Transcatheter Aortic Valve Implantation (TAVI) Planning with Dual-Layer Spectral CT Using Virtual Monoenergetic Image (VMI) Reconstructions and 20 mL of Contrast Media
Abstract
:1. Introduction
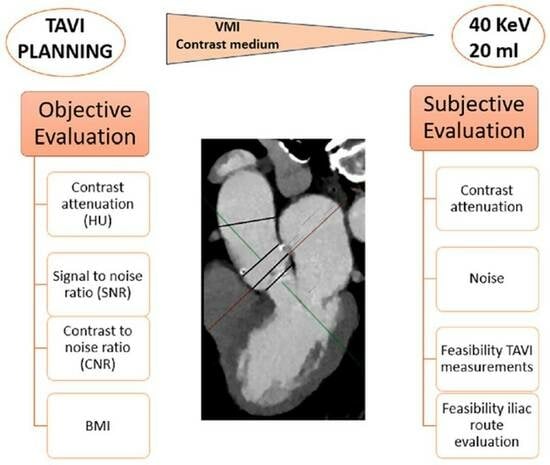
2. Materials and Methods
3. Results
3.1. Population Characteristics
3.2. Quantitative and Qualitative Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, R.; Ponticelli, F.; Giannini, F.; Galvani, M. Transcatheter Aortic Valve Implantation for Severe Pure Aortic Regurgitation Due to Active Aortitis. Catheter. Cardiovasc. Interv. 2021, 97, 950–954. [Google Scholar] [CrossRef]
- Banovic, M.; Iung, B.; Wojakowski, W.; Van Mieghem, N.; Bartunek, J. Asymptomatic Severe and Moderate Aortic Stenosis: Time for Appraisal of Treatment Indications. Struct. Heart 2023, 7, 100201. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Usui, K.; Katsunuma, Y.; Honda, H.; Hatakeyama, K. Reducing Contrast Dose Using Virtual Monoenergetic Imaging for Aortic CTA. J. Appl. Clin. Med. Phys. 2020, 21, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.N.; Spasiano, C.M.; Maino, C.; De Ponti, E.; Ragusi, M.; Giandola, T.; Terrani, S.; Peroni, M.; Corso, R.; Ippolito, D. Principles and Applications of Dual-Layer Spectral CT in Gastrointestinal Imaging. Diagnostics 2023, 13, 1740. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT Imaging: Technical Principles of Dual-Energy CT and Multi-Energy Photon-Counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Lanzafame, L.R.M.; Micari, A.; Blandino, A.; Yel, I.; Koch, V.; Gruenewald, L.D.; Vogl, T.J.; Booz, C.; Bucolo, G.M.; et al. Improved Coronary Artery Visualization Using Virtual Monoenergetic Imaging from Dual-Layer Spectral Detector CT Angiography. Diagnostics 2023, 13, 2675. [Google Scholar] [CrossRef]
- van Ommen, F.; de Jong, H.W.A.M.; Dankbaar, J.W.; Bennink, E.; Leiner, T.; Schilham, A.M.R. Dose of CT Protocols Acquired in Clinical Routine Using a Dual-Layer Detector CT Scanner: A Preliminary Report. Eur. J. Radiol. 2019, 112, 65–71. [Google Scholar] [CrossRef]
- Majeed, N.F.; Ali, S.M.; Therrien, J.; Wald, C.; Wortman, J.R. Virtual Monoenergetic Spectral Detector CT for Preoperative CT Angiography in Liver Donors. Curr. Probl. Diagn. Radiol. 2022, 51, 517–523. [Google Scholar] [CrossRef]
- Patino, M.; Parakh, A.; Lo, G.C.; Agrawal, M.; Kambadakone, A.R.; Oliveira, G.R.; Sahani, D.V. Virtual Monochromatic Dual-Energy Aortoiliac CT Angiography With Reduced Iodine Dose: A Prospective Randomized Study. AJR Am. J. Roentgenol. 2019, 212, 467–474. [Google Scholar] [CrossRef]
- Chalian, H.; Kalisz, K.; Rassouli, N.; Dhanantwari, A.; Rajiah, P. Utility of Virtual Monoenergetic Images Derived from a Dual-Layer Detector-Based Spectral CT in the Assessment of Aortic Anatomy and Pathology: A Retrospective Case Control Study. Clin. Imaging 2018, 52, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Bartorelli, A.L.; Berti, S.; Golino, P.; Esposito, G.; Musumeci, G.; Petronio, S.; Tamburino, C.; Tarantini, G.; Ussia, G.; et al. Updated Clinical Indications for Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis: Expert Opinion of the Italian Society of Cardiology and GISE. J. Cardiovasc. Med. 2018, 19, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Alsara, O.; Alsarah, A.; Laird-Fick, H. Advanced Age and the Clinical Outcomes of Transcatheter Aortic Valve Implantation. J. Geriatr. Cardiol. 2014, 11, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.A.; Leipsic, J.A.; Shivalkar, B.; Ardies, L.; Van Herck, P.L.; Op de Beeck, B.J.; Vrints, C.; Rodrigus, I.; Parizel, P.M.; Bosmans, J. Preprocedural CT Evaluation of Transcatheter Aortic Valve Replacement: What the Radiologist Needs to Know. Radiographics 2014, 34, 1491–1514. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Hague, C.J.; Gurvitch, R.; Ajlan, A.M.; Labounty, T.M.; Min, J.K. MDCT to Guide Transcatheter Aortic Valve Replacement and Mitral Valve Repair. Cardiol. Clin. 2012, 30, 147–160. [Google Scholar] [CrossRef]
- Webb, J.G.; Altwegg, L.; Masson, J.-B.; Al Bugami, S.; Al Ali, A.; Boone, R.A. A New Transcatheter Aortic Valve and Percutaneous Valve Delivery System. J. Am. Coll. Cardiol. 2009, 53, 1855–1858. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.M.; Ascenti, V.; Barbera, S.; Fontana, F.; Aricò, F.M.; Piacentino, F.; Coppola, A.; Cicero, G.; Marino, M.A.; Booz, C.; et al. Virtual Non-Contrast Spectral CT in Renal Masses: Is It Time to Discard Conventional Unenhanced Phase? J. Clin. Med. 2023, 12, 4718. [Google Scholar] [CrossRef]
- Zorzetto, G.; Coppola, A.; Molinelli, V.; Angeretti, M.G.; Casarin, J.; Fontana, F.; Piacentino, F.; Carcano, G.; Ghezzi, F.; Venturini, M. Spectral CT in Peritoneal Carcinomatosis from Ovarian Cancer: A Tool for Differential Diagnosis of Small Nodules? Eur. Radiol. Exp. 2022, 6, 45. [Google Scholar] [CrossRef]
- Curti, M.; Fontana, F.; Piacentino, F.; Ossola, C.; Coppola, A.; Carcano, G.; Venturini, M. Dual-Layer Spectral CT Fusion Imaging for Lung Biopsies: More Accurate Targets, Diagnostic Samplings, and Biomarker Information? Eur. Radiol. Exp. 2022, 6, 34. [Google Scholar] [CrossRef]
- Lenga, L.; Czwikla, R.; Wichmann, J.L.; Leithner, D.; Albrecht, M.H.; Booz, C.; Arendt, C.T.; Yel, I.; D’Angelo, T.; Vogl, T.J.; et al. Dual-Energy CT in Patients with Colorectal Cancer: Improved Assessment of Hypoattenuating Liver Metastases Using Noise-Optimized Virtual Monoenergetic Imaging. Eur. J. Radiol. 2018, 106, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lenga, L.; Czwikla, R.; Wichmann, J.L.; Leithner, D.; Albrecht, M.H.; D’Angelo, T.; Arendt, C.T.; Booz, C.; Hammerstingl, R.; Vogl, T.J.; et al. Dual-Energy CT in Patients with Abdominal Malignant Lymphoma: Impact of Noise-Optimised Virtual Monoenergetic Imaging on Objective and Subjective Image Quality. Clin. Radiol. 2018, 73, 833.e19–833.e27. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.M.; D’Angelo, T.; Yel, I.; Koch, V.; Gruenewald, L.D.; Othman, A.E.; Alizadeh, L.S.; Overhoff, D.P.; Waldeck, S.; Martin, S.S.; et al. Virtual Monoenergetic Imaging of Lower Extremities Using Dual-Energy CT Angiography in Patients with Diabetes Mellitus. Diagnostics 2023, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, W.; Huang, W.; Luo, M.; Xiao, W.; Qin, C.; Dong, S.; Liu, H.; Li, Z.; Diao, K. Dual-Layer Spectral Computed Tomography Aortography Using a Seventy-Five-Percent-Reduced Iodine Dose Protocol and Multiparameter Spectral Imaging: Comparison with Conventional Computed Tomography Imaging. Quant. Imaging Med. Surg. 2023, 13, 6456–6467. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual Energy Computed Tomography Virtual Monoenergetic Imaging: Technique and Clinical Applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef]
- Dubourg, B.; Caudron, J.; Lestrat, J.-P.; Bubenheim, M.; Lefebvre, V.; Godin, M.; Tron, C.; Eltchaninoff, H.; Bauer, F.; Dacher, J.-N. Single-Source Dual-Energy CT Angiography with Reduced Iodine Load in Patients Referred for Aortoiliofemoral Evaluation before Transcatheter Aortic Valve Implantation: Impact on Image Quality and Radiation Dose. Eur. Radiol. 2014, 24, 2659–2668. [Google Scholar] [CrossRef]
- Martin, S.S.; Albrecht, M.H.; Wichmann, J.L.; Hüsers, K.; Scholtz, J.-E.; Booz, C.; Bodelle, B.; Bauer, R.W.; Metzger, S.C.; Vogl, T.J.; et al. Value of a Noise-Optimized Virtual Monoenergetic Reconstruction Technique in Dual-Energy CT for Planning of Transcatheter Aortic Valve Replacement. Eur. Radiol. 2017, 27, 705–714. [Google Scholar] [CrossRef]
- Mangold, D.; Salatzki, J.; Riffel, J.; Kauczor, H.-U.; Weber, T.F. Dual-Layer Spectral CTA for TAVI Planning Using a Split-Phase Protocol and Low-keV Virtual Monoenergetic Images: Improved Image Quality in Comparison with Single-Phase Conventional CTA. Rofo 2022, 194, 652–659. [Google Scholar] [CrossRef]
- Langenbach, I.L.; Langenbach, M.C.; Mayrhofer, T.; Foldyna, B.; Maintz, D.; Klein, K.; Wienemann, H.; Krug, K.B.; Hellmich, M.; Adam, M.; et al. Reduction of Contrast Medium for Transcatheter Aortic Valve Replacement Planning Using a Spectral Detector CT: A Prospective Clinical Trial. Eur. Radiol. 2023, 1–11. [Google Scholar] [CrossRef]
- Bruce, R.J.; Djamali, A.; Shinki, K.; Michel, S.J.; Fine, J.P.; Pozniak, M.A. Background Fluctuation of Kidney Function versus Contrast-Induced Nephrotoxicity. AJR Am. J. Roentgenol. 2009, 192, 711–718. [Google Scholar] [CrossRef]
- Mack, M.J.; Brennan, J.M.; Brindis, R.; Carroll, J.; Edwards, F.; Grover, F.; Shahian, D.; Tuzcu, E.M.; Peterson, E.D.; Rumsfeld, J.S.; et al. Outcomes Following Transcatheter Aortic Valve Replacement in the United States. JAMA 2013, 310, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Webb, J.G.; Nietlispach, F.; Dumont, E.; De Larochellière, R.; Doyle, D.; Masson, J.-B.; Gutiérrez, M.J.; Clavel, M.-A.; Bertrand, O.F.; et al. Acute Kidney Injury Following Transcatheter Aortic Valve Implantation: Predictive Factors, Prognostic Value, and Comparison with Surgical Aortic Valve Replacement. Eur. Heart J. 2010, 31, 865–874. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.-F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.W.; et al. Post-Contrast Acute Kidney Injury—Part 1: Definition, Clinical Features, Incidence, Role of Contrast Medium and Risk Factors. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Szewczyk-Bieda, M.; Greenhalgh, R.; Drinkwater, K. Preventing Post-Contrast Acute Kidney Injury and Hypersensitivity Reactions: UK National Audit. Clin. Radiol. 2023, 78, E898–E907. [Google Scholar] [CrossRef]
- Namasivayam, S.; Kalra, M.K.; Torres, W.E.; Small, W.C. Adverse Reactions to Intravenous Iodinated Contrast Media: A Primer for Radiologists. Emerg. Radiol. 2006, 12, 210–215. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, C.M.; Leung, A.M.; Braverman, L.E.; Brent, G.A.; Pearce, E.N. A Review: Radiographic Iodinated Contrast Media-Induced Thyroid Dysfunction. J. Clin. Endocrinol. Metab. 2015, 100, 376–383. [Google Scholar] [CrossRef]





| PSOAS | LV | SNR/CNR | ATA | SNR/CNR | DTA | SNR/CNR | AA | SNR/CNR | CIA | SNR/CNR | EIA | SNR/CNR | BMI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 (12) | 300 (10) | 30/23.2 | 280 (8) | 35/26.5 | 304 (9) | 33.7/26.2 | 287 (12) | 23.9/18.2 | 279 (11) | 25.3/19.1 | 263 (6) | 43.8/32.5 | 22.4 |
| 2 | 52 (19) | 248 (15) | 16.5/13 | 283 (10) | 28.3/23.1 | 290 (10) | 29/23.8 | 282 (13) | 21.7/17.7 | 244 (11) | 22.1/17.4 | 261 (14) | 18.6/14.9 | 23.3 |
| 3 | 72 (10) | 333 (9) | 37/29 | 306 (7) | 43.7/33.4 | 310 (9) | 34.4/26.4 | 328 (10) | 32.8/25.6 | 282 (10) | 28.2/21 | 292 (13) | 22.4/16.9 | 21.9 |
| 4 | 57 (16) | 333 (12) | 27.75/23 | 366 (11) | 33.2/28 | 376 (14) | 26.8/22.7 | 361 (15) | 24/20.2 | 323 (19) | 17/14 | 253 (26) | 9.7/7.5 | 20.9 |
| 5 | 70 (9) | 335 (10) | 33.5/26.5 | 343 (9) | 38.1/30.3 | 350 (8.5) | 41.1/33 | 334 (12) | 27.8/22 | 301 (10) | 30.1/23.1 | 258 (12.5) | 20.6/15 | 22.4 |
| 6 | 56 (18) | 199 (22) | 9/6.5 | 252 (16) | 15.7/12.2 | 287 (16) | 17.9/14.4 | 264 (19) | 13.9/10.9 | 281 (22) | 12.7/10.2 | 222 (17) | 13/9.7 | 25.6 |
| 7 | 75 (18) | 200 (19) | 10.5/6.6 | 240 (14) | 17/11.7 | 245 (14) | 17.5/12.1 | 228 (18) | 12.6/8.5 | 243 (19) | 12.7/8.8 | 226 (14) | 16.1/10.7 | 26.0 |
| 8 | 82 (20) | 238 (14) | 17/11.1 | 300 (17) | 17.6/12.8 | 314 (19) | 16.5/12.2 | 223 (22) | 10.1/6.4 | 167 (18) | 9.2/4.7 | 151 (17) | 8.8/40 | 26.6 |
| 9 | 65 (18) | 234 (16) | 14.6/10.5 | 229 (13) | 17.6/12.6 | 233 (15) | 15.5/11.2 | 240 (18) | 13.3/9.7 | 206 (12) | 17.1/11.7 | 160 (17) | 9.4/5.5 | 28.5 |
| 10 | 50 (24) | 290 (14) | 20.7/17.1 | 283 (10) | 28.3/23.3 | 264 (9) | 29.3/23.7 | 280 (16) | 17.5/14.3 | 259 (14) | 18.5/14.9 | 190 (10) | 19/14 | 21.6 |
| 11 | 70 (15) | 251 (8) | 31/22.6 | 274 (8) | 34.2/25.5 | 278 (9) | 30.8/23.1 | 278 (15) | 18.5/13.8 | 289 (18) | 16/12.1 | 266 (16) | 16.6/12.2 | 29.3 |
| 12 | 50 (17) | 230 (18) | 12.7/10 | 287 (8) | 35.8/29.6 | 281 (10) | 28.1/23.1 | 250 (13) | 19.2/15.3 | 200 (15) | 13.3/10 | 191 (11) | 17.3/12.8 | 23.4 |
| 13 | 58 (10) | 263 (10) | 26.3/20.5 | 297 (10) | 29.7/23.9 | 311 (10) | 31.1/25.3 | 313 (16) | 19.5/15.9 | 304 (18) | 16.8/13.6 | 295 (13) | 22.6/18.2 | 27.1 |
| 14 | 69 (18) | 275 (13) | 21.1/15.8 | 279 (13) | 21.4/16.1 | 280 (12) | 23.3/17.5 | 275 (17) | 16.1/12.1 | 252 (19) | 13.2/9.6 | 253 (15) | 16.8/12.2 | 24.7 |
| 15 | 72 (17) | 329 (13) | 25.3/19.7 | 330 (12) | 27.5/21.5 | 346 (10) | 34.6/27.4 | 320 (16) | 20/15.5 | 242 (17) | 14.2/10 | 180 (10) | 18/10.8 | 25.7 |
| 16 | 54 (10) | 268 (11) | 24.3/19.4 | 303 (7) | 43.2/35.5 | 309 (10) | 30.9/25.5 | 296 (10) | 29.6/24.2 | 280 (9) | 31.1/25.1 | 261 (13) | 20/15.9 | 20.7 |
| 17 | 38 (20) | 240 (15) | 16/13.4 | 289 (12) | 24/20.9 | 299 (13) | 23/20 | 229 (16) | 14.3/11.9 | 214 (14) | 15.2/12.5 | 172 (13) | 13.2/10.3 | 20.7 |
| 18 | 57 (5) | 336 (8) | 42/34.8 | 343 (8) | 42.8/35.7 | 337 (7) | 48.1/40 | 329 (10) | 32.9/27.2 | 297 (9) | 33/26.6 | 182 (9) | 20.2/13.8 | 14.5 |
| 19 | 47 (7) | 336 (7) | 48/41.2 | 340 (5) | 68/58.6 | 333 (8) | 41.6/35.7 | 291 (10) | 29.1/24.4 | 277 (10) | 27.7/23 | 180 (21) | 8.5/6.3 | 15.6 |
| 20 | 49 (10) | 333 (10) | 33.3/28.4 | 331 (13) | 25.4/21.7 | 314 (11) | 28.5/24.1 | 294 (13) | 22.6/18.8 | 263 (16) | 16.4/13.4 | 288 (15) | 19.2/15.9 | 22.0 |
| 21 | 44 (10) | 265 (8) | 33.1/27.6 | 267 (9) | 29.6/24.7 | 258 (10) | 25.8/21.4 | 261 (13) | 20.1/16.7 | 274 (18) | 15.2/12.8 | 218 (17) | 12.8/10.2 | 24.2 |
| 22 | 54 (9) | 307 (8) | 38.4/31.6 | 364 (7) | 52/44.3 | 366 (8) | 45.7/39 | 325 (8) | 40.6/33.9 | 274 (17) | 16.1/13 | 198 (10) | 19.8/14.4 | 26.6 |
| 23 | 52 (6) | 288 (8) | 36/29.5 | 298 (6) | 49.6/41 | 299 (8) | 37.3/30.9 | 277 (14) | 19.8/16.1 | 253 (10) | 25.3/20.1 | 173 (5) | 34.6/24.2 | 25.7 |
| 24 | 53 (9) | 238 (12) | 19.8/15.4 | 258 (8) | 32.2/25.6 | 250 (12) | 20.8/16.4 | 233 (9) | 25.9/20 | 184 (13) | 14.1/10.1 | 175 (12) | 14.6/10.2 | 25.8 |
| 25 | 33 (10) | 244 (11) | 22.2/19.2 | 259 (9) | 28.8/25.1 | 240 (10) | 24/20.7 | 254 (19) | 13.4/11.6 | 241 (11) | 21.9/18.9 | 196 (12) | 16.3/13.6 | 25 |
| 26 | 43 (13) | 368 (11) | 33.4/29.5 | 380 (10) | 38/33.7 | 382 (12) | 31.8/28.2 | 363 (8) | 45.4/40 | 319 (13) | 24.5/21.2 | 305 (19) | 16/13.8 | 24.9 |
| 27 | 42 (6) | 323 (10) | 32.3/28.1 | 299 (7) | 42.7/36.7 | 284 (12) | 23.6/20.2 | 303 (10) | 30.3/26.1 | 286 (8) | 35.7/30.5 | 234 (9) | 26/21.3 | 24.4 |
| 28 | 51 (18) | 330 (24) | 13.7/11.6 | 326 (14) | 23.3/19.6 | 328 (12) | 27.3/23.1 | 342 (24) | 14.2/12.1 | 288 (21) | 13.7/11.3 | 257 (27) | 9.5/7.6 | 24.2 |
| 29 | 55 (11) | 372 (8) | 46.5/39.6 | 366 (6) | 61/51.8 | 344 (11) | 31.3/26.3 | 372 (8) | 46.5/39.6 | 350 (24) | 14.6/12.3 | 354 (14) | 25.3/21.3 | 18.7 |
| 30 | 57 (7) | 173 (8) | 21.6/14.5 | 256 (7) | 36.6/28.4 | 170 (10) | 17/11.3 | 197 (12) | 16.4/11.6 | 152 (11) | 13.8/8.6 | 132 (11) | 12/6.8 | 26.4 |
| 31 | 55 (28) | 535 (26) | 20.6/18.5 | 526 (21) | 25/22.4 | 503 (29) | 17.3/15.5 | 500 (27) | 18.5/16.5 | 490 (24) | 20.4/18.1 | 469 (29) | 16.2/14.3 | 15.9 |
| Mean | 290.7 | 24.9/16.9 | 308.2 | 40/30 | 305.9 | 23.2/3 | 294.5 | 19.3/23.2 | 268.5 | 21/15.6 | 234.2 | 17.7/23 | 23.4 | |
| SD | 68.6 | 10.7/6.8 | 56.5 | 18.5/10.1 | 58.5 | 4.9/9.2 | 58.5 | 5/12.1 | 61.2 | 10.4/5.5 | 68 | 4.7/14.7 | 3.6 |
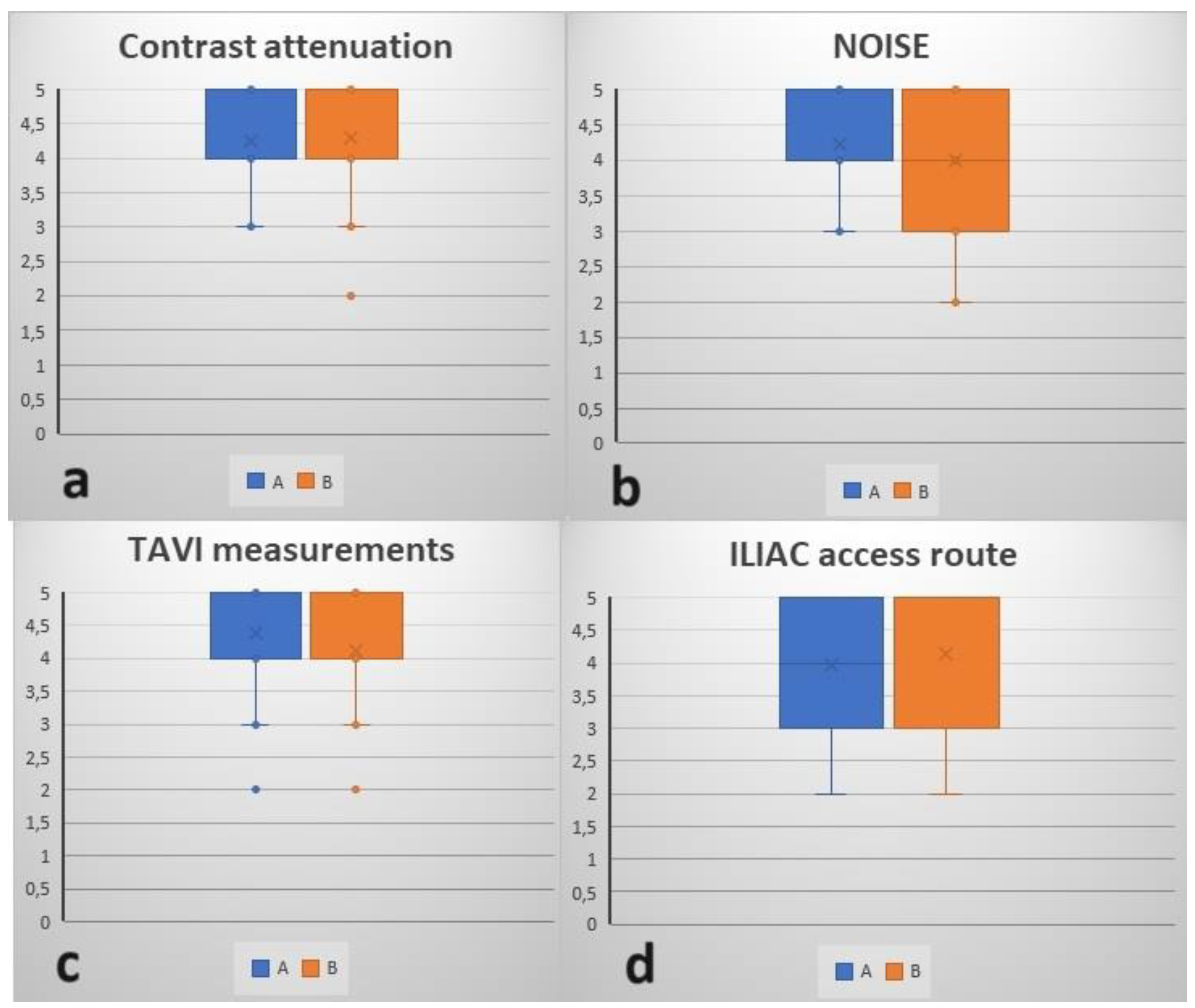
| CA | NOISE | TAVI Measurements | ILIAC Route | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | |
| 1 | 3 | 3 | 3 | 4 | 3 | 2 | 4 | 4 |
| 2 | 4 | 4 | 5 | 5 | 4 | 2 | 5 | 5 |
| 3 | 3 | 3 | 4 | 3 | 5 | 3 | 3 | 2 |
| 4 | 4 | 4 | 3 | 2 | 4 | 4 | 4 | 4 |
| 5 | 5 | 5 | 4 | 3 | 5 | 5 | 4 | 5 |
| 6 | 4 | 4 | 5 | 3 | 5 | 4 | 3 | 4 |
| 7 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 |
| 8 | 4 | 5 | 3 | 3 | 5 | 3 | 4 | 3 |
| 9 | 3 | 4 | 4 | 3 | 5 | 4 | 3 | 5 |
| 10 | 3 | 3 | 5 | 5 | 3 | 3 | 2 | 2 |
| 11 | 3 | 2 | 5 | 5 | 5 | 4 | 3 | 3 |
| 12 | 4 | 3 | 3 | 2 | 4 | 4 | 4 | 3 |
| 13 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 14 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 5 |
| 15 | 5 | 5 | 3 | 4 | 3 | 4 | 5 | 5 |
| 16 | 4 | 4 | 5 | 5 | 4 | 5 | 5 | 5 |
| 17 | 5 | 4 | 4 | 4 | 3 | 5 | 5 | 4 |
| 18 | 5 | 5 | 4 | 4 | 2 | 2 | 3 | 3 |
| 19 | 4 | 5 | 5 | 5 | 5 | 4 | 2 | 2 |
| 20 | 5 | 5 | 3 | 3 | 4 | 4 | 5 | 5 |
| 21 | 5 | 5 | 4 | 4 | 5 | 4 | 4 | 4 |
| 22 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 23 | 4 | 4 | 5 | 4 | 4 | 5 | 2 | 3 |
| 24 | 4 | 5 | 4 | 4 | 5 | 5 | 4 | 5 |
| 25 | 5 | 4 | 4 | 3 | 5 | 4 | 5 | 5 |
| 26 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 27 | 4 | 4 | 4 | 4 | 5 | 5 | 4 | 5 |
| 28 | 5 | 5 | 4 | 4 | 5 | 5 | 4 | 4 |
| 29 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 30 | 3 | 3 | 5 | 5 | 5 | 5 | 3 | 3 |
| 31 | 5 | 5 | 3 | 3 | 4 | 4 | 5 | 5 |
| Mean | 4.26 | 4.29 | 4.23 | 4 | 4.39 | 4.13 | 3.97 | 4.13 |
| SD | 0.77 | 0.86 | 0.80 | 0.97 | 0.84 | 0.96 | 0.98 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, F.; Piacentino, F.; Gnesutta, A.; Macchi, E.; Coppola, A.; Saccomanno, A.; Gatta, T.; Recaldini, C.; Minenna, M.; Tamborini, C.; et al. Transcatheter Aortic Valve Implantation (TAVI) Planning with Dual-Layer Spectral CT Using Virtual Monoenergetic Image (VMI) Reconstructions and 20 mL of Contrast Media. J. Clin. Med. 2024, 13, 524. https://doi.org/10.3390/jcm13020524
Fontana F, Piacentino F, Gnesutta A, Macchi E, Coppola A, Saccomanno A, Gatta T, Recaldini C, Minenna M, Tamborini C, et al. Transcatheter Aortic Valve Implantation (TAVI) Planning with Dual-Layer Spectral CT Using Virtual Monoenergetic Image (VMI) Reconstructions and 20 mL of Contrast Media. Journal of Clinical Medicine. 2024; 13(2):524. https://doi.org/10.3390/jcm13020524
Chicago/Turabian StyleFontana, Federico, Filippo Piacentino, Aroa Gnesutta, Edoardo Macchi, Andrea Coppola, Angiola Saccomanno, Tonia Gatta, Chiara Recaldini, Manuela Minenna, Claudio Tamborini, and et al. 2024. "Transcatheter Aortic Valve Implantation (TAVI) Planning with Dual-Layer Spectral CT Using Virtual Monoenergetic Image (VMI) Reconstructions and 20 mL of Contrast Media" Journal of Clinical Medicine 13, no. 2: 524. https://doi.org/10.3390/jcm13020524
APA StyleFontana, F., Piacentino, F., Gnesutta, A., Macchi, E., Coppola, A., Saccomanno, A., Gatta, T., Recaldini, C., Minenna, M., Tamborini, C., Dossi, F., Ascenti, V., Barbera, S., Cicero, G., Carcano, G., Ascenti, G., Castiglioni, B., & Venturini, M. (2024). Transcatheter Aortic Valve Implantation (TAVI) Planning with Dual-Layer Spectral CT Using Virtual Monoenergetic Image (VMI) Reconstructions and 20 mL of Contrast Media. Journal of Clinical Medicine, 13(2), 524. https://doi.org/10.3390/jcm13020524