Post-Irradiation Breast Angiosarcoma: All the Possible Treatments and Electrochemotherapy. Case Report and Literature Review
Abstract
:1. Introduction
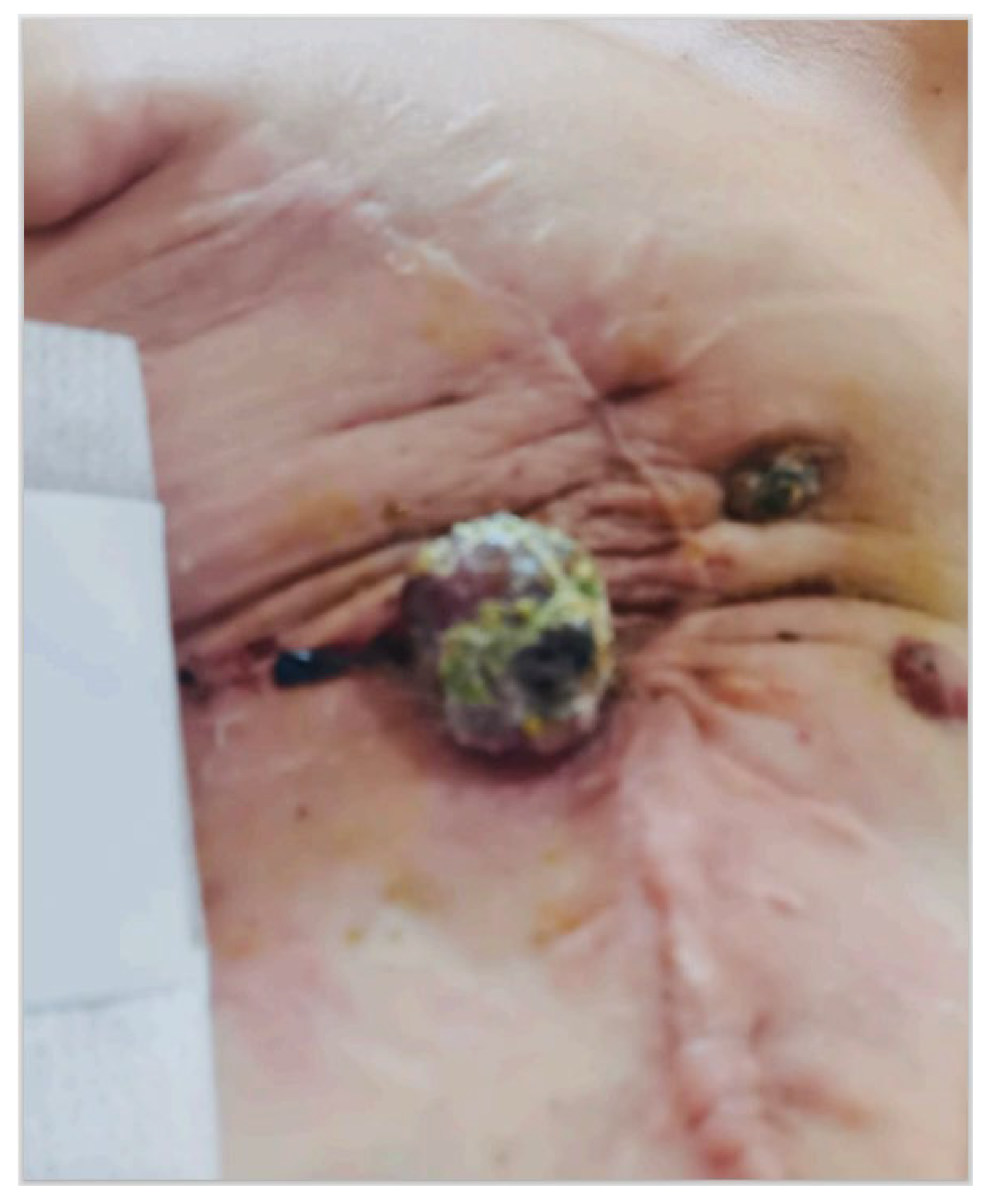
2. Case Presentation
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shah, S.; Rosa, M. Radiation-associated angiosarcoma of the breast. Clinical and pathologic features. Arch. Pathol. Lab. Med. 2016, 140, 477–481. [Google Scholar] [CrossRef]
- Seo, I.S.; Min, K.-W. Postirradiation epithelioid angiosarcoma of the breast. A case report with immunohistochemical and electron microscopic study. Ultrastruct. Pathol. 2003, 27, 197–203. [Google Scholar] [CrossRef]
- Desbiens, C.; Hogue, J.C.; Lévesque, Y. Primary breast angiosarcoma: Avoiding a common trap. Case Rep. Oncol. Med. 2011, 2011, 517047. [Google Scholar] [CrossRef]
- Arora, T.K.; Terracina, K.P.; Soong, J.; Idowu, M.O.; Takabe, K. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014, 3, 28–34. [Google Scholar]
- Brenn, T.; Fletcher, C.D. Postradiation vascular proliferations: An increasing problem. Histopathology 2006, 48, 106–114. [Google Scholar] [CrossRef]
- Laé, M.; Lebel, A.; Hamel-Viard, F.; Asselain, B.; Trassard, M.; Sastre, X.; Kirova, Y.M. Can c-myc amplification reliably discriminate post-radiation from primary angiosarcoma of the breast? Cancer Radiother. 2015, 19, 168–174. [Google Scholar] [CrossRef]
- Singh, R.; Chufal, K.S.; Pahuja, A.K.; Suresh, T.; Chowdhary, R.L.; Ahmad, I. Primary angiosarcoma of the breast: A radiation oncologist’s perspective with a concise review of the literature. BMJ Case Rep. 2019, 12, e227036. [Google Scholar] [CrossRef]
- Bousquet, G.; Confavreux, C.; Magné, N.; de Lara, C.T.; Poortmans, P.; Senkus, E.; de Lafontan, B.; Bolla, M.; Largillier, R.; Lagneau, E.; et al. Outcome and prognostic factors in breast sarcoma: A multicenter study from the rare cancer network. Radiother. Oncol. 2007, 85, 355–361. [Google Scholar] [CrossRef]
- Tozon, N.; Sersa, G.; Cemazar, M. Electrochemotherapy: Potentiation of local antitumor effectiveness of cisplatin in dogs and cats. Anticancer Res. 2001, 21, 2483–2488. [Google Scholar]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumors and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–213. [Google Scholar] [CrossRef]
- Campana, L.G.; Kis, E.; Bottyán, K.; Orlando, A.; de Terlizzi, F.; Mitsala, G.; Careri, R.; Curatolo, P.; Snoj, M.; Sersa, G.; et al. Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the International Network for Sharing Practices of electrochemotherapy (InspECT). Int. J. Surg. 2019, 72, 34–42. [Google Scholar] [CrossRef]
- Coindre, J.M.; Trojani, M.; Contesso, G.; David, M.; Rouesse, J.; Bui, N.B.; Bodaert, A.; De Mascarel, I.; De Mascarel, A.; Goussot, J.F. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer 1986, 58, 306–309. [Google Scholar] [CrossRef]
- Cencelj-Arnez, R.; Novak, J.; Klevisar Ivancic, A.; Bosnjak, M.; Cemazar, M.; Snoj, M. Radiotherapy-associated angiosarcoma in the breast reconstructed by autologous Free-flap and treated with electrochemotherapy. Radiol. Oncol. 2020, 55, 77–81. [Google Scholar] [CrossRef]
- Zhou, G.; Mei, Z. Electrochemotherapy for advanced cutaneous angiosarcoma: A european register-based cohort study from the international network for sharing practices of electrochemotherapy (InspECT)—An invited commentary. Int. J. Surg. 2019, 72, 232–233. [Google Scholar] [CrossRef]
- Alagkiozidis, I. A Commentary on “Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the International Network for Sharing Practices of Electrochemotherapy (InspECT)”. Int. J. Surg. 2019, 72, 196–197. [Google Scholar] [CrossRef]
- Benevento, R.; Carafa, F.; Di Nardo, D.; Pellino, G.; Letizia, A.; Taddeo, M.; Gambardella, A.; Canonico, S.; Santoriello, A. Angiosarcoma of the breast: A new therapeutic approach? Int. J. Surg. Case Rep. 2015, 13, 30–32. [Google Scholar] [CrossRef]
- Guida, M.; Ruggieri, E.; Fucci, L.; Ressa, M.; D’Aluisio, L.; Fanelli, G.; Strippoli, S. Image Gallery: A case of cutaneous giant angiosarcoma treated successfully with electrochemotherapy. Br. J. Dermatol. 2017, 177, e27. [Google Scholar] [CrossRef]
- Guida, M.; Campana, L.G.; Curatolo, P.; Strippoli, S.; Bonadies, A.; Grilz, G.; Cabula, C.; Rotunno, R.; Bucher, S.; Solari, N.; et al. Local treatment with electrochemotherapy of superficial angiosarcomas: Efficacy and safety results from a multi-institutional retrospective study. J. Surg. Oncol. 2016, 114, 246–253. [Google Scholar] [CrossRef]
- Borgognoni, L.; Pescitelli, L.; Gerlini, G.; Brandani, P.; Gelli, R.; Giannotti, V.; Bellucci, F.; Sestini, S. Efficacy of Electrochemotherapy in the Treatment of Cutaneous Melanoma Metastases and Rare Non-melanoma Skin Cancer. Anticancer Res. 2020, 40, 6485–6492. [Google Scholar] [CrossRef]
- Campana, L.G.; Valpione, S.; Tosi, A.; Rastrelli, M.; Rossi, C.R.; Aliberti, C. Angiosarcoma on Lymphedema (Stewart-Treves Syndrome): A 12-Year Follow-up after Isolated Limb Perfusion, Limb Infusion, and Electrochemotherapy. J. Vasc. Interv. Radiol. 2016, 27, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Spagnolo, F.; Ponte, E.; Quaglia, A.; Lillini, R.; Battista, M.; Queirolo, P.; Cafiero, F. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: A series of 39 patients treated with palliative intent. J. Surg. Oncol. 2014, 109, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Mocerino, C.; Iannaci, G.; Sapere, P.; Luise, R.; Canonico, S.; Gambardella, A. Multidisciplinary approach to breast angiosarcoma in an elderly patient: Repeated local relapses and significant objective responses. Int. J. Immunopathol. Pharmacol. 2016, 29, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Laurino, S.; Omer, L.C.; Albano, F.; Marino, G.; Bianculli, A.; Solazzo, A.P.; Sgambato, A.; Falco, G.; Russi, S.; Bochicchio, A.M. Radiation-induced sarcomas: A single referral cancer center experience and literature review. Front. Oncol. 2022, 12, 986123. [Google Scholar] [CrossRef]
- Di Meo, N.; Drabeni, M.; Gatti, A.; Trevisan, G. A Stewart-Treves syndrome of the lower limb. Dermatol. Online J. 2012, 18, 14. [Google Scholar]
- Parisi, S.; Ruggiero, R.; Gualtieri, G.; Volpe, M.L.; Rinaldi, S.; Nesta, G.; Bogdanovich, L.; Lucido, F.S.; Tolone, S.; Parmeggiani, D.; et al. Combined LOCalizer™ and Intraoperative Ultrasound Localization: First Experience in Localization of Non-palpable Breast Cancer. In Vivo 2021, 35, 1669–1676. [Google Scholar] [CrossRef]
- Parisi, S.; Gambardella, C.; Conzo, G.; Ruggiero, R.; Tolone, S.; Lucido, F.S.; Iovino, F.; Fisone, F.; Brusciano, L.; Parmeggiani, D.; et al. Advanced Localization Technique for Non-Palpable Breast Cancer: Radiofrequency alone VS Combined Technique with Ultrasound. J. Clin. Med. 2023, 12, 5076. [Google Scholar] [CrossRef]
- Parisi, S.; Gambardella, C.; Ruggiero, R.; Tolone, S.; Lucido, F.S.; Docimo, L. Radiofrequency Identification—RFID using LOCalizer-Tag in Non-palpable Breast Lump. Indian J. Surg. 2022, 85, 934–938. [Google Scholar] [CrossRef]
- Dogan, A.; Kern, P.; Schultheis, B.; Häusler, G.; Rezniczek, G.A.; Tempfer, C.B. Radiogenic angiosarcoma of the breast: Case report and systematic review of the literature. BMC Cancer 2018, 18, 463. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Munhoz, R.R.; Kuk, D.; Landa, J.; Hartley, E.W.; Bonafede, M.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Crago, A.M.; et al. Outcomes of systemic therapy for patients with metastatic angiosarcoma. Oncology 2015, 89, 205–214. [Google Scholar] [CrossRef]
- Depla, A.L.; Scharloo-Karels, C.H.; de Jong, M.A.A.; Oldenborg, S.; Kolff, M.W.; Oei, S.B.; van Coevorden, F.; van Rhoon, G.C.; Baartman, E.A.; Scholten, R.J.; et al. Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: A systematic review. Eur. J. Cancer 2014, 50, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadithy, N.; Dehnel, A.; George, A.; Kisiel, R.; Lunt, C.; Stone, C. Patient reported outcomes in prospective cohort study of Electrochemotherapy. Int. J. Surg. 2018, 52, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Balestrieri, N.; Menin, N. Adjuvant skin-sparing electrochemotherapy in a breast cancer patient with a prosthetic implant: 5-year follow-up outcomes. J. Surg. Case Rep. 2022, 5, rjac199. [Google Scholar] [CrossRef] [PubMed]
- Cote, G.M.; He, J.; Choy, E. Next-Generation Sequencing for Patients with Sarcoma: A Single Center Experience. Oncologist 2018, 23, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.; Chi, P.; Tap, W.D.; Rosenbaum, E.; D’Angelo, S.; Alektiar, K.M.; Antonescu, C.R. Distinct genomic landscapes in radiation-associated angiosarcoma compared with other radiation-associated sarcoma histologies. J. Pathol. 2023, 260, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.; Perrone, A.M.; Buwenge, M.; Arcelli, A.; Vadala’, M.; Fionda, B.; Malato, M.C.; De Iaco, P.; Zamagni, C.; Cammelli, S.; et al. Combination of Electrochemotherapy with Radiotherapy: A Comprehensive, Systematic, PRISMA-Compliant Review of Efficacy and Potential Radiosensitizing Effects in Tumor Control. Curr. Oncol. 2023, 30, 9895–9905. [Google Scholar] [CrossRef]
- Espejo-Freire, A.P.; Elliott, A.; Rosenberg, A.; Costa, P.A.; Barreto-Coelho, P.; Jonczak, E.; D’Amato, G.; Subhawong, T.; Arshad, J.; Diaz-Perez, J.A.; et al. Genomic Landscape of Angiosarcoma: A Targeted and Immunotherapy Biomarker Analysis. Cancers 2021, 13, 4816. [Google Scholar] [CrossRef]
- Fraga-Guedes, C.; André, S.; Mastropasqua, M.G.; Botteri, E.; Toesca, A.; Rocha, R.M.; Peradze, N.; Rotmensz, N.; Viale, G.; Veronesi, P.; et al. Angiosarcoma and atypical vascular lesions of the breast: Diagnostic and prognostic role of MYC gene amplification and protein expression. Breast Cancer Res. Treat. 2015, 151, 131–140. [Google Scholar] [CrossRef]


| Study | Type of Study | Patients | Age | History | First Treatment | Latency Period Months | Clinical Features | Skin Region | Re-Treatment | Histology | C myc Amplification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cencelj-Arnez 2020 [14] | CR | 1 | 63 | Syncronus bilateral Luminal BC (right breast) | Mastectomy + 6 cycles x 5-fluorouracil, epirubicin, Cyclophosphamide + letrozole + RT 25Gy | 60 | Ulcerated red lesion | Lower-medial quadrant In right breast | Excision + ECT + doxorubicin | High grade RAS | Yes |
| Campana 2019 [12] | CS | 20 (10 breast RAS) | / | / | / | / | / | / | / | / | / |
| Benevento 2015 [17] | CR | 1 | 76 | Invasive ductal carcinoma Luminal BC (left breast) pT1 pN0 M0 G2 | BCS + 50 Gy in 25 fractions of 200 cGy/daily with boost of 10 Gy in 5 fractions of 200 cGy/daily + Tamoxifene | 48 | painful, violet, multi-nodular mass | Left > right breast | Excision + Mastectomy (after 4 years) + doxorubicin | grade-II RAS | / |
| Guida 2016 [19] | RS | 19 (6 breast RAS) | 69 | / | / | 96 | / | Scalp (5) Breast (8) Skin (3) Soft tissue (3) | ECT (19/19)+ Surgery (17/19) + RT (5/19) + CT (3/19) | RAS | / |
| Mocerino 2016 [23] | CR | 1 | 77 | invasive ductal carcinoma pT1N0M0 ER + 15%; PgR + 30%; HER2 IHC 1 + (left breast) | BCL + 60 Gy in 30 fractions + tamoxifen | 84 | ecchymotic lesion (1.3 cm) | near the scar | Excision + left mastectomy (after 1 year) + right mastectomy (after 2 years) + ECT + 69 Gy + Doxorubicin | low-grade RAS | / |
| Laurino 2022 [24] | CR | 1 | 61 | infiltrating ductal carcinoma, pT1cN0, grade G2, ER 98%, PGR 20%, HER2 +, left breast | BCL + 50 Gy in 25 fractions + 10 Gy in 5 fractions by photons + Adjuvant CT + letrozole | 72 | / | Left breast | Neoadjuvant CT + mastectomy (after 1 year) + ECT + Re-excision | high-grade RAS (G3), positive for Factor VIII and CD31, with extensive areas of necrosis and ulceration. | / |
| Laurino 2022 [24] | CR | 1 | 63 | infiltrating ductal breast cancer pT1cN1(1/18), G2, ER: 90%, PGR: 60%, Ki67 index at 15%, and HER2 negative Left breast | BCS+ 5-fluorouracil, epidoxorubicin, and cyclophosphamide+ 50 Gy in 25 fractions + 10 Gy in 5 fractions by photons+ letrozole | 108 | ulcerated and bleeding left breast lump, 7 cm in diameter, adherent to the chest wall | Left breast | Radiofrequency termoablation + gemcitabine and docetaxel + ECT + | RAS | / |
| Current case report | CR | 1 | 59 | breast invasive ductal Luminal B carcinoma pT1c N0 M0 (right breast) | BCL + 60 Gy in 30 fractions+ femara | 60 | exophytic lump | near the scar | Excision + right mastectomy + Paclitaxel (doxorubicin contraindicated) + 40.5 Gy in 15 fractions + ECT | Grade II RAS | / |
| Study | ECT | ||||||
|---|---|---|---|---|---|---|---|
| Cycles | Drug | Dose | Adverse Reactions | Overall Survival | Free-Progression Survival (Months) | Results | |
| Cencelj-Arnez 2020 [14] | 3 | Bleomicin | 30,000 | edema | 19 | 1 | CR = 100% |
| Campana 2019 [12] | 24 (10 Breast RAS; 1 ECT per patient) | Bleomicin | 250–1000 IU/cm3 or 15,000/m2 | Skin ulceration (25%) pain (30%) | 12.5 | 1.8 | CR 40% (8/20) PR, 40% (8/20) |
| Benevento 2015 [17] | 8 | Bleomicin | 15,000 IU/m2 | / | 18 | 18 | CR = 100% |
| Guida 2016 [19] | / | Bleomicin | 15,000 IU/m2 | Pain | 29.9 | / | CR = 42% PR = 21% |
| Mocerino 2016 [23] | 2 | Bleomicin | 15,000 IU/m2 | / | 21 | 21 | CR = 100% |
| Laurino 2022 [24] | / | / | / | / | 3 | 3 | Local condition improvement |
| Laurino 2022 [24] | 2 | / | / | / | 24 | 24 | Local condition improvement |
| Parisi 2023 | 3 | Bleomicin | 15,000 IU/m2 | Pain Edema necrosis | 24 | 17 | Local condition improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, S.; Gambardella, C.; Iovino, F.; Ruggiero, R.; Lucido, F.S.; Nesta, G.; Tolone, S.; Brusciano, L.; Fisone, F.; Mongardini, F.M.; et al. Post-Irradiation Breast Angiosarcoma: All the Possible Treatments and Electrochemotherapy. Case Report and Literature Review. J. Clin. Med. 2024, 13, 567. https://doi.org/10.3390/jcm13020567
Parisi S, Gambardella C, Iovino F, Ruggiero R, Lucido FS, Nesta G, Tolone S, Brusciano L, Fisone F, Mongardini FM, et al. Post-Irradiation Breast Angiosarcoma: All the Possible Treatments and Electrochemotherapy. Case Report and Literature Review. Journal of Clinical Medicine. 2024; 13(2):567. https://doi.org/10.3390/jcm13020567
Chicago/Turabian StyleParisi, Simona, Claudio Gambardella, Francesco Iovino, Roberto Ruggiero, Francesco Saverio Lucido, Giusiana Nesta, Salvatore Tolone, Luigi Brusciano, Francesca Fisone, Federico Maria Mongardini, and et al. 2024. "Post-Irradiation Breast Angiosarcoma: All the Possible Treatments and Electrochemotherapy. Case Report and Literature Review" Journal of Clinical Medicine 13, no. 2: 567. https://doi.org/10.3390/jcm13020567
APA StyleParisi, S., Gambardella, C., Iovino, F., Ruggiero, R., Lucido, F. S., Nesta, G., Tolone, S., Brusciano, L., Fisone, F., Mongardini, F. M., Cozzolino, G., Della Corte, C. M., Napolitano, S., Orditura, M., Esposito, R., & Docimo, L. (2024). Post-Irradiation Breast Angiosarcoma: All the Possible Treatments and Electrochemotherapy. Case Report and Literature Review. Journal of Clinical Medicine, 13(2), 567. https://doi.org/10.3390/jcm13020567









