J.G. Retinal deimination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD). Exp. Eye Res. 2013, 111, 71–78. [Google Scholar] [CrossRef]
- Niazi, S.; Krogh Nielsen, M.; Sørensen, T.L.; Subhi, Y. Neutrophil-to-lymphocyte ratio in age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2019, 97, 558–566. [Google Scholar] [CrossRef]
- Shen, M.; Tao, Y.; Feng, Y.; Liu, X.; Yuan, F.; Zhou, H. Quantitative proteomic analysis of mice corneal tissues reveals angiogenesis-related proteins involved in corneal neovascularization. Biochim. Biophys. Acta 2016, 1864, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Padmanabhan, A.; Vaidya, T.; Watson, A.M.; Bhutto, I.A.; Hose, S.; Shang, P.; Stepicheva, N.; Yazdankhah, M.; Weiss, J.; et al. Neutrophils homing into the retina trigger pathology in early age-related macular degeneration. Commun. Biol. 2019, 2, 348. [Google Scholar] [CrossRef]
- An, Z.; Li, J.; Yu, J.; Wang, X.; Gao, H.; Zhang, W.; Wei, Z.; Zhang, J.; Zhang, Y.; Zhao, J.; et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle 2019, 18, 2928–2938. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeeb, S.; Arabi, T.Z.; Shah, H.; Alsalameh, S.; Abu-Shaar, M.; El-Sibai, A.M.; Alkattan, K.; Yaqinuddin, A. Unveiling the Web: Exploring the Multifaceted Role of Neutrophil Extracellular Traps in Ocular Health and Disease. J. Clin. Med. 2024, 13, 512. https://doi.org/10.3390/jcm13020512
Adeeb S, Arabi TZ, Shah H, Alsalameh S, Abu-Shaar M, El-Sibai AM, Alkattan K, Yaqinuddin A. Unveiling the Web: Exploring the Multifaceted Role of Neutrophil Extracellular Traps in Ocular Health and Disease. Journal of Clinical Medicine. 2024; 13(2):512. https://doi.org/10.3390/jcm13020512
Chicago/Turabian StyleAdeeb, Salma, Tarek Ziad Arabi, Hassan Shah, Sulaiman Alsalameh, Mylia Abu-Shaar, Abduljalil Mohamed El-Sibai, Khaled Alkattan, and Ahmed Yaqinuddin. 2024. "Unveiling the Web: Exploring the Multifaceted Role of Neutrophil Extracellular Traps in Ocular Health and Disease" Journal of Clinical Medicine 13, no. 2: 512. https://doi.org/10.3390/jcm13020512
APA StyleAdeeb, S., Arabi, T. Z., Shah, H., Alsalameh, S., Abu-Shaar, M., El-Sibai, A. M., Alkattan, K., & Yaqinuddin, A. (2024). Unveiling the Web: Exploring the Multifaceted Role of Neutrophil Extracellular Traps in Ocular Health and Disease. Journal of Clinical Medicine, 13(2), 512. https://doi.org/10.3390/jcm13020512






