Transcatheter Aortic Valve Implantation to Treat Degenerated Aortic, Mitral and Tricuspid Bioprosthesis
Abstract
1. Introduction
2. TAVI for Degenerated Surgical Aortic Bioprosthetic Valves: Valve-in-Valve (ViV)-TAVI
2.1. Pre-Procedural Considerations for ViV-TAVI
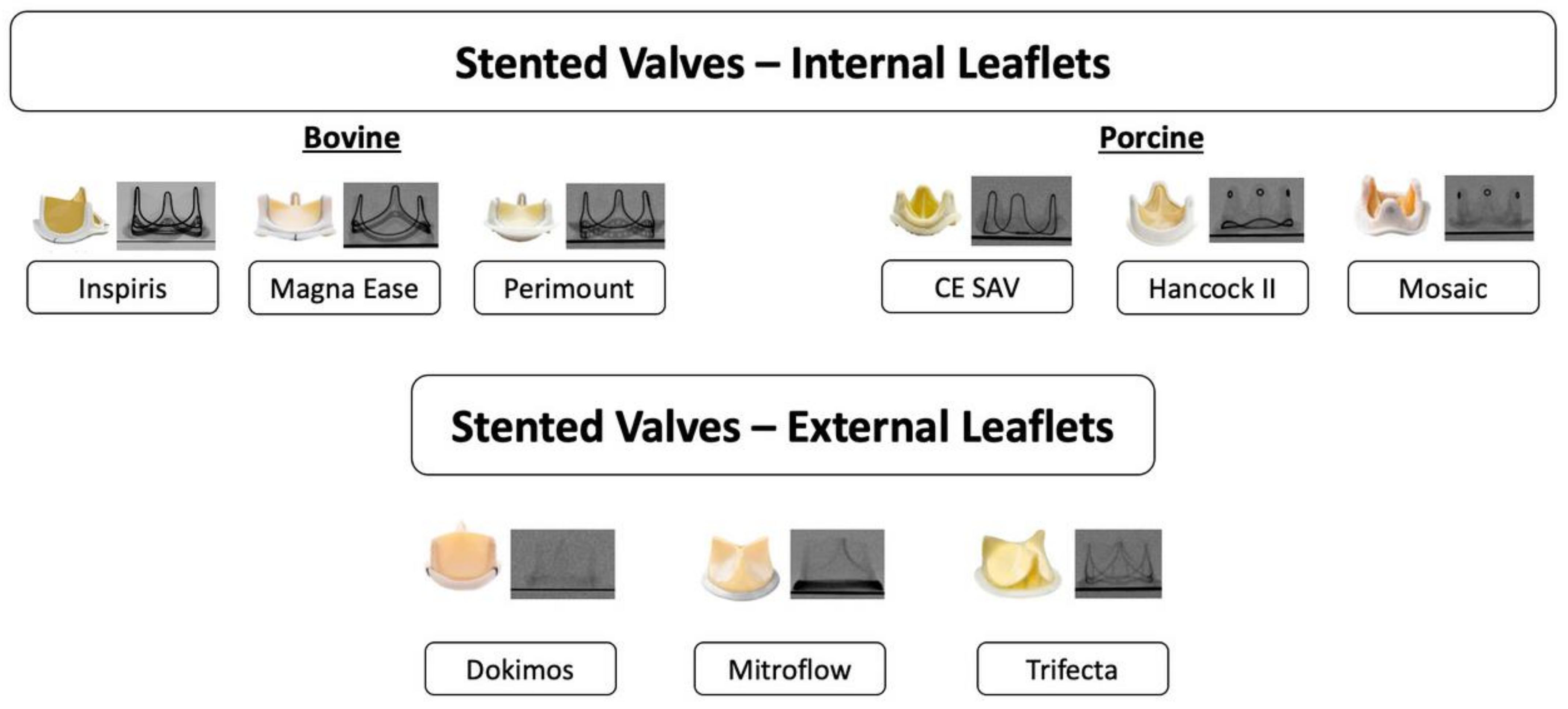
2.1.1. Type of Surgical Aortic Bioprosthesis
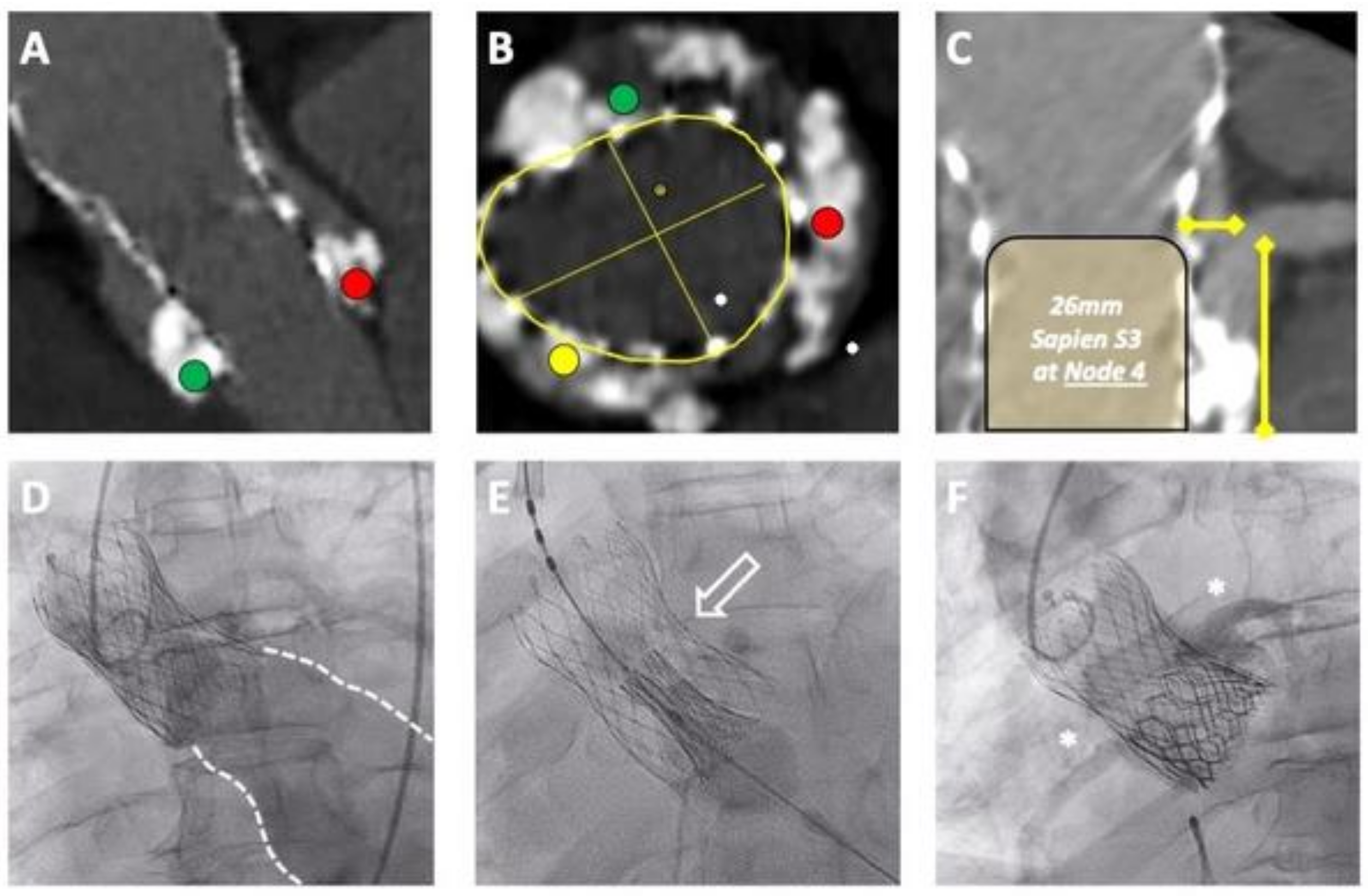
2.1.2. Pre-Procedural Assessment
2.2. Procedural Considerations
2.3. Future Considerations Regarding ViV
3. TAVI for Degenerated Transcatheter Aortic Valves: Redo TAVI
3.1. Clinical Experience
3.2. Principles of Redo TAVI
3.2.1. Leaflet Neoskirt
3.2.2. Leaflet Overhang
3.2.3. Frame Anchoring
3.2.4. Frame Alignment
3.2.5. Frame Distortion
3.3. Pre-Procedural Planning for Redo TAVI
Index THV
3.4. Leaflet Modification for Redo TAVI
3.5. Coronary Access after Redo TAVI
3.6. Future Perspectives
4. TAVI for Treatment of Degenerated Mitral Bioprosthesis
4.1. Current Evidence
4.2. Procedural Considerations
4.2.1. LVOT Obstruction
4.2.2. Procedural Steps
4.3. Future Perspectives
5. TAVI for Treating Degenerated Tricuspid Bioprosthesis
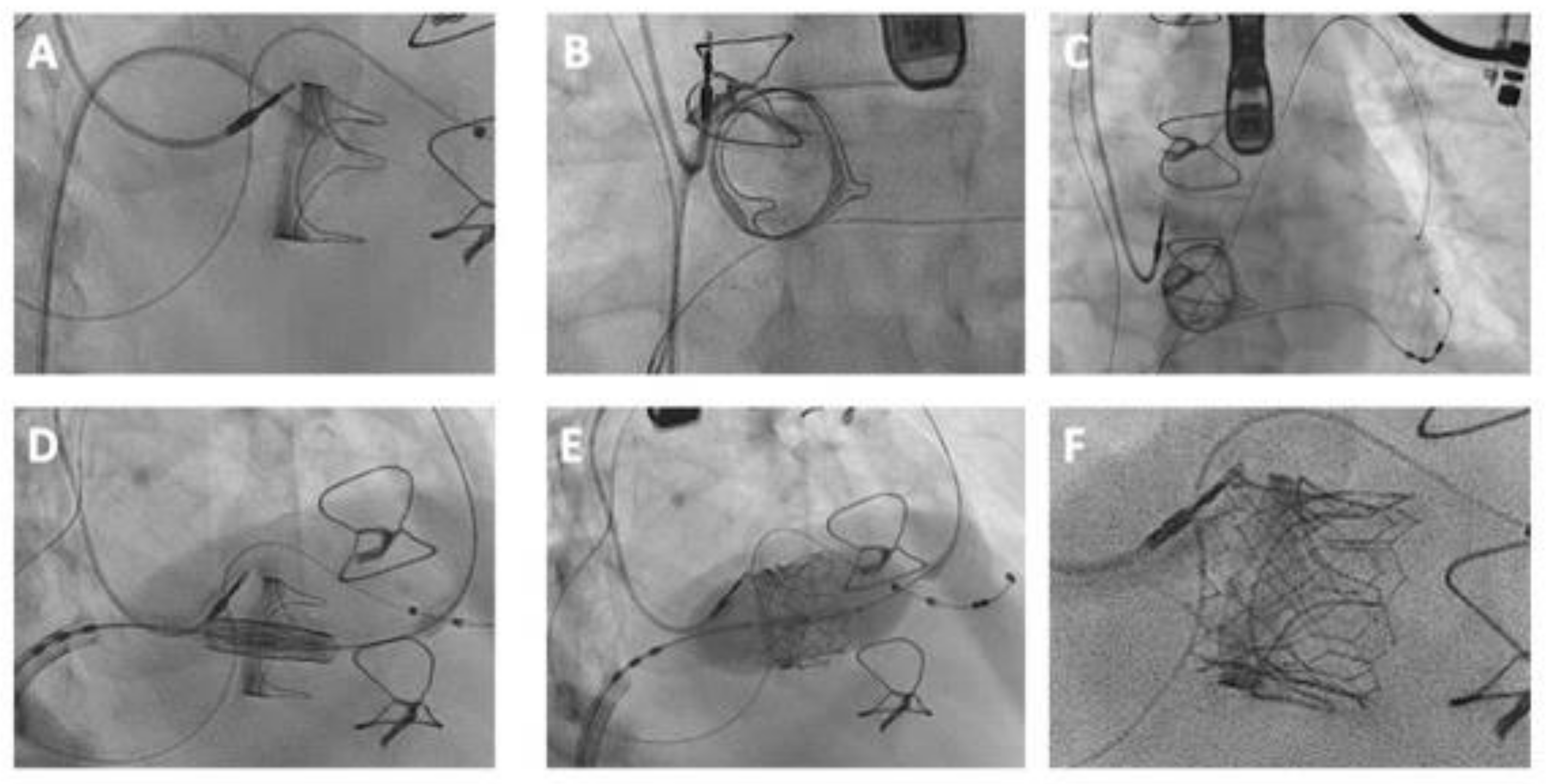
5.1. Comprehensive Procedural Guide for TViV and TViR Interventions
5.2. Preprocedural Assessment
5.2.1. Imaging Modalities
5.2.2. Planning Considerations
5.2.3. Computed Tomography (CT) for Anatomical Assessment
5.2.4. Valve Deployment
5.3. Procedural Considerations for TViVs and TViRs
5.3.1. Vascular Access and Procedural Approach
5.3.2. Crossing the Tricuspid Valve
5.3.3. Wire Positioning
5.3.4. Valve Sizing and Selection
5.4. Valve Implantation
- Valve Preparation: Mounting the valve onto the delivery catheter involves a series of steps ensuring the correct orientation of the valve. For instance, the Melody valve is positioned as per standard protocols used in pulmonary valve implantation [97].
- Implantation Techniques: Deploying the valve involves a methodical approach tailored to the specific valve being used. The Melody valve typically requires dilatation of the inner balloon, ensuring approximately 40% of the stent frame is aligned within the right atrium. Subsequent inflation of the outer balloon aligns the valve into the correct tricuspid position. Conversely, the Sapien 3 valve may necessitate overexpansion up to a diameter of 31 mm, thanks to its design features like longer leaflets and a taller stent height. Proper positioning concerning the surgical valve stent frame or other markers is critical for optimal deployment [90,92].
- Balloon Sizing and Positioning: In challenging cases involving very stenotic valves, pre-dilation of the prosthesis might be considered to facilitate subsequent valve deployment. Specific guidelines dictate the positioning of the central marker of the Sapien 3 valve concerning the surgical valve stent frame or other anatomical markers, ensuring accurate alignment and minimizing the risk of malposition or paravalvular leakage [73].
- Procedural Modifications: Adjustments during the procedure might be necessary based on real-time observations. Techniques like retracting the Sapien 3 delivery catheter pusher or slightly inflating the balloon for better tracking of the system can aid in overcoming obstacles encountered during valve crossing or deployment [99].
5.5. Future Perspectives
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; Tchétché, D.; Huang, J.; et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J. Am. Coll. Cardiol. 2023, 82, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Ternacle, J.; Denimal, T.; Shen, M.; Redfors, B.; Delhaye, C.; Simonato, M.; Debry, N.; Verdier, B.; Shahim, B.; et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis. Circulation 2021, 143, 1043–1061. [Google Scholar] [CrossRef]
- Poletti, E.; De Backer, O.; Scotti, A.; Costa, G.; Bruno, F.; Fiorina, C.; Buzzatti, N.; Latini, A.; Rudolph, T.K.; Dorpel, M.M.v.D.; et al. Transcatheter Aortic Valve Replacement for Pure Native Aortic Valve Regurgitation: The PANTHEON International Project. JACC Cardiovasc. Interv. 2023, 16, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Mack, M.J.; White, J.M.; Dvir, D.; Blanke, P.; Herrmann, H.C.; Leipsic, J.; Kodali, S.K.; Makkar, R.; Miller, D.C.; et al. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J. Am. Coll. Cardiol. 2017, 69, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Deeb, G.M.; Chetcuti, S.J.; Reardon, M.J.; Patel, H.J.; Grossman, P.M.; Schreiber, T.; Forrest, J.K.; Bajwa, T.K.; O’hair, D.P.; Petrossian, G.; et al. 1-Year Results in Patients Undergoing Transcatheter Aortic Valve Replacement With Failed Surgical Bioprostheses. JACC Cardiovasc. Interv. 2017, 10, 1034–1044. [Google Scholar] [CrossRef]
- Tuzcu, E.M.; Kapadia, S.R.; Vemulapalli, S.; Carroll, J.D.; Holmes, D.R., Jr.; Mack, M.J.; Thourani, V.H.; Grover, F.L.; Brennan, J.M.; Suri, R.M.; et al. Transcatheter Aortic Valve Replacement of Failed Surgically Implanted Bioprostheses: The STS/ACC Registry. J. Am. Coll. Cardiol. 2018, 72, 370–382. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Isaacs, A.J.; Shuhaiber, J.; Salemi, A.; Isom, O.W.; Sedrakyan, A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J. Thorac. Cardiovasc. Surg. 2015, 149, 1262–1269.e3. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Abbas, A.E.; Serra, V.; Vilalta, V.; Nombela-Franco, L.; Regueiro, A.; Al-Azizi, K.M.; Iskander, A.; Conradi, L.; Forcillo, J.; et al. Balloon- vs Self-Expanding Valve Systems for Failed Small Surgical Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2022, 80, 681–693. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Park, J.K.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2017, 39, 687–695. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Tanaka, A.; Finkelstein, A.; Mack, M.; Tamburino, C.; Van Mieghem, N.; de Backer, O.; Testa, L.; Gatto, P.; Purita, P.; et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Komatsu, I.; Tzemach, L.; Simonato, M.; Wolak, A.; Blanke, P.; Dvir, D. Risk of coronary obstruction and the need to perform BASILICA: The VIVID classification. EuroIntervention 2021, 16, e757–e759. [Google Scholar] [CrossRef] [PubMed]
- Yerasi, C.; Rogers, T.; Forrestal, B.J.; Case, B.C.; Khan, J.M.; Ben-Dor, I.; Satler, L.F.; Garcia-Garcia, H.M.; Cohen, J.E.; Kitahara, H.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Young, Low-Risk Patients With Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2021, 14, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Okuno, T.; Unbehaun, A.; Mack, M.; Kapadia, S.; Falk, V. Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation? Eur. Heart J. 2022, 43, 2729–2750. [Google Scholar] [CrossRef]
- Khokhar, A.A.; Ponticelli, F.; Zlahoda-Huzior, A.; Zakrzewski, P.; Mikhail, G.; Dudek, D.; Giannini, F. Coronary access techniques following ACURATE neo2 implantation in surgical bioprosthesis. EuroIntervention 2022, 18, 820–821. [Google Scholar] [CrossRef]
- Khokhar, A.K.; Ponticelli, F.; Zlahoda-Huzior, A.; Ruggiero, R.; Kim, W.-K.; Mangieri, A.; Colombo, A.; Dudek, D.; Giannini, F. A novel three-dimensional imaging approach to evaluate coronary access before transcatheter aortic valve-in-valve implantation. EuroIntervention 2022, 17, 1238–1239. [Google Scholar] [CrossRef]
- Khokhar, A.A.; Giannini, F.; Zlahoda-Huzior, A.; Mikhail, G.; Dudek, D. Coronary access after ACURATE neo2 implantation for valve-in-valve TAVR: Insights from ex vivo simulations. Catheter. Cardiovasc. Interv. 2022, 100, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Banbury, M.K.; Cosgrove, D.M.; White, J.A.; Blackstone, E.H.; Frater, R.W.M.; Okies, J. Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann. Thorac. Surg. 2001, 72, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Mokhles, M.M.; Osnabrugge, R.L.; Pibarot, P.; Mack, M.J.; Takkenberg, J.J.; Bogers, A.J.; Kappetein, A.P. The impact of prosthesis–patient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur. Heart J. 2012, 33, 1518–1529. [Google Scholar] [CrossRef]
- Pibarot, P.; Simonato, M.; Barbanti, M.; Linke, A.; Kornowski, R.; Rudolph, T.; Spence, M.; Moat, N.; Aldea, G.; Mennuni, M.; et al. Impact of Pre-Existing Prosthesis-Patient Mismatch on Survival Following Aortic Valve-in-Valve Procedures. JACC Cardiovasc. Interv. 2018, 11, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, J.; Fraser, R.; Hatoum, H.; Barlow, A.M.; Stanová, V.; Allen, K.B.; Chhatriwalla, A.K.; Rieu, R.; Pibarot, P.; Dasi, L.P.; et al. A bench test study of bioprosthetic valve fracture performed before versus after transcatheter valve-in-valve intervention. EuroIntervention 2020, 15, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Meier, D.; Payne, G.W.; Payne, G.W.; Mostaço-Guidolin, L.B.; Mostaço-Guidolin, L.B.; Bouchareb, R.; Bouchareb, R.; Rich, C.; Rich, C.; et al. Timing of bioprosthetic valve fracture in transcatheter valve-in-valve intervention: Impact on valve durability and leaflet integrity. EuroIntervention 2023, 18, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, I.; Leipsic, J.; Webb, J.G.; Blanke, P.; Mackensen, G.B.; Don, C.W.; McCabe, J.M.; Rumer, C.; Tan, C.W.; Levin, D.B.; et al. Coronary ostial eccentricity in severe aortic stenosis: Guidance for BASILICA transcatheter leaflet laceration. J. Cardiovasc. Comput. Tomogr. 2020, 14, 516–519. [Google Scholar] [CrossRef]
- Curio, J.; Khokhar, A.A.; Beneduce, A.; Mylotte, D.; Fezzi, S.; Kim, W.; Zlahoda-Huzior, A.; Giannini, F.; Dudek, D. Patient-specific commissural alignment for ACURATE neo2 implantation in degenerated surgical bioprostheses. Catheter. Cardiovasc. Interv. 2023, 102, 1401–1405. [Google Scholar] [CrossRef]
- Westermann, D.; Ludwig, S.; Kalbacher, D.; Spink, C.; Linder, M.; Bhadra, O.D.; Nikorowitsch, J.; Waldschmidt, L.; Demal, T.; Voigtländer, L.; et al. Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: The Hamburg BASILICA experience. Clin. Res. Cardiol. 2021, 110, 1900–1911. [Google Scholar] [CrossRef]
- Mercanti, F.; Rosseel, L.; Neylon, A.; Bagur, R.; Sinning, J.-M.; Nickenig, G.; Grube, E.; Hildick-Smith, D.; Tavano, D.; Wolf, A.; et al. Chimney Stenting for Coronary Occlusion During TAVR. JACC Cardiovasc. Interv. 2020, 13, 751–761. [Google Scholar] [CrossRef]
- Khan, J.M.; Dvir, D.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Aldea, G.; Reisman, M.; Mackensen, G.B.; Eng, M.H.; Paone, G.; et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc. Interv. 2018, 11, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Krishnan, A.M.; Lahoud, R.; Polomsky, M.; Dauerman, H.L. National Trends in TAVR and SAVR for Patients With Severe Isolated Aortic Stenosis. J. Am. Coll. Cardiol. 2022, 80, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Vanhaverbeke, M.; Nuyens, P.; Bække, P.S.; Maaranen, P.E.; Wang, X.; Bieliauskas, G.; De Backer, O.; Sondergaard, L. Temporal Trends in Survival Rates After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2022, 15, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Webb, J.G.; Tamburino, C.; Van Mieghem, N.M.; Makkar, R.R.; Piazza, N.; Latib, A.; Sinning, J.-M.; Won-Keun, K.; Bleiziffer, S.; et al. Outcomes of Redo Transcatheter Aortic Valve Replacement for the Treatment of Postprocedural and Late Occurrence of Paravalvular Regurgitation and Transcatheter Valve Failure. Circ. Cardiovasc. Interv. 2016, 9, e003930. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.; Agnifili, M.; Van Mieghem, N.M.; Tchétché, D.; Asgar, A.W.; De Backer, O.; Latib, A.; Reimers, B.; Stefanini, G.; Trani, C.; et al. Transcatheter Aortic Valve Replacement for Degenerated Transcatheter Aortic Valves: The TRANSIT International Project. Circ. Cardiovasc. Interv. 2021, 14, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Zaid, S.; Kleiman, N.S.; Goel, S.S.; Fukuhara, S.; Marin-Cuartas, M.; Kiefer, P.; Abdel-Wahab, M.; De Backer, O.; Søndergaard, L.; et al. Explant vs. Redo-TAVR After Transcatheter Valve Failure: Mid-Term Outcomes From the EXPLANTORREDO-TAVR International Registry. JACC Cardiovasc. Interv. 2023, 16, 927–941. [Google Scholar] [CrossRef]
- Percy, E.D.; Harloff, M.T.; Hirji, S.; McGurk, S.; Yazdchi, F.; Newell, P.; Malarczyk, A.; Sabe, A.; Landes, U.; Webb, J.; et al. Nationally Representative Repeat Transcatheter Aortic Valve Replacement Outcomes: Report From the Centers for Medicare and Medicaid Services. JACC Cardiovasc. Interv. 2021, 14, 1717–1726. [Google Scholar] [CrossRef]
- Landes, U.; Sathananthan, J.; Witberg, G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Holzhey, D.; Kim, W.-K.; Hamm, C.; Buzzatti, N.; et al. Transcatheter Replacement of Transcatheter versus Surgically Implanted Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2021, 77, 1–14. [Google Scholar] [CrossRef]
- Landes, U.; Webb, J.G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Crusius, L.; Kim, W.-K.; Hamm, C.; Buzzatti, N.; Montorfano, M.; et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Kapadia, S.; Chakravarty, T.; Cubeddu, R.J.; Kaneko, T.; Mahoney, P.; Patel, D.; Gupta, A.; Cheng, W.; Kodali, S.; et al. Outcomes of repeat transcatheter aortic valve replacement with balloon-expandable valves: A registry study. Lancet 2023, 402, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Landes, U.; Richter, I.; Danenberg, H.; Kornowski, R.; Sathananthan, J.; De Backer, O.; Søndergaard, L.; Abdel-Wahab, M.; Yoon, S.-H.; Makkar, R.R.; et al. Outcomes of Redo Transcatheter Aortic Valve Replacement according to the Initial and Subsequent Valve Type. JACC Cardiovasc. Interv. 2022, 15, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Akodad, M.; Landes, U.; Barlow, A.M.; Chatfield, A.G.; Lai, A.; Tzimas, G.; Tang, G.H.; Puehler, T.; Lutter, G.; et al. Coronary Access Following Redo TAVR: Impact of THV Design, Implant Technique, and Cell Misalignment. JACC Cardiovasc. Interv. 2022, 15, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Sellers, S.; Gulsin, G.S.; Tzimas, G.; Landes, U.; Chatfield, A.G.; Chuang, A.; Meier, D.; Leipsic, J.; Blanke, P.; et al. Leaflet and Neoskirt Height in Transcatheter Heart Valves: Implications for Repeat Procedures and Coronary Access. JACC Cardiovasc. Interv. 2021, 14, 2298–2300. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Sellers, S.; Landes, U.; Meier, D.; Tang, G.H.; Gada, H.; Rogers, T.; Caskey, M.; Rutkin, B.; Puri, R.; et al. Balloon-Expandable Valve for Treatment of Evolut Valve Failure: Implications on Neoskirt Height and Leaflet Overhang. JACC Cardiovasc. Interv. 2022, 15, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Meier, D.; Sellers, S.; de Backer, O.; Mylotte, D.; Landes, U.; Frawley, C.; Lynch, L.; Tang, G.H.; Sondergaard, L.; et al. A bench study of balloon-expandable valves for the treatment of self-expanding valve failure. EuroIntervention 2023, 19, 93–102. [Google Scholar] [CrossRef]
- Tarantini, G.; Sathananthan, J.; Fabris, T.; Landes, U.; Bapat, V.N.; Khan, J.M.; Fovino, L.N.; Zaid, S.; Van Mieghem, N.M.; Latib, A.; et al. Transcatheter Aortic Valve Replacement in Failed Transcatheter Bioprosthetic Valves. JACC Cardiovasc. Interv. 2022, 15, 1777–1793. [Google Scholar] [CrossRef]
- Tarantini, G.; Delgado, V.; de Backer, O.; Sathananthan, J.; Treede, H.; Saia, F.; Blackman, D.; Parma, R. Redo-Transcatheter Aortic Valve Implantation Using the SAPIEN 3/Ultra Transcatheter Heart Valves—Expert Consensus on Procedural Planning and Techniques. Am. J. Cardiol. 2023, 192, 228–244. [Google Scholar] [CrossRef]
- Fukui, M.; Okada, A.; Thao, K.R.; Burns, M.R.; Koike, H.; Wang, C.; Phichaphop, A.; Lesser, J.R.; Sorajja, P.; Cavalcante, J.L.; et al. Feasibility of Redo-Transcatheter Aortic Valve Replacement in Sapien Valves Based on In Vivo Computed Tomography Assessment. Circ. Cardiovasc. Interv. 2023, 16, e013497. [Google Scholar] [CrossRef]
- Bieliauskas, G.; Wong, I.; Bajoras, V.; Wang, X.; Kofoed, K.F.; De Backer, O.; Søndergaard, L. Patient-Specific Implantation Technique to Obtain Neo-Commissural Alignment With Self-Expanding Transcatheter Aortic Valves. JACC Cardiovasc. Interv. 2021, 14, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, J.; Fraser, R.; Landes, U.; Rich, C.; Sellers, S.L.; Leipsic, J.; Blanke, P.; Lutter, G.; Frank, D.; Puehler, T.; et al. Repeat transcatheter aortic valve implantation and implications for transcatheter heart valve performance: Insights from bench testing. EuroIntervention 2021, 17, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Grubb, K.J.; Shekiladze, N.; Spencer, J.; Perdoncin, E.; Tang, G.H.L.; Xie, J.; Lisko, J.; Sanchez, J.Z.; Lucas, L.M.; Sathananthan, J.; et al. Feasibility of redo-TAVI in self-expanding Evolut valves: A CT analysis from the Evolut Low Risk Trial substudy. EuroIntervention 2023, 19, e330–e339. [Google Scholar] [CrossRef] [PubMed]
- Vanhaverbeke, M.; Kim, W.-K.; Mylotte, D.; Bieliauskas, G.; Janarthanan, S.; Sondergaard, L.; De Backer, O. Procedural considerations for transcatheter aortic valve-in-valve implantation in a degenerated ACURATE neo prosthesis. EuroIntervention 2023, 18, 1436–1438. [Google Scholar] [CrossRef]
- Khan, J.M.; Bruce, C.G.; Babaliaros, V.C.; Greenbaum, A.B.; Rogers, T.; Lederman, R.J. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc. Interv. 2020, 13, 787–789. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Kamioka, N.; Vavalle, J.P.; Lisko, J.C.; Gleason, P.T.; Paone, G.; Grubb, K.J.; Bruce, C.G.; Lederman, R.J.; Babaliaros, V.C. Balloon-Assisted BASILICA to Facilitate Redo TAVR. JACC Cardiovasc. Interv. 2021, 14, 578–580. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Kitamura, M.; Krieghoff, C.; Lauten, P.; Komatsu, I.; Thiele, H.; Holzhey, D.; Dvir, D. BASILICA for a Degenerated Self-Expanding Transcatheter Heart Valve: Structural Considerations for Supra-Annular Prosthetic Leaflets. JACC Cardiovasc. Interv. 2020, 13, 778–781. [Google Scholar] [CrossRef]
- Damlin, A.; Meduri, C.; Manouras, A.; Verouhis, D.; Linder, R.; Rück, A.; Settergren, M. BASILICA Procedure Prior to Valve-in-Valve TAVR in a Supra-Annular TAV Prosthesis. JACC Case Rep. 2023, 11, 101777. [Google Scholar] [CrossRef]
- De Backer, O.; Landes, U.; Fuchs, A.; Yoon, S.-H.; Mathiassen, O.N.; Sedaghat, A.; Kim, W.-K.; Pilgrim, T.; Buzzatti, N.; Ruile, P.; et al. Coronary Access After TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2528–2538. [Google Scholar] [CrossRef]
- Fovino, L.N.; Scotti, A.; Massussi, M.; Fabris, T.; Cardaioli, F.; Rodinò, G.; Matsuda, Y.; Frigo, F.; Fraccaro, C.; Tarantini, G. Incidence and feasibility of coronary access after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2020, 96, E535–E541. [Google Scholar] [CrossRef]
- Tang, G.H.; Spencer, J.; Rogers, T.; Grubb, K.J.; Gleason, P.; Gada, H.; Mahoney, P.; Dauerman, H.L.; Forrest, J.K.; Reardon, M.J.; et al. Feasibility of Coronary Access Following Redo-TAVR for Evolut Failure: A Computed Tomography Simulation Study. Circ. Cardiovasc. Interv. 2023, 16, e013238. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Grayburn, P.A.; Badhwar, V.; Afonso, L.C.; Carroll, J.D.; Elmariah, S.; Kithcart, A.P.; Nishimura, R.A.; Ryan, T.J.; Schwartz, A.; et al. 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2017, 70, 2421–2449. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.S.; Badhwar, V.; He, X.; Jacobs, J.P.; Gammie, J.S.; Furnary, A.P.; Fazzalari, F.L.; Han, J.; O’Brien, S.M.; Shahian, D.M. The Society of Thoracic Surgeons Mitral Valve Repair/Replacement Plus Coronary Artery Bypass Grafting Composite Score: A Report of The Society of Thoracic Surgeons Quality Measurement Task Force. Ann. Thorac. Surg. 2017, 103, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Vongpatanasin, W.; Hillis, L.D.; Lange, R.A. Prosthetic Heart Valves. N. Engl. J. Med. 1996, 335, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef]
- Paradis, J.-M.; Del Trigo, M.; Puri, R.; Rodés-Cabau, J. Transcatheter valve-in-valve and valve-in-ring for treating aortic and mitral surgical prosthetic dysfunction. J. Am. Coll. Cardiol. 2015, 66, 2019–2037. [Google Scholar] [CrossRef]
- Urena, M.; Brochet, E.; Lecomte, M.; Kerneis, C.; Carrasco, J.L.; Ghodbane, W.; Abtan, J.; Alkhoder, S.; Raffoul, R.; Iung, B.; et al. Clinical and haemodynamic outcomes of balloon-expandable transcatheter mitral valve implantation: A 7-year experience. Eur. Heart J. 2018, 39, 2679–2689. [Google Scholar] [CrossRef]
- Mehaffey, H.J.; Hawkins, R.B.; Schubert, S.; Fonner, C.; Yarboro, L.T.; Quader, M.; Speir, A.; Rich, J.; Kron, I.L.; Ailawadi, G. Contemporary outcomes in reoperative mitral valve surgery. Heart 2018, 104, 652–656. [Google Scholar] [CrossRef]
- Kwedar, K.; McNeely, C.; Zajarias, A.; Markwell, S.; Vassileva, C.M. Outcomes of Early Mitral Valve Reoperation in the Medicare Population. Ann. Thorac. Surg. 2017, 104, 1516–1521. [Google Scholar] [CrossRef]
- Onorati, F.; Mariscalco, G.; Reichart, D.; Perrotti, A.; Gatti, G.; De Feo, M.; Rubino, A.; Santarpino, G.; Biancari, F.; Detter, C.; et al. Hospital Outcome and Risk Indices of Mortality after redo-mitral valve surgery in Potential Candidates for Transcatheter Procedures: Results From a European Registry. J. Cardiothorac. Vasc. Anesthesia 2018, 32, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Taramasso, M. Mitral valve-in-valve, valve-in-ring, and valve-in-MAC: The Good, the Bad, and the Ugly. Eur. Heart J. 2019, 40, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V. Valve-in-valve apps: Why and how they were developed and how to use them. EuroIntervention 2014, 10, U44–U51. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Wei, Z.A.; Sadri, V.; Khan, J.M.; Lisko, J.C.; Netto, T.; Greenbaum, A.B.; Blanke, P.; Oshinski, J.N.; Lederman, R.J.; et al. Dynamic nature of the LVOT following transcatheter mitral valve replacement with LAMPOON: New insights from post-procedure imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 650–662. [Google Scholar] [CrossRef]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.-H.; Leon, M.B.; Piazza, N.; et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions A Consensus Document from the Mitral Valve Academic Research Consortium. J. Am. Coll. Cardiol. 2015, 66, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Ben Zekry, S.; Turaga, M.; Tarazi, S.; Bax, J.J.; Wang, D.D.; Piazza, N.; Bapat, V.N.; Ihdayhid, A.R.; Cavalcante, J.L.; et al. Neo-LVOT and Transcatheter Mitral Valve Replacement: Expert Recommendations. JACC Cardiovasc. Imaging 2020, 14, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Foerst, J.R.; Yazdani, S.; McCabe, J.M.; Paone, G.; Eng, M.H.; Leshnower, B.G.; Gleason, P.T.; et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2521–2534. [Google Scholar] [CrossRef]
- Pirelli, L.; Hong, E.; Steffen, R.; Vahl, T.P.; Kodali, S.K.; Bapat, V. Mitral valve-in-valve and valve-in-ring: Tips, tricks, and outcomes. Ann. Cardiothorac. Surg. 2021, 10, 96–112. [Google Scholar] [CrossRef]
- Killu, A.M.; Collins, J.D.; Eleid, M.F.; Alkhouli, M.; Simard, T.; Rihal, C.; Asirvatham, S.J.; Guerrero, M. Preemptive Septal Radiofrequency Ablation to Prevent Left Ventricular Outflow Tract Obstruction With Transcatheter Mitral Valve Replacement: A Case Series. Circ. Cardiovasc. Interv. 2022, 15, 824–831. [Google Scholar] [CrossRef]
- Eleid, M.F.; Collins, J.D.; Mahoney, P.; Williamson, E.E.; Killu, A.M.; Whisenant, B.K.; Rihal, C.S.; Guerrero, M.E. Emerging Approaches to Management of Left Ventricular Outflow Obstruction Risk in Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 885–895. [Google Scholar] [CrossRef]
- Khan, J.M.; Bruce, C.G.; Greenbaum, A.B.; Babaliaros, V.C.; Jaimes, A.E.; Schenke, W.H.; Ramasawmy, R.; Seemann, F.; Herzka, D.A.; Rogers, T.; et al. Transcatheter Myotomy to Relieve Left Ventricular Outflow Tract Obstruction: The Septal Scoring Along the Midline Endocardium Procedure in Animals. Circ. Cardiovasc. Interv. 2022, 15, e011686. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.S.; Demir, O.M.; Pabari, P.; Sutaria, N.; Chukwuemeka, A.; Gopalan, D.; Hadjiloizou, N.; Sen, S.; Mikhail, G.W.; Ruparelia, N. Double Utility of a Buddy Wire in Transseptal Transcatheter Mitral Intervention. JACC Cardiovasc. Interv. 2019, 12, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, D.B.; Aboulhosn, J.A.; Dvir, D.; Whisenant, B.; Zhang, Y.; Eicken, A.; Ribichini, F.; Tzifa, A.; Hainstock, M.R.; Martin, M.H.; et al. Mid-Term Valve-Related Outcomes After Transcatheter Tricuspid Valve-in-Valve or Valve-in-Ring Replacement. J. Am. Coll. Cardiol. 2019, 73, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; O’Kane, H.; Gladstone, D.J.; Sarsam, M.A.; Campalani, G.; MacGowan, S.W.; Cleland, J.; Cran, G.W. Repeat heart valve surgery: Risk factors for operative mortality. J. Thorac. Cardiovasc. Surg. 2001, 122, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Saha-Chaudhuri, P.; Rankin, J.S.; Conte, J.V. Trends and Outcomes of Tricuspid Valve Surgery in North America: An Analysis of More Than 50,000 Patients From The Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2013, 96, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Dearani, J.A.; Danielson, G.K.; Cetta, F.; Connolly, H.M.; Warnes, C.A.; Li, Z.; Hodge, D.O.; Driscoll, D.J. Comparison of the Outcome of Porcine Bioprosthetic Versus Mechanical Prosthetic Replacement of the Tricuspid Valve in the Ebstein Anomaly. Am. J. Cardiol. 2009, 103, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, C.M.; Shabosky, J.; Boley, T.; Markwell, S.; Hazelrigg, S. Tricuspid valve surgery: The past 10 years from the Nationwide Inpatient Sample (NIS) database. J. Thorac. Cardiovasc. Surg. 2012, 143, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Guenther, T.; Noebauer, C.; Mazzitelli, D.; Busch, R.; Tassani-Prell, P.; Lange, R. Tricuspid valve surgery: A thirty-year assessment of early and late outcome. Eur. J. Cardio Thoracic Surg. 2008, 34, 402–409. [Google Scholar] [CrossRef]
- Aboulhosn, J.; Cabalka, A.K.; Levi, D.S.; Himbert, D.; Testa, L.; Latib, A.; Makkar, R.R.; Boudjemline, Y.; Kim, D.W.; Kefer, J.; et al. Transcatheter Valve-in-Ring Implantation for the Treatment of Residual or Recurrent Tricuspid Valve Dysfunction After Prior Surgical Repair. JACC Cardiovasc. Interv. 2017, 10, 53–63. [Google Scholar] [CrossRef]
- Sanon, S.; Cabalka, A.K.; Babaliaros, V.; Rihal, C.; Gafoor, S.; Webb, J.; Latib, A. Transcatheter Tricuspid Valve-in-Valve and Valve-in-Ring Implantation for Degenerated Surgical Prosthesis. JACC Cardiovasc. Interv. 2019, 12, 1403–1412. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Bhudia, S.K.; Rajeswaran, J.; Hoercher, K.J.; Lytle, B.W.; Cosgrove, D.M.; Blackstone, E.H. Tricuspid valve repair: Durability and risk factors for failure. J. Thorac. Cardiovasc. Surg. 2004, 127, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Van Garsse, L.A.; ter Bekke, R.M.; van Ommen, V.G. Percutaneous transcatheter Valve-in-Valve implantation in stenosed tricuspid valve bioprosthesis. Circulation 2011, 123, e219–e221. [Google Scholar] [CrossRef] [PubMed]
- Taggart, N.W.; Cabalka, A.K.; Eicken, A.; Aboulhosn, J.A.; Thomson, J.D.; Whisenant, B.; Bocks, M.L.; Schubert, S.; Jones, T.K.; Asnes, J.D.; et al. Outcomes of Transcatheter Tricuspid Valve-in-Valve Implantation in Patients With Ebstein Anomaly. Am. J. Cardiol. 2018, 121, 262–268. [Google Scholar] [CrossRef]
- Eng, M.H.; Yadav, P.; Thourani, V.; Fang, K. Transcatheter Tricuspid Valve Replacement for Surgical Failures. Interv. Cardiol. Clin. 2022, 11, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Shivaraju, A.; Kodali, S.; Thilo, C.; Ott, I.; Schunkert, H.; von Scheidt, W.; Leon, M.B.; Kastrati, A.; Kasel, A.M. Overexpansion of the SAPIEN 3 Transcatheter Heart Valve. JACC Cardiovasc. Interv. 2015, 8, 2041–2043. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, D.B.; Cabalka, A.K.; Aboulhosn, J.A.; Eicken, A.; Boudjemline, Y.; Schubert, S.; Himbert, D.; Asnes, J.D.; Salizzoni, S.; Bocks, M.L.; et al. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves: An International, Multicenter Registry Study. Circulation 2016, 133, 1582–1593. [Google Scholar] [CrossRef]
- Godart, F.; Baruteau, A.-E.; Petit, J.; Riou, J.-Y.; Sassolas, F.; Lusson, J.R.; Fraisse, A.; Boudjemline, Y. Transcatheter tricuspid valve implantation: A multicentre French study. Arch. Cardiovasc. Dis. 2014, 107, 583–591. [Google Scholar] [CrossRef]
- Krishnan, S.; Daniels, D.; McCabe, J.M. Novel bipolar preshaped left ventricular pacing wire for transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2018, 92, 1015–1017. [Google Scholar] [CrossRef]
- Cocchieri, R.; Wiegerinck, E.M.; de Groot, J.R.; Bouma, B.J.; Marsman, M.; de Mol, B.A.; Baan, J. Troubleshooting in Transatrial Tricuspid Valve-in-Valve Implantation. Ann. Thorac. Surg. 2012, 94, 1349–1352. [Google Scholar] [CrossRef]



| Data on ViV Outcomes | |||||
|---|---|---|---|---|---|
| Authors | Year | No. of Patients | Patient Cohort | Procedures Performed | Main Outcomes |
| Dvir, D., et al. [6] | 2014 | 459 | VIVID Registry (prospective, multinational, 55 centers, all-comer) | BEV (Sapien) and SEV (mainly Evolut) ViV | 30-day mortality 7.6%, 1.7% stroke; 1-year mortality 16.8%, higher mortality in patients with smaller valves and predominant stenosis of SAVR valve |
| Webb, J.G., et al. [7] | 2017 | 365 | PARTNER 2 ViV Registry (multicenter, continued access) | BEV (using 23- or 26 mm Sapien XT) | 30-day mortality 2.7%, 2.7% stroke; 1-year mortality 12.4%, mean gradient 17.6 mmHg, ≥moderate PVL 1.9% |
| Deeb, G.M., et al. [8] | 2017 | 233 | CoreValve U.S. Expanded Use Study (prospective, nonrandomized) | SEV (23- to 31 mm CoreValve) | 30-day mortality 2.2%, 0.4% stroke; 1-year mortality 14.6%, mean gradient 16.6 mmHg (higher gradients in smaller SAVR, stenosis as mode of failure, patient prosthesis mismatch) |
| Tuzcu EM et al. [9] | 2018 | 1150 | STS/ACC Registry (consecutive patients undergoing TAVI in US; 1:2 matched with native TAVI) | BEV and SEV ViV | 30-day mortality 2.9%, 1.7% stroke; 1-year mortality 11.7%; higher gradients (16 vs. 9 mmHg; highest in small & stenotic SAVRs) compared with native TAVI, but less moderate/severe AR (3.2% vs. 6.6% in native TAVI) |
| Randomized Data on ViV Outcomes | |||||
| Rodés-Cabau, et al. [13] | 2022 | 102 | Patients with small SAVR (≤23 mm) randomized between BEV (n = 46) & SEV (n = 52) | BEV and SEV ViV | Similar clinical outcomes (no death, no stroke) at 30 days; lower mean gradients in SEV vs. BEV (15 vs. 23 mmHg; on echo but not via invasive hemodynamics), tendency to less PPM (44% vs. 64%) |
| Author | Study Period | Cohort | Redo TAVI as % of Total TAVI | Index THV | Redo THV Type | THV Failure | Reported Outcomes |
|---|---|---|---|---|---|---|---|
| Barbanti et al., 2016 [36] | 2014–2016 | Redo-TAVI 14 centers N = 50 | 50/13,876 (0.4%) | SEV: 92% BEV: 8% | SEV: 60% BEV: 40% | PVL: 50% Pure AR: 26% Pure AS: 18% Combined AS/AR: 6% | In-hospital Mortality: 0% Stroke: 2% PPM: 8.6% |
| Landes et al., 2020 [41] | 2008–2021 | Redo-TAVR 37 centers N = 212 | 212/63,876 (<0.01%) | SEV: 61% BEV: 39% | SEV: 50% BEV: 50% | Pure AR: 44.8% Pure AS: 29.7% Combined AS/AR: 25.4% | Peri-procedural Stroke: 1.4% Malposition: 3.3% CO: 0.9% PPM: 9.6% 30-day mortality: 2.8% 1-year mortality: 13.5% |
| Testa et al., 2021 [37] | 2008–2020 | TRANSIT 28 centres N = 172 | 172/40,000 (<0.01%) | SEV: 65% BEV: 35% | SEV: 61% | Pure AR: 56% Pure AS: 33% Combined AS/AR: 10% | In-hospital Mortality: 4.1% Stroke: 3.5% PPM: 8.6% MI: 1.2% 30-day mortality: 7% |
| Percy et al, 2021 [39] | 2012–2017 | US Medicare N = 617 | n = 617/133,650 (<0.01%) | N/A | N/A | N/A | 30-day mortality: 6.2% 1-year mortality: 21% |
| Tanget al, 2023 [38] | 2009–2022 | EXPLANT OR REDO-TAVR 29 centres N = 215 | 215/66,760 (<0.01%) | SEV: 54% BEV: 40% MEV: 6% | SEV: 47% BEV: 50% MEV: 3% | SVD: 63.7% PVL: 32.8% PPM: 0.5% PVT: 0.39% Delayed migration: 0.5% | In-hospital Mortality: 2.8% PPM: 11.1% Stroke 3% CO: 0.5% 30-day mortality: 8% 1-year mortality: 22.3% |
| Makkar et al., 2023 [42] | 2011–2022 | STS/TVT registry All BEVN = 1320 | 1320/350,591 (<0.01%) | SEV: 61% BEV: 39% | BEV: 100% | Mod-severe AR: 64.8% Mean AV gradient: 36.7 | In-hospital Mortality: 3.4% Stroke 1.6% PPM: 6.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokhar, A.A.; Curio, J.; Sticchi, A.; Hartley, A.; Demir, O.M.; Ruparelia, N. Transcatheter Aortic Valve Implantation to Treat Degenerated Aortic, Mitral and Tricuspid Bioprosthesis. J. Clin. Med. 2024, 13, 592. https://doi.org/10.3390/jcm13020592
Khokhar AA, Curio J, Sticchi A, Hartley A, Demir OM, Ruparelia N. Transcatheter Aortic Valve Implantation to Treat Degenerated Aortic, Mitral and Tricuspid Bioprosthesis. Journal of Clinical Medicine. 2024; 13(2):592. https://doi.org/10.3390/jcm13020592
Chicago/Turabian StyleKhokhar, Arif A., Jonathan Curio, Alessandro Sticchi, Adam Hartley, Ozan M. Demir, and Neil Ruparelia. 2024. "Transcatheter Aortic Valve Implantation to Treat Degenerated Aortic, Mitral and Tricuspid Bioprosthesis" Journal of Clinical Medicine 13, no. 2: 592. https://doi.org/10.3390/jcm13020592
APA StyleKhokhar, A. A., Curio, J., Sticchi, A., Hartley, A., Demir, O. M., & Ruparelia, N. (2024). Transcatheter Aortic Valve Implantation to Treat Degenerated Aortic, Mitral and Tricuspid Bioprosthesis. Journal of Clinical Medicine, 13(2), 592. https://doi.org/10.3390/jcm13020592






