Fluid Status Assessment in Critically Ill Patients with COVID-19: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Fluid Status Evaluation in Critical Care Areas
2.2.1. Body Composition and Hydration Assessment with Bioelectrical Impedance Analysis
2.2.2. Cumulative Fluid Balance (CFB) and Fluid Overload (FO)
2.3. Venous Congestion Assessed by Ultrasound Protocol (VExUS)
2.4. Definition of Acute Kidney Injury (AKI)
2.5. Statistical Analysis
3. Results
3.1. Performance of Mortality Predictors
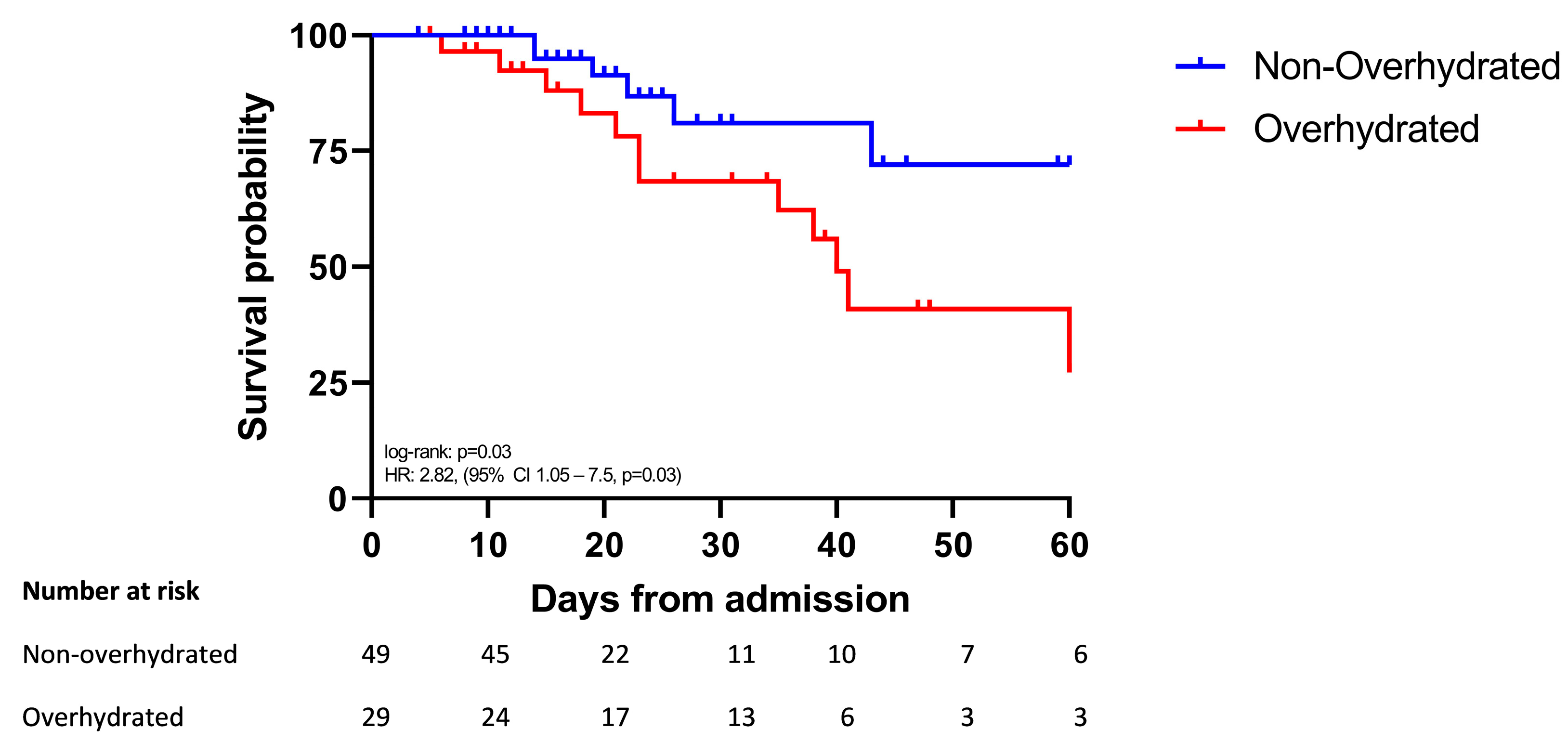
3.2. Survival Was Shorter in Overhydrated Patients
3.3. Overhydration and Persistent Overhydration Were Risk Factors for Mortality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazory, A.; Ronco, C.; McCullough, P.A. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Bayl. Univ. Med. Cent. Proc. 2020, 33, 370–375. [Google Scholar] [CrossRef]
- Hasanin, A.; Mostafa, M. Evaluation of fluid responsiveness during COVID-19 pandemic: What are the remaining choices? J. Anesth. 2020, 34, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.S.; Zingg, C.; Müller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Crit. Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Gorman, E.A.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. Lancet 2022, 400, 1157–1170. [Google Scholar] [CrossRef]
- Seitz, K.P.; Caldwell, E.S.; Hough, C.L. Fluid management in ARDS: An evaluation of current practice and the association between early diuretic use and hospital mortality. J. Intensive Care 2020, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L. Fluid management in the critically ill. Kidney Int. 2019, 96, 52–57. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Rodríguez-Moguel, N.C.; Rodríguez-Llamazares, S.; Aguilar-Vargas, A.; Casas-Aparicio, G.A.; Ríos-Ayala, M.A.; Hernández-Cardenas, C.M. Low phase angle is associated with 60-day mortality in critically ill patients with COVID-19. J. Parenter. Enter. Nutr. 2022, 46, 828–835. [Google Scholar] [CrossRef]
- Argaiz, E.R. VExUS Nexus: Bedside Assessment of Venous Congestion. Adv. Chronic Kidney Dis. 2021, 28, 252–261. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef]
- Mulasi, U.; Kuchnia, A.J.; Cole, A.J.; Earthman, C.P. Bioimpedance at the bedside: Current applications, limitations, and opportunities. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2015, 30, 180–193. [Google Scholar] [CrossRef]
- Moonen, H.P.F.X.; Van Zanten, A.R.H. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr. Opin. Crit. Care 2021, 27, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, J.H.; Jang, D.H.; Kim, J.; Hwang, B.R.; Kim, S.; Lee, J.E.; Jo, Y.H. Assessment of body water distribution in patients with sepsis during fluid resuscitation using multi-frequency direct segmental bioelectrical impedance analysis. Clin. Nutr. 2020, 39, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Scalfi, L.; Terracciano, V.; De Nicola, L.; Minutolo, R.; Marra, M.; Guida, B.; Cianciaruso, B.; Conte, G.; Di Iorio, B.R. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J. Am. Soc. Nephrol. JASN 2006, 17, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Padilla, I.A.; Borja-Magno, A.I.; Leal-Escobar, G.; Verdugo-Hernández, S. Validación de Ecuaciones de Estimación de Peso y Talla con Circunferencias Corporales en Adultos Mayores Mexicanos [Validation of Predictive Equations for Weight and Height Using Body Circumferences in Mexican Elderlys]. Nutr. Hosp. 2015, 32, 2898–2902. [Google Scholar] [CrossRef] [PubMed]
- A Healthy Lifestyle WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 2 October 2023).
- Bouchard, J.; Soroko, S.B.; Chertow, G.M.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Mehta, R.L. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009, 76, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Dennhardt, S.; Jüttner, F.; Hopf, H.B. Cumulative changes in weight but not fluid volume balances reflect fluid accumulation in ICU patients. Acta Anaesthesiol. Scand. 2017, 61, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Vignon, P.; Evrard, B.; Asfar, P.; Busana, M.; Calfee, C.S.; Coppola, S.; Demiselle, J.; Geri, G.; Jozwiak, M.; Martin, G.S.; et al. Fluid administration and monitoring in ARDS: Which management? Intensive Care Med. 2020, 46, 2252–2264. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, M.; Xu, B.; Li, C.; Chen, H.; Gong, D. The association of bioimpedance analysis parameters with the outcomes of critically ill patients. Clin. Nutr. 2020, 39, 2848–2855. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef]
- Slobod, D.; Yao, H.; Mardini, J.; Natkaniec, J.; Correa, J.A.; Jayaraman, D.; Weber, C.L. Bioimpedance-measured volume overload predicts longer duration of mechanical ventilation in intensive care unit patients. Une surcharge volémique telle que mesurée par bio-impédance prédit une durée prolongée de la ventilation mécanique chez les patients à l’unité de soins intensifs. Can. J. Anaesth. 2019, 66, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Káňová, M.; Petřeková, K.; Borzenko, N.; Rusková, K.; Nytra, I.; Dzurňáková, P. Bioelectrical impedance analysis to assess hydration in critically ill patients: A practical guide demonstrating its use on artificially ventilated COVID patients. Neuro Endocrinol. Lett. 2023, 44, 271–282, Advance online publication. [Google Scholar]
- O’Connor, M.E.; Prowle, J.R. Fluid Overload. Crit. Care Clin. 2015, 31, 803–821. [Google Scholar] [CrossRef]
- Malbrain, M.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, R.; Teeter, W.; Sullivan, S.; Tupchong, K.; Mohammed, N.; Sutherland, M.; Leibner, E.; Rola, P.; Galvagno, S.M., Jr.; Murthi, S.B. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit. Care 2020, 24, 615. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Eljaiek, R.; Cavayas, Y.A.; Rodrigue, E.; Desjardins, G.; Lamarche, Y.; Toupin, F.; Denault, A.Y.; Beaubien-Souligny, W. High postoperative portal venous flow pulsatility indicates right ventricular dysfunction and predicts complications in cardiac surgery patients. Br. J. Anaesth. 2019, 122, 206–214. [Google Scholar] [CrossRef]
- Andrei, S.; Bahr, P.A.; Nguyen, M.; Bouhemad, B.; Guinot, P.G. Prevalence of systemic venous congestion assessed by Venous Excess Ultrasound Grading System (VExUS) and association with acute kidney injury in a general ICU cohort: A prospective multicentric study. Crit. Care 2023, 27, 224. [Google Scholar] [CrossRef]
- Prager, R.; Argaiz, E.; Pratte, M.; Rola, P.; Arntfield, R.; Beaubien-Souligny, W.; Denault, A.Y.; Haycock, K.; Miralles Aguiar, F.; Bakker, J.; et al. Doppler identified venous congestion in septic shock: Protocol for an international, multi-centre prospective cohort study (Andromeda-VEXUS). BMJ Open 2023, 13, e074843. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.E.; Teboul, J.L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.; Teboul, J.L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R.; Cecconi, M.; Chew, M.S.; De Backer, D.; Douglas, I.; Edwards, M.; Hamzaoui, O.; Hernandez, G.; Martin, G.; Monnet, X.; et al. Effective hemodynamic monitoring. Crit. Care 2022, 26, 294. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 79) | Survivors (n = 61) | Non-Survivors (n = 18) | p Value | |
|---|---|---|---|---|
| Age (years) | 51.8 ± 16.4 | 48.1 ± 15.2 | 64.3 ±14.1 | <0.001 |
| Men % | 60 (75) | 48 (78.6) | 12 (66.7) | 0.290 |
| Diabetes % | 20 (26) | 15 (24) | 5 (18%) | 0.870 |
| Hypertension % | 22 (29) | 17 (28%) | 5 (28%) | 0.860 |
| Obesity % | 23 (29) | 22 (36%) | 1 (6%) | 0.010 |
| Laboratories | ||||
| Hemoglobin (g/dL) | 13.6 ± 1.6 | 13.7 ± 1.5 | 13.3 ± 2.2 | 0.470 |
| Creatinine (mg/dL) | 0.91 (0.72–1.37) | 0.9 (0.71–1.37) | 0.98 (0.78–1.43) | 0.360 |
| BUN (mg/dL) | 28 (21–39) | 27.5 (18–36) | 38 (24–54) | 0.080 |
| Sodium (mmol/L) | 141 ± 5 | 141 ± 3.3 | 140 ± 9.2 | 0.620 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.5 ± 0.6 | 0.620 |
| Phosphorus (mg/dL) | 3.9 ± 1.0 | 3.9 ± 1.0 | 3.9 ± 1.0 | 0.770 |
| Serum albumin (gr/dL) | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.7 ± 0.3 | 0.330 |
| D-dimer (µg/mL) | 0.9 (0.4–1.9) | 0.9 (0.4–1.69) | 1.5 (0.9–2.8) | 0.055 |
| Procalcitonin (ng/mL) | 0.14 (0.08–0.46) | 0.13 (0.07–0.34) | 0.26 (0.2–0.46)) | 0.100 |
| C-reactive protein (CRP) (mg/dL) | 10.8 (4.3–15.8) | 10.2 (3.8–15.6) | 11.5 (9.3–16.4) | 0.290 |
| P/F ratio (PaO2/FiO2) | 165 ± 45 | 168 ± 4 | 155 ± 47 | 0.320 |
| AKI n (%) | 29 (37%) | 24 (39%) | 5 (28%) | 0.570 |
| Hemodialysis (HD) n (%) | 2 (3%) | 1 (2%) | 1 (5.5%) | 0.320 |
| Outcomes | ||||
| Hospital stay, Days | 19 (14–35) | 13 (8–25) | 23 (14–40) | 0.013 |
| Invasive mechanical ventilation (IMV), Days | 15 (9–27) | 13 (8–25) | 22 (14–35) | 0.017 |
| Survivors (n = 61) | Non-Survivors (n = 18) | p Value | |
|---|---|---|---|
| Fluid balance | |||
| CFB, baseline (mL) | 840 (227–1402) | 913 (−319–1898) | 0.860 |
| CFB mL/kg, baseline | 10.4 (3.6–18.6) | 9.5 (−6.0–23.3) | 0.790 |
| FO > 5%, baseline | 2 (4%) | 0 | 0.470 |
| CFB, day 7 (mL) | 2717 (1333–4577) | 1852 (475–4389) | 0.420 |
| CFB mL/kg, day 7 | 27.3 (15.5–59.4) | 26.9 (6.4–53.6) | 0.510 |
| FO > 5%, day 7 | 13 (21) | 4 (22) | 0.950 |
| CFB, day 14 (mL) | 6140 (1719–8944) | 7131 (2920–16,995) | 0.400 |
| CFB mL/kg, day 14 | 67.8 (19.6–144.9) | 124.3 (48.2–184.6) | 0.250 |
| FO > 5%, day 14 | 17 (28) | 5 (28) | 0.760 |
| BIA | |||
| TBW, baseline (L) | 41.7 ± 7.7 | 36.9 ± 6.3 | 0.019 |
| TBW, day 7 (L) | 40.0 ± 8.8 | 36.2 ± 6.4 | 0.100 |
| TBW, day 14 (L) | 41.4 ± 8.0 | 35.2 ± 5.0 | 0.045 |
| ECW/TBW, baseline | 0.38 ± 0.01 | 0.39 ± 0.008 | 0.004 |
| ECW/TBW, day 7 | 0.39 ±0.01 | 0.40 ± 0.009 | 0.020 |
| ECW/TBW, day 14 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.002 |
| ICW/TBW, baseline | 0.61 ± 0.01 | 0.60 ± 0.008 | 0.001 |
| ICW/TBW, day 7 | 0.60 ± 0.01 | 0.59 ± 0.009 | 0.003 |
| ICW/TBW, day 14 | 0.60 ± 0.001 | 0.59 ± 0.009 | 0.009 |
| VExUS | |||
| VExUS 2–3 (%), baseline a | 11 (20%) | 5 (36%) | 0.410 |
| VExUS 2–3 (%), day 7 b | 7 (18%) | 3 (23%) | 0.710 |
| VExUS 2–3 (%), day 14 c | 4 (16%) | 2 (28%) | 0.460 |
| AUC | CI 95% | Cut-Off Point | Sensitivity % | Specificity % | |
|---|---|---|---|---|---|
| CFB mL/kg | 0.47 | 0.28–0.66 | >8 | 64% | 43% |
| Fluid Overload (FO) > 5% | 0.48 | 0.45–0.50 | >1 | 0% | 96% |
| VExUS 2–3 | 0.57 | 0.43–0.71 | >1 | 36% | 80% |
| ECW/TBW | 0.78 | 0.67–0.89 | 0.394 | 72% | 68% |
| ICW/TBW | 0.21 | 0.10–0.32 | 0.595 | 56% | 13% |
| Overhydrated | Persistently Overhydrated | |
|---|---|---|
| Model 1 | 5.0 (1.6–15.6, p < 0.01) | 6.0 (1.4–25.1, p = 0.01) |
| Model 2 | 6.2 (1.2–30.6, p = 0.02) | 7.6 (1.11–52.3, p = 0.03) |
| Model 3 | 6.2 (1.2–32.6, p = 0.02) | 9.5 (1.18–77.5, p = 0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Moguel, N.; Osuna-Padilla, I.A.; Piekarska, K.B.; Negrete-García, M.-F.; Hernández-Muñoz, A.; Contreras-Marín, J.A.; Montaño-Mattar, R.; Casas-Aparicio, G. Fluid Status Assessment in Critically Ill Patients with COVID-19: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 540. https://doi.org/10.3390/jcm13020540
Rodríguez-Moguel N, Osuna-Padilla IA, Piekarska KB, Negrete-García M-F, Hernández-Muñoz A, Contreras-Marín JA, Montaño-Mattar R, Casas-Aparicio G. Fluid Status Assessment in Critically Ill Patients with COVID-19: A Retrospective Cohort Study. Journal of Clinical Medicine. 2024; 13(2):540. https://doi.org/10.3390/jcm13020540
Chicago/Turabian StyleRodríguez-Moguel, Nadia, Ivan Armando Osuna-Padilla, Karolina Bozena Piekarska, María-Fernanda Negrete-García, Andrea Hernández-Muñoz, Julián Andrés Contreras-Marín, Roberto Montaño-Mattar, and Gustavo Casas-Aparicio. 2024. "Fluid Status Assessment in Critically Ill Patients with COVID-19: A Retrospective Cohort Study" Journal of Clinical Medicine 13, no. 2: 540. https://doi.org/10.3390/jcm13020540









